Abstract
The existence of a skin resistance for adsorbate mass transport at the surface of a pelletized adsorbent particle or at the surface of adsorbent crystals within a particle has been proven by many macroscopic and microscopic experiments. An isothermal and isobaric column dynamic test method may be used to approximately estimate the relative magnitude of the skin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The potential of existence of a surface barrier (skin resistance) for adsorbate mass transport at the surface of an adsorbent particle is well documented in the literature. The barrier may exist at the surface of (a) a pelletized adsorbent (amorphous or crystalline) particle (with or without a binder), and (b) the individual crystals within an adsorbent particle. Several instances of visible surface barrier of type (a) in pelletized extruded and beaded, abrasion-resistant zeolites have been reported by using scanning electron microscopes (Ogawa et al. 1983; Kumar and Sircar 1986; Moran et al. 2018). Formation of microscopically invisible surface barriers of type (b) have been reported on various types of zeolite crystals by PFG NMR study (Vasenkov et al. 2001); interference microscopy (Kortunov et al. 2004); oscillating microbalance and IR micro imaging (Zhang et al. 2009); thermal frequency response method (Bourdin et al. 1996); zero length chromatography (Teixeira et al. 2013): etc. micro imaging technique has also revealed that the surface barriers on the individual crystals of a zeolite can be different (micro diversity), even though the shape and size of the crystals are similar (Saint Remi et al. 2016). Such a distribution is known to lead to problems on discriminating the existence of surface barriers by “bulk” techniques, which are applied to assemblages of crystals/particles—rather than to a particular crystal/particle (as a unique property of micro-imaging). For circumventing this problem, Brandani et al. have introduced the so-called “partial loading technique” (Brandani et al. 1995). This technique makes use of the characteristic differences in the molecular distribution inside the crystal/particle for diffusion and barrier limitation, which are predominantly affected by the mean value (rather than by the distribution) of the surface resistances. Such differences become, as a matter of course, immediately visible in micro-imaging experiments with the individual crystals/particles (Chmelik et al. 2009).
Analysis of adsorbate uptake curves measured by various gravimetric methods using a transport model, which incorporates both intra crystalline diffusion and surface barrier at the crystal surface, also provide direct quantification of surface barriers at the crystal surfaces (Gao et al. 2019). Interestingly, the traditionally observed large difference between the diffusivity of a gas measured by a macroscopic method (gravimetric or volumetric) and that measured by a microscopic method (NMR pulsed field gradient) is now explained in terms of the presence of a surface barrier (Ruthven 2012). Thus, the existence of a surface barrier is well proven. Consequently, it is important to know the magnitude of the surface barrier resistance (if any) for design of an adsorptive separation process.
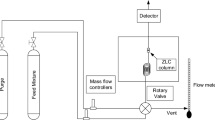
Approximate values of the surface barrier resistances for adsorption of pure N2, O2, and Ar into small particles of pelletized LiLSX zeolite were recently reported (Wu et al. 2014, 2015). Information on this adsorbate-adsorbent combination is practically useful for design of a PSA process for air separation (Sircar and Myers 2003) The protocol used to estimate the surface barrier resistance consisted of (i) measuring the isothermal–isobaric column break-through data at pressure P and temperature T for a pure adsorbate in the Henry’s law region displacing pure non-adsorbing helium at P and T in a column packed with the adsorbent pellets, (ii) estimating an overall, effective mass transfer co-efficient (\({k}_{e}\), s−1) for an adsorbate at P and T using the assumptions that (a) a constant pattern mass transfer zone is formed in the column, and (b) the linear driving force (LDF) model can describe the effective gas to adsorption site mass transfer of the adsorbate, and finally, (iii) subtracting out the contributions of various other series resistances in the transport path offered by the external film, the macropore, the micropore, as well as the resistance equivalence created by the axial dispersion in the packed column (Wu et al. 2014). The following resistances in series model, which is justified when the adsorbate has a linear adsorption isotherm (Henry’s law region), was used (Wu et al. 2014; Ruthven 1984):
where \({k}_{sk}\), \({k}_{ax}\), \({k}_{M}\), \({k}_{m}\) and \({k}_{f}\) are, respectively, the effective mass transfer coefficients (s−1) for the surface skin [type (a), type (b), or combination], the axial dispersion of the adsorbate in the inter-particle gas phase in the packed column, the adsorbent macropores, the adsorbent micropores, and the external gas film around the particles.
Published models (Ruthven 1984) were used for the evaluation of the individual mass transfer coefficients listed inside the parenthesis on the right-hand side of Eq. 1. Clearly, the estimation of \({k}_{sk}\) depends on the accuracy and reliability of these models (Ackley 2019). For example, the possible uncertainty in model estimation of \({k}_{M}\) is discussed elsewhere (Rama Rao and Sircar 2017).
A popular correlation for estimation of \({k}_{ax}\) is given below (Ruthven 1984).
where \({D}_{L}\) (cm2/s) is the effective axial dispersion coefficient for the pure adsorbate in the packed column at P and T, The variable K is the dimensionless Henry’s law constant for the adsorbate at T, the parameter ε (cm3/cm3) is the inter -particle void fraction in the packed column and V (cm/s) is the linear velocity of the gas through the external void space in the column. Equation 2 shows that the effective resistance to adsorbate mass transfer due to axial dispersion (\(1/k_{ax}\)) is directly proportional to \({D}_{L}.\) In other words, a smaller \({D}_{L}\) (larger \({k}_{ax}\)) is preferable for an efficient adsorptive separation process design.
2 Model estimation of \({D}_{L}\)
A vast body of literature exists on dispersion in packed beds (Delgado 2006). A simple empirical model which is frequently-used for practical purposes is given below (Ruthven 1984):
where \({D}_{m}\) (cm2/s) is the molecular diffusivity of a pure adsorbate gas at P and T, and \({d}_{p}\) (cm) is the diameter of the particle in the packed column. The dimensionless group \({P}_{e}\)[ = \({P}_{e}\,[=\,V{d}_{p}/D_{L}]\) is the Peclet number. The variables \({\gamma }_{1}\) and \({\gamma }_{2}\) are empirical constants. It is generally accepted that \({\gamma }_{1}\) is equal to 0.7 (Ruthven 1984). On the other hand, Langer et al. (1978) correlated experimental data from various authors to observe that the limiting value of \({P}_{e}\) (= \({P}_{e}^{\infty }\)) when V is very large, is approximately (a) equal to 2 when \({d}_{p}\) > 0.3 cm and (b) proportional to \({d}_{p}\) (\({P}_{e}^{\infty }\) = 6.67 \({d}_{p}\)) in the region where 0 ≤ \({d}_{p}\) ≤ 0.3 cm. It then follows from Eq. 3 that \({\gamma }_{2}\) = 0.5 when \({d}_{p}\) > 0.3 cm and \({\gamma }_{2}\) = 0.15/\({d}_{p}\) when 0≤ \({d}_{p}\) ≤ 0.3 cm. Thus:
Equations 2 and 4 indicate that \({D}_{L}\) increases and \({k}_{ax}\) decreases with increasing \({d}_{p}\) when \({d}_{p}\) ≥ 0.3 cm, while Eqs. 2 and 5 indicate that both \({D}_{L}\)and \({k}_{ax}\) are independent of \({d}_{p}\) when \({d}_{p}\) < 0.3 cm.
No clear physical explanation can be found for the observed linear dependence of \({P}_{e}^{\infty }\) on \({d}_{p}\) when \({d}_{p}\) ≤ 0.3 cm. In particular, the consequential lack of dependence of \({D}_{L }\)on \({d}_{p}\) is strange and awkward. Agglomeration (cluster formation) of small particles, aggravated channeling, gas maldistribution in column, etc. have been cited as possible reasons (Langer et al. 1978; Ruthven 1984). These effects depend on the nature and compactness of the packing in the column, particle size and shape distribution, vessel design, etc. which may be difficult to reproduce in experiments and nearly impossible to model reliably. Thus, estimation of \({D}_{L}\) or \({k}_{ax}\) using Eqs. 2 and 5 may be uncertain.
3 Sample calculation of \({k}_{ax}\)
It was reported earlier that the effective skin resistance (\(1/{k}_{sk}\)) for adsorption of pure O2 in the Henry’s law region into small zeolite particles of LiLSX (\({d}_{p}\) = 0.0525 cm) at P = 2.0 atm and T = 303.1 K was ~ 62% of the overall resistance (\(1/{{k}_{e}}\)) for mass transport (Table I, Wu et al. 2014). The details of the column size and the experimental conditions can be found elsewhere (Wu et al. 2014). Equation 4 was chosen over Eq. 5 to estimate \({D}_{L}\) even though \({d}_{p}\) was less than 0.3 cm, for the reasons given earlier. An additional support for selection of Eq. 4 was provided by Edwards and Richardson (1968) who reported the following empirical expression to describe experimentally measured values of \({D}_{L}\) for particles diameters \(\left({d}_{p}\right)\) ranging between 0.0377 and 0.60 cm and Reynolds number (Re) ranging between 0.008 and 50:
The relative values of \({D}_{L}\) for axial dispersion of O2 in He calculated by Eqs. 4 and 6 were comparable in magnitude, being respectively, ~ 0.41 and 0.28 cm2/s under the relevant conditions (P = 2.0 atm, T = 303.1 K, \({d}_{p}\) = 0.0525 cm, V = 6.0 cm/s, Re ~ 1.5). On the other hand, the corresponding value of \({D}_{L}\) estimated by Eq. 5 was ~ 1.16 cm2/s which was ~ 2.8 times larger than that estimated by Eq. 4.
Nonetheless, it can be shown that the skin resistance would still be ~ 35% of the overall resistance if Eq. 5 was chosen for estimation of \({D}_{L}\). Thus, the skin resistance for this system was not negligible as suggested by Moran et al. 2018) even when Eq. 5 was used for the estimation of \({D}_{L}\). It was also suggested by the same authors that a large skin resistance should be visible in a SEM picture. That, of course depends on how thick the type (a) skin is. A high skin resistance can be exhibited by a very thin skin depending on the imperviousness of the skin. A type (b) skin, on the other hand, cannot be seen by a microscope.
4 Summary
The existence of a skin resistance for adsorbate mass transport at the surface of a pelletized adsorbent particle or at the surface of the adsorbent crystals within a particle has been proven by many macroscopic and microscopic experiments. An isobaric–isothermal column dynamic test method may be used for approximate estimation of the magnitude of the skin resistance for transport of a pure gas into an adsorbent particle in the Henry’s law region. A protocol for data analysis is described. The skin resistance is extracted from the experimentally measured over-all transport resistance by subtracting out the contributions of other resistances in the path of transport, which are estimated using standard models published in the literature. Thus, the goodness of these individual models may be important.
It is reconfirmed that a substantial skin resistance exists for adsorption of O2 into small particles of LiLSX zeolite even when extreme models for estimation of the coefficient of axial dispersion of O2 in packed columns are used.
References
Ackley, M.D.: Medical oxygen concentrator: a review of progress in air separation technology. Adsorption 213, 235–245 (2019)
Bourdin, V., Grenier, Ph, Meunier, F., Sun, L.M.: Thermal frequency response method for the study of mass transfer kinetics in adsorbents. AIChE J. 42, 700 (1996)
Brandani, S., Hufton, J., Ruthven, D.: Self-diffusion of propane and propylene in 5A and 13X zeolite crystals studied by the tracer ZLC method. Zeolites 15(7), 624 (1995)
Chmelik, C., Heinke, L., Kortunov, P., Li, J., Olson, D., Tzoulaki, D., Weitkamp, J., Kärger, J.: Ensemble measurement of diffusion: novel beauty and evidence. ChemPhysChem 10, 2623 (2009)
Delgado, J.M.P.Q.: A critical review of dispersion in packed bed. Heat Mass Transfer 42, 229 (2006)
Edwards, M.F., Richardson, J.F.: Gas dispersion in packed beds. Chem. Eng. Sci. 23, 109 (1968)
Gao, M., Li, H., Yang, M., Gao, S., Wu, P., Tian, P., Xu, S., Ye, M., Liu, Z.: Direct quantification of surface barriers for mass transport in nano-porous crystalline materials. Commun. Chem. 2, 43 (2019)
Kortunov, P., Vasenkov, S., Chemlik, C., Karger, J., Ruthven, D.M., Wloch, J.: Influence of defects on the external crystal surface on molecular uptake into MFI type zeolite. Chem. Mater. 18, 3552–3558 (2004)
Kumar, R., Sircar, S.: Skin resistance for adsorbate mass transfer into extruded adsorbent pellets. Chem. Eng. Sci. 41, 2215 (1986)
Langer, G., Roethe, A., Roethe, K.P., Gelbin, D.: Heat and mass transfer in packed beds—III. Axial mass dispersion. Int. J. Heat Mass Transfer 21, 751 (1978)
Moran, A., Patel, M., Talu, O.: Axial dispersion effects with small diameter adsorbent particles. Adsorption 24, 333 (2018)
Ogawa, M., Tetsuo, M., Goto, K., Sugai, H., Takahashi, M.: Abrasion-resistant granular zeolite and process for preparation thereof. U.S. Patent 4,420,419 (1983)
Rama Rao, V., Sircar, S.: Comments on the reliability of model simulation of a rapid pressure swing adsorption process for high purity product. I & EC Res. 56, 8991 (2017)
Ruthven, D.M.: Principles of Adsorption and Adsorption Processes. Wiley, New York (1984)
Ruthven, D.M.: Diffusion in type A zeolites: new insights from old data. Microporous Mesoporous Mater. 162, 69 (2012)
Saint Remi, J.C., Lauerer, A., Chmelik, C., Vandendael, I., Terryn, H., Baron, G.V., Denaver, J.F., Karger, J.: The role of crystal diversity in understanding mass transfer in nano-porous materials. Nat. Mater. 15, 401 (2016)
Sircar, S., Myers, A.L.: In: Aurbach, S.M., Carrado, K.A., Dutta, P.K. (eds.) Gas Separation by Zeolites, Chap. 22 in Handbook of Zeolite, Catalysts, and Microporous Materials, pp. 1063–1105. Marcell Dekker, New York (2003)
Teixeira, A.R., Chang, C.C., Coogan, T., Kendall, R., Fan, W., Dauenhauer, P.J.: Dominance of surface barriers in molecular transport through silicalite—1. J. Phys. Chem. C 117, 25545 (2013)
Vasenkov, S., Bohlmann, W., Galvosas, P., Geier, O., Liu, H., Karger, K.: PFG NMR study of diffusion in MFI type zeolites: evidence of the existence of intracrystalline transport barriers. J. Phys. Chem. B 105, 5922–5927 (2001)
Wu, C.-W., Kothare, M.V., Sircar, S.: Column dynamic study of mass transfer of pure N2 and O2 into small particles of pelletized LiLSX zeolite. I & EC Res. 53, 17806 (2014)
Wu, C.W., Kothare, M.V., Sircar, S.: Equilibrium isotherm and mass transfer coefficient for adsorption of pure argon on small particles of pelletized lithium exchanged low silica zeolite. I & EC Res. 54, 2385 (2015)
Zhang, L., Chmelik, C., van Laak, A.N.C., Karger, J., de Jongh, P.E., de Jong, K.P.: Direct assessment of molecular transport in mordenite: dominance of surface resistances. Chem. Commun. (2009). https://doi.org/10.1039/B914391B
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sircar, S. Remarks on adsorbent surface barrier to adsorbate mass transport. Adsorption 27, 167–170 (2021). https://doi.org/10.1007/s10450-020-00234-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-020-00234-3