Abstract
The use of solid wastes and industrial by-products to prepare CO2 adsorbents is an alternative to conventional reagent grade raw materials that has recently gained interest. Among waste materials, slag has a high content of silica and calcium and is the largest solid by-product from iron and steel industry, thus its use can reduce the production costs of CO2 adsorbent materials, such as lithium silicates, which are applied in capture processes at high temperatures. Li4SiO4 has potential applications in post-combustion CO2 capture as well as in H2 production by sorption enhanced steam reforming process. In this study, Li4SiO4 was prepared using solid-state reaction and two iron and steel slags as SiO2 sources to evaluate their characteristics and CO2 capture capacities. The slag-derived lithium silicates (S1-Li4SiO4 and S2-Li4SiO4) were characterized by XRD, adsorption-desorption N2 and SEM. Different capture tests at CO2 partial pressures (\(P_{{{\text{CO}}_{2} }}\)) of 0.05, 0.10, 0.15 and 0.20 were performed using thermogravimetric (TG) and temperature programmed (TPC-TPDC) techniques. The kinetic parameters of the CO2 capture process were obtained by fitting the experimental results to the Avrami–Erofeev model. Finally, the cyclic behavior of S1-Li4SiO4 and S2-Li4SiO4 was analyzed in \(P_{{{\text{CO}}_{2} }}\) of 0.2 and 0.05. XRD patterns showed that Li4SiO4 was the main crystal phase (60 wt%) present in S1-Li4SiO4 and S2-Li4SiO4 in addition to calcium phases such as Li2CaSiO4, Ca3SiO5 and CaO. According to the TG and TPC-TPDC tests, the derived lithium silicates showed CO2 uptake three times greater than the values recorded for Li4SiO4 (134 mgCO2/g sorbent for S1-Li4SiO4) produced from pure reagents, at \(P_{{{\text{CO}}_{2} }}\) between 0.2 and 0.05 and 650 °C. Furthermore, these materials had kinetic constants at least one order of magnitude higher than those reported for Li4SiO4, at the aforementioned operating conditions. Both materials exhibited an excellent stability during 20 cycles of CO2 adsorption/desorption. These results showed that slags can be used as silica source to produced adsorbents with better performance and stability in the CO2 capture process at high temperature than the one of Li4SiO4 produced from pure reagents, at \(P_{{{\text{CO}}_{2} }}\) of 0.2–0.05.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Industrial by-products are materials produced during the manufacture of a primary product and its use has been encouraged in order to reduce CO2 emissions, avoid disposing wastes in landfills, increase resource efficiency and generate revenue. Over the past 20 years, the use of the steel industry’s by-products has increased significantly. The main by-products generated during iron and steel production are slags (90% by mass), dust and sludge. According to Euroslag, ferrous slag is considered a by-product in liquid state, directly after its manufacture, with or without processing steps; it is first considered as waste but ceases to be a residue after a number of recovery measures (EUROSLAG 2008). On average, the production of one tonne of crude steel results in around 170 or 400 kg of slags for electric arc furnace (EAF) or blast furnace (BF) routes, respectively. It is estimated that global iron slag output in 2017 was around 300 to 360 million tons, while steel slag was around 170 to 250 million tons (WorldSteel 2016). In 2016, the iron and steel slag production in Europe was of 41 Mt where the mainly uses were cement and concrete additive (46.8%), road construction (29.8%), metallurgical use (6.8%), hydraulic engineering (1%), fertilizer (1.2%), interim storage (4.2%), landfill (5.7%) among others.
At the same time, there is a continuous increase of the worldwide energy consumption and therefore of CO2 emissions. The average CO2 concentration in the world on February 2019 was 411.75 ppm, 47% higher than prior to industrial revolution (280 ppm) (U.S. Department of Commerce 2019). In addition to the improvement in energy and process efficiencies in the industry, the reduction of anthropogenic greenhouse gas emissions is one of the main challenges for the coming years. Besides to energy efficiency, other strategies such as emission efficiency (including fuel and feedstock switching and CO2 capture and storage (CCS), material use efficiency (e.g., less scrap), recycling and re-use of raw materials and products are required (IPCC 2014).
Post-combustion capture provides a short-term approach to mitigate the concentration of the CO2, generated by power plants, metallurgical and cement industries, among others. In post-combustion, the gas stream contains mainly CO2 and N2, with a partial pressure of CO2 of around 0.05–0.2 bar, where the temperature range depends on the concerned industrial sector. CO2 capture has also been used in the sorption enhanced methane steaming reforming (SESMR) process, which aims to enrich the hydrogen concentration in the gas stream (95%) (Albo Sánchez 2015; Yancheshmeh et al. 2015). In post-combustion and also in SESMR, high-temperature (450–700 °C) solid sorbents are more cost-effective and efficient than low-temperature amine-based materials as the direct separation of CO2 from the high-temperature exhaust gases saves large amounts of energy (Dou et al. 2016; Ochoa-Fernández et al. 2005; Yancheshmeh et al. 2015). Recently, Garcia et al. (2017), carried out the integration of lithium looping post-combustion carbon capture technology in a NGCC (natural gas combined cycle) power plant following the EBTF methodology. The results showed that lithium looping have approximately 0.6 percentage points lower energy penalty compared to the best performing chemical absorption capture system. Among high-temperature CO2 sorbents, lithium-based materials have aroused great interest due to their high CO2 capture capacity, selectivity, fast kinetics and good regeneration properties. Lithium orthosilicate (Li4SiO4), the most studied lithium based ceramic, shows suitable reactivity, thermal stability during several sorption-desorption cycles and the fastest CO2 sorption rate over a wide range of temperatures and CO2 concentrations (Albo Sánchez 2015; Amorim et al. 2016; Hu et al. 2019; Kaniwa et al. 2018; Rodríguez-Mosqueda and Pfeiffer 2010; Zhang et al. 2019). Kato et al. (2005) reported that the CO2 adsorption on Li4SiO4 was up to 30 times faster than on Li2ZrO3 at 500 °C and 20 vol% CO2. Although its theoretical adsorption capacity (8.34 mmol CO2/g) is lesser than the value of calcined limestone (17.8 mmol CO2/g), this material is a promising adsorbent since shows lower values of energy requirements for regeneration (< 750 °C vs 950 °C) and capture temperature of CO2 emissions, with respect to CaO (Chen et al. 2016; Kato et al. 2005; Seggiani et al. 2013). The double shell model is widely accepted to explain the CO2 sorption mechanism on Li4SiO4, as well as on other alkaline ceramics, and comprises two stages (e. g. Na2ZrO3, Li5AlO4, Li8SiO6) (Alcérreca-Corte et al. 2008; Avalos-Rendón et al. 2009; Castillo Villa et al. 2015; Durán-Muñoz et al. 2013; Martínez-dlCruz and Pfeiffer 2012; Zhang et al. 2019). In the first stage, the CO2 reacts with the surface of lithium silicate particles to form an external shell composed of Li2CO3 and Li2SiO3, according to the following reaction (Amorim et al. 2016; Seggiani et al. 2011; Zhang et al. 2019):
In a second stage, the reactants have to diffuse through the external shell to react with each other. The bulk diffusion process begins, and the CO2 continues reacting with the Li4SiO4 particles that remain unreacted. This last stage has been explained from two different perspectives; some works propose that the CO2 diffusion through the external shell is the dominant process, while others put forward that the intercrystalline diffusion of Li+ and O2− ions is the main phenomena (Kato et al. 2005; López Ortiz et al. 2014). To enhance its CO2 chemisorption Li4SiO4 has been modified with different elements such as K, Na, Al, Fe, and V, among others. This promotes the diffusion of lithium and oxygen ions and/or CO2 due to the formation of vacancies, eutectic phases, or different lithium secondary phases after carbonation process (Albo Sánchez 2015; Gao et al. 2017; Gauer and Heschel 2006; Ortiz-Landeros et al. 2012; Seggiani et al. 2011; Wang et al. 2017a, b). In addition, a Ca-Li4SiO4 sorbent was synthetized, considering the high CO2 capture capacity demonstrated by CaO (0.78 \({\text{g}}_{{{\text{CO}}_{2} }}\) g/CaO); it was shown that the transformation of the Ca species from Ca2SiO4 to Li2CaSiO4 during the CO2 adsorption process promotes the transfer of CO2 to Li4SiO4, and then the inverse process favors the CO2 desorption (Chen et al. 2016).
Moreover, there is enough evidence that the different sources of silica used in the synthesis of Li4SiO4 produce changes in the particle size and microstructure, thus generating characteristic behavior of CO2 sorption (Hu et al. 2019). Bearing in mind that the development of low-cost CO2 adsorbent materials will undoubtedly enhance the competitiveness of CO2 capture technologies and other applications such as sorption enhanced steam fuel reforming for hydrogen production and thermochemical energy storage. Some scientific papers have been published on the use of residues and by-products, generated from industrial and agricultural operations, as raw materials for CO2 adsorbents. These materials are low-cost and abundant, and therefore their use may contribute to reduce the total costs of CO2 capture technologies and at the same time, show promising CO2 capture capacities. For this reason different silica sources such as fly ash, rice husk ash and, recently, blast furnace slag, have been used to prepare lithium orthosilicate; the results indicate that, as expected, the adsorption capacity is influenced by the silica source (Olivares-Marín and Maroto-Valer 2012; Sanna et al. 2015; Wang et al. 2011, 2018). Olivares-Marin et al. (2010) investigated lithium-based sorbents made from fly ashes for CO2 capture at high temperatures. The obtained Li4SiO4-based sorbents did not show CO2 adsorption in 100 vol% CO2, however, the addition of K2CO3 enhanced the sorption capacity to 107 mg CO2/g sorbent (at 600 °C and 40 mol% K2CO3). In addition, Izquierdo et al. (2018), studied the effect of the silica source (pure reagent or fly ash) and the preparation method (solid state reaction and precipitation method) on the CO2 uptake of the derived Li4SiO4. The material prepared from fly ash and Li2CO3 by solid state reaction showed just a 5.9 wt% of CO2 uptake (in 92% CO2), as the calcium silicates formed limit the CO2 capture on the CaO. In another work performed by Wang et al. (2018), silica extracted with an acid leaching method from a blast furnace slag was also used to prepare Li4SiO4 through the solid state reaction method with Li2CO3 at 873 °C. The adsorption capacity (in pure CO2) obtained at 600–650 °C was 100.8 mg CO2/g sorbent (28% conversion) and increased to 98% conversion at 700 °C. This was associated to the small particle size and metal impurities present in the slag, such as potassium and calcium.
Based on the above information, so the use of waste or by-product materials as source of silica to prepare Li4SiO4 has aroused growing interest due to its potential applications in high temperature post-combustion CO2 capture as well as in H2 production by SESR process. In addition to reducing the cost of the sorbent, the use of iron and steel slags, as SiO2 source, also introduces other elements in to Li4SiO4 crystal lattice which could modify the CO2 capture capacity and the kinetic behavior at low CO2 partial pressure. Therefore, in this work Li4SiO4 was prepared from two different iron and steel slags by solid-state reaction method. The slag-derived lithium silicates were characterized and tested as CO2 adsorbents at \(P_{{{\text{CO}}_{2} }}\) of 0.05, 0.10, 0.15 and 0.20. Furthermore, the kinetic parameters of the CO2 process and the cyclic performance of the adsorbents were also determined and compared with the Li4SiO4 prepared with analytical grade reagents.
2 Experimental
2.1 Sorbent preparation and characterization
Two slag samples, named S1 and S2, were obtained from different iron and steel Mexican industries. S1 is a blast furnace slag while S2 is an electric arc furnace slag. The chemical composition was previously determined by X-ray fluorescence and is shown in Table 1 (Mercado-Borrayo et al. 2013).
The slag-derived lithium silicates were prepared using the solid-state reaction method by mixing Li2CO3 reagent grade (99.8%, Meyer) with the iron and steel slags (S1 and S2), as SiO2 sources, in a Li2CO3:SiO2 molar ratio of 2.1:1 (an excess of 10 mole percent was added due to the lithium sublimation). Both mixtures were calcined at 850 °C for 8 h, and they were named S1-Li4SiO4 and S2-Li4SiO4. For comparison purposes, pure lithium orthosilicate (Li4SiO4) was also prepared from Li2CO3 and SiO2 reagent grade (325 mesh, 99.5%, Aldrich) using the same calcination conditions. All calcined materials were homogenized in an agate mortar to identify their mineralogical phases by the X-ray diffraction (XRD) technic in an Empyrean diffractometer with CuKα radiation and PIXcel3D detector. The XRD measurements were carried out over a 2-θ angle of 5°–70° in steps of 0.003° and 40 s integration time. The identification of phases was performed using the Inorganic Crystal Structure Database (ICSD). The normalized Relative Intensity Ratio (RIR) method was used to conduct a semi-quantitative determination of these phases. In the RIR method, the variation of peak intensities with concentration is considered nonlinear and the former is derived by standards Chung (1974). Recognized that if all phases in a mixture are known and if RIR is known for all of those phases, then the addition of the fractions of all the phases must be equal to 1. N2 adsorption–desorption isotherms of the materials were obtained on a Minisorp II instrument (Bel Japan) at 77 K using the multipoint technique (N2 from Praxair, grade 4.8) and the specific surface area was calculated by the Brunauer–Emmett–Teller (BET) method. Prior to analysis, the samples were degassed at room temperature in N2 flow for 12 h. The microstructural characterization was completed with scanning electron microscopy (SEM); the backscattered electron images were obtained from a Philips XL 20 instrument.
2.2 CO2 sorption tests
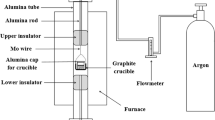
The CO2 capture capacity of the resultant slag-derived lithium silicates was evaluated by thermogravimetric and temperature programmed techniques. Before the CO2 sorption tests, the samples were pretreated in N2 flow (60 mL/min) at 700 °C to eliminate any previous carbonation. Then, temperature programed carbonation-decarbonation (TPC-TPDC) experiments were carried out using a Belcat B (Bel Japan) equipped with a thermal conductivity detector (TCD), which records the changes in CO2 concentration in the outlet gas stream. In these experiments, 50 mg of sorbents were kept in contact with a gas stream containing 60 mL/min of 5%mol CO2 (He balance, Praxair, certificated standard), and were heated up to 800 °C using a temperature ramp of 5 °C/min. In addition, thermogravimetric experiments were performed on a Labsys Evo TG analyzer from Setaram, with 20 mg of sample. The dynamic performance of CO2 sorption was obtained by heating the samples from room temperature to 850 °C at 5 °C/min using \(P_{{{\text{CO}}_{2} }}\) of 0.2 and 0.05 (balance with N2). The CO2 sorption over the time was measured between 580 and 700 °C. In each test, the temperature was increased in N2 flow (60 mL/min); afterwards, the flow was switched to a \(P_{{{\text{CO}}_{2} }}\) of 0.05 for 180 min. Besides, the influence of different \(P_{{{\text{CO}}_{2} }}\) values in the gas mixture (\(P_{{{\text{CO}}_{2} }}\) = 0.2, 015, 0.1 and 0.05) was evaluated at the best CO2 sorption temperature for each material.
2.3 Cyclic performance tests
The cyclic performance of each material was analyzed by consecutive stages of sorption-desorption. Sorption was carried out in \(P_{{{\text{CO}}_{2} }}\) of 0.2 and 0.05 at 600 and 650 °C for S2-Li4SiO4 and S1-Li4SiO4, respectively, and the desorption process was performed at 750 °C in N2 flow.
3 Results and discussion
3.1 Slag characterization
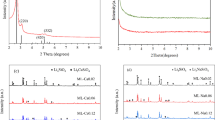
Figure 1 shows the XRD patterns of iron and steel slags. The XRD profiles of both slags presented characteristic peaks of SiO2, in addition to aluminite (Al2SO4(OH)4·7H2O) and tricalcium silicate (Ca3SiO5) in S1, and dicalcium silicate (Ca2SiO4) and brownmillerite (Ca2(AlFe)2O5) in S2. The textural characterization of both slags is shown in Fig. 2. The N2 adsorption−desorption isotherms correspond to type II, with a narrow H3-type hysteresis loop, according to the IUPAC classification (Lowell et al. 2004); this behavior corresponds to nonporous materials. The specific surface area was calculated using the BET method with values for S1 and S2 of 4.4 and 1.2 m2/g, respectively.
In addition, the morphology of the particles was observed by SEM. The backscattering electron micrographs corroborated that both slags were nonporous solids, with leaves and needles shapes for S1, and polyhedral particles for S2.
3.2 Sorbents characterization
Figure 3 shows the presence of Li4SiO4 on the XRD patterns of slags-derived lithium silicates. Also, other lithium and calcium compounds (CaO, Li2CaSiO4, Ca3SiO5 and β-LiAlSiO4) were identified. According to the semiquantitative analysis of the crystalline phases using a normalized RIR method, Li4SiO4 was the main phase in both slag-derived products with 60% w/w, while CaO was the secondary phase with 25 and 14%, in S1-Li4SiO4 and S2-Li4SiO4, respectively. The Li2CaSiO4 can be produced during the synthesis process through a chemical reaction between CaO/CaCO3, Li2CO3 and SiO2 (Chen et al. 2016). Moreover, the calcium phases such as CaO, Ca3SiO5 and Li2CaSiO4 might enhance the CO2 uptake in the capture tests at high temperature. Bejarano Peña (2018), obtained a similar percentage of Li4SiO4 crystalline phase (59 w/w%) after the synthesis of another batch using the same steel slag.
The N2 adsorption–desorption isotherms of lithium silicates presented in Fig. 4 correspond to type II isotherms with a very narrow H3-type hysteresis loop, according to the IUPAC classification (Lowell et al. 2004); this behavior is in accordance with the synthesis method which produces nonporous materials. The specific surface area calculated with the BET method was 0.6 m2/g for the pure Li4SiO4 and 1.0 and 1.2 m2/g for S1-Li4SiO4 and S2-Li4SiO4, respectively. These surface area values are similar to those obtained in other alkaline ceramics prepared in the same way and used as CO2 sorbents.
3.3 CO2 sorption tests
The evaluation of the CO2 capture properties of the synthesized materials started with a dynamic thermogravimetric test in \(P_{{{\text{CO}}_{2} }}\) of 0.2 and 0.05, with N2 balance (Fig. 5). All samples showed a similar behavior in the CO2 concentrations tested. The weight for the three materials increased slowly in a low temperature range, from around 150 to 300 °C, and then sharply at 350 and 450 °C, in slag-derived materials and pure Li4SiO4, respectively. The diffusion process was activated above 450 °C and CO2 uptake increased in all samples. Li4SiO4 reached the maximum adsorption of 56.4 mgCO2/g material at 555 °C and 29.8 mgCO2/g material at 631 °C, for \(P_{{{\text{CO}}_{2} }}\) of 0.2 and 0.05, respectively. At higher temperatures, the pure Li4SiO4 started to release CO2 rapidly. In addition, this material diminished by 47% its CO2 capture capacity when the \(P_{{{\text{CO}}_{2} }}\) decreases from 0.20 to 0.05. On the contrary, S1-Li4SiO4 and S2-Li4SiO4 presented outstanding improvements in CO2 capture capacities, with values at least two times greater than the capacity of pure Li4SiO4. For the CO2 partial pressure of 0.2, the maximum CO2 uptakes from 600 °C to T ≥ 815 °C for S1-L4SiO4 and S2-L4SiO4, were 115.8 mgCO2/g material and 130.8 mgCO2/g material, respectively. After this temperature, the desorption process began for both materials. A decrease in the CO2 concentration (\(P_{{{\text{CO}}_{2} }}\) = 0.05) diminished the CO2 adsorption by almost 15%, obtaining values of 99.7 and 115.8 mgCO2/g material, in S1-L4SiO4 and S2-L4SiO4, respectively. In addition, when \(P_{{{\text{CO}}_{2} }}\) decreased from 0.2 to 0.05, the desorption temperature shifted from ≥ 815 °C to 770 °C in S1-L4SiO4 and 720 °C in S2-L4SiO4. These thermal shifts of the desorption process can be related to CO2 adsorption-desorption equilibrium changes generated by the CO2 concentration in the gas mixture.
The differences in the CO2 adsorption capacities, between pure Li4SiO4 and slag-derived lithium silicates are related to the presence of calcium phases in the slag-derived materials. Dicalcium and tricalcium silicates can transfer CO2 to Li4SiO4 during the sorption process to generate CaCO3 and SiO2, and then CaCO3 reacts with Li4SiO4 to produce Li2CaSiO4 and Li2CO3 (Chen et al. 2016). It is important to note that the CO2 adsorption behavior of Li4SiO4 is affected by the type of SiO2 used, the synthesis method and, as expected, by the CO2 concentration. Some studies showed a very low CO2 adsorption capacities, such as 40 mgCO2/g material (Rodríguez-Mosqueda and Pfeiffer 2010; Romero-Ibarra et al. 2013), even in saturated CO2 atmosphere, while in others works, it is reported that they almost reached the maximum theoretical CO2 capture capacity, between 300 and 350 mgCO2/g material (Chen et al. 2016; Gao et al. 2017; Wang et al. 2016, 2017b, 2018).
The sorption behavior was also observed in the TPC and TPDC results, as illustrated in Fig. 6, which is useful to determine the CO2 adsorption-desorption capacity and the inversion temperature. In the carbonation process for the pure Li4SiO4, a double peak could be observed; the first (420 °C) is assigned to the CO2 adsorption on the surface and the second (500 °C) to the CO2 adsorption in the bulk of the material controlled by diffusive processes. This produces the formation of an external shell of Li2CO3 and Li2SiO3 (Chowdhury et al. 2013; Qi et al. 2013). The inversion temperature was identified at 515 °C, which is in agreement to the thermodynamic calculations reported by Chowdhury et al. (Chowdhury et al. 2013) for the same condition (500 °C and a CO2 partial pressure of 0.05). Duan et al. (Duan et al. 2013; Duan et al. 2012) also reported the turnover temperature for pure Li4SiO4 in pre- and post-combustion conditions, as the temperature above at which lithium silicate cannot adsorb CO2 and starts to release it according to the CO2 partial pressure.
In addition, some differences in the TPC-TPDC profiles were observed between pure Li4SiO4 and lithium silicates derived from slags. A third peak of adsorption was identified after 500 °C, which can be associated to the CO2 adsorption on the calcium phases present. Also, the CO2 adsorption peaks for S1-Li4SiO4 appeared at lower temperatures, the maximum TPC peak is observed at 381 °C, while the desorption of CO2 started after 588 °C. For S2-Li4SiO4, the inversion temperature increased slightly to 600 °C with the maximum TPC peak at 540 °C. The desorption peak, in both slags-derived lithium silicates, showed the maximum value at 770 °C.
The effect of temperature and of different \(P_{{{\text{CO}}_{2} }}\) (0.05, 0.10, 0.15 and 0.20) was evaluated by performing TG experiments in which CO2 adsorption was measured over time. First, the CO2 capture performance of lithium silicates using a \(P_{{{\text{CO}}_{2} }}\) of 0.05 and temperatures from 580 to 700 °C (according with the dynamic TG results), is shown in Fig. 7. The maximum CO2 capture, of both derived lithium silicates, was reached at very short times, such as 15 min, despite the low CO2 concentration used in the tests. Figure 7a shows the S1-Li4SiO4 curves where, as expected, the amount of adsorbed CO2 increased with the rise in temperature as follows: 84.5, 97.3 and 134.4 mgCO2/g material at 580, 600 and 650 °C, respectively. The highest CO2 capture value was observed at 650 °C. Afterwards, an increase in the temperature to 700 °C resulted in a decrease of the CO2 capture by 40 percent (79.0 mgCO2/g material), associated with the desorption process and the sintering of the material.
S2-Li4SiO4 (Fig. 7b) shows a similar behavior for the CO2 uptake to that of S1-Li4SiO4. The adsorption capacities values of S2-Li4SiO4 were 106.5, 118.6, 103.0 and 97.1 mgCO2/g material at 580, 600, 650 and 700 °C, respectively. According to these results, the best CO2 capture was reached at 600 °C, which is a lower temperature than that of S1-Li4SiO4, nevertheless, the capture was higher for S1-Li4SiO4 than the one of S2-Li4SiO4. The adsorption of CO2 on S2-Li4SiO4 decreased above 600 °C, that is, the adsorption-desorption equilibrium is different and the desorption process started at lower temperatures compared to those of S1-Li4SiO4. Considering as calculation basis the theoretical maximum CO2 capture for the pure Li4SiO4 is 8.3 molCO2/kg sorbent (367 mgCO2/g sorbent) (Zhang et al. 2019), the slag derived-lithium silicates reached an efficiency of 36.57 and 32.37%, for S1-Li4SiO4 and S2-Li4SiO4, respectively, using a \(P_{{{\text{CO}}_{2} }}\) = 0.05. Figure 8 shows the CO2 uptake as function of time for the pure Li4SiO4, which is compared with that of derived silicates, under the same experimental conditions. With a \(P_{{{\text{CO}}_{2} }}\) = 0.20 (Fig. 8a) the CO2 uptake was very slow and low, where the equilibrium was not reached after 3 h (between 500 and 600 °C). The maximum CO2 adsorption was 60 mgCO2/g sorbent (550 °C), similar to data previously reported (Hu et al. 2019; Monica et al. 2013). In addition, when the CO2 concentration decreased from 0.2 to 0.05, the CO2 uptake became slower and decreased to only 5 mgCO2/g sorbent in \(P_{{{\text{CO}}_{2} }}\) = 0.05.
Figure 9 shows the CO2 capture as a function of time on the slag-derived lithium silicates, at the best adsorption temperature (650 °C for S1-Li4SiO4 and 600 °C for S2-Li4SiO4), using \(P_{{{\text{CO}}_{2} }}\) of 0.05, 0.10, 015 and 0.20. In both materials, the CO2 capture increased as the CO2 concentration augmented from 0.1 to 0.2, but this behavior was not observed for \(P_{{{\text{CO}}_{2} }}\) = 0.05. In fact, the highest adsorption capacity was obtained for \(P_{{{\text{CO}}_{2} }}\) = 0.05 on S1-Li4SiO4. Figure 9a shows the CO2 adsorption for S1-Li4SiO4, and it is evident that when the \(P_{{{\text{CO}}_{2} }}\) increased the process became faster during the first 12 min. Thus, for \(P_{{{\text{CO}}_{2} }}\) values of 0.10, 0.15 and 0.2, the CO2 capture increased from 78.6 to 94.7 and 114.2 mgCO2/g sorbent, respectively. To verify the reproducibility of these results, a second batch of S1-Li4SiO4 was prepared and the CO2 uptake capacities were 79.1, 95.35 and 111.24 mgCO2/g for \(P_{{{\text{CO}}_{2} }}\) = 0.10, 0.15 and 0.20, respectively. A difference lower than 3% was estimated between both batches. A similar behavior was observed for S2-Li4SiO4 (Fig. 9b), since the CO2 sorption process became faster in the first 10 min and the CO2 capture capacity increased with an increment in the \(P_{{{\text{CO}}_{2} }}\) (88.3, 98.6 and 118 mgCO2/g material for \(P_{{{\text{CO}}_{2} }}\) = 0.10, 0.15 and 0.20, respectively). S2-Li4SiO4 showed the same CO2 sorption capacities in the upper and lower partial pressures values (0.05 and 0.20). It is important to highlight that the reaction rates observed in both materials for the different \(P_{{{\text{CO}}_{2} }}\) used in this work were faster than that reported for pure Li4SiO4 with low CO2 concentration (Seggiani et al. 2013; Seggiani et al. 2011; Zhang et al. 2014).
3.4 Kinetic analysis
In order to investigate the effect of the CO2 concentration on the kinetic behavior of the prepared materials, the experimental data of S1-Li4SiO4 and S2-Li4SiO4 showed in Fig. 9 were analyzed according to the Avrami–Erofeev model (Qi et al. 2013; Zhang et al. 2014). The Avrami–Erofeev model is associated with the reaction mechanism of the formation and growth of reaction product crystals and is based on the typical model for gas-solid reactions:
where
where α is the degree of conversion (refers to the conversion of sorbent material towards carbonation products, that is, the ratio between the CO2 adsorption capacity at given time t and the maximum theoretical CO2 adsorption), t is the time, K is the kinetic constant and n is the kinetic parameter.
thus
where \(k={K}^{n}\)
The double logarithmic form of Eq. 3 (Eq. 4) can be successfully applied to the experimental results of the CO2 adsorption by linear plot of \(\text{ln}\left(-\text{ln}\left(1-\alpha \right)\right)\) vs \(\text{ln}\,t\) with slope n. This equation was used to estimate the specific kinetic parameters, K and n, where n is a fractional number that accounts for possible changes of the adsorption mechanism during the adsorption process. When n > 1, the carbonation reaction is controlled by the formation rate and growth of the product layer, and, with n < 1, the reaction proceeds under diffusion control (Qi et al. 2013; Zhang et al. 2014; Zhao et al. 2018). Plots of \(\text{ln}\left[-\text{ln}\left(1-\alpha \right)\right]\) vs \(\text{ln}\,t\) for both slag- derived lithium silicates at different CO2 partial pressures are shown in Fig. 10. The kinetic parameters n and K were determined using the slope and intercept and are summarized in Table 2.
For both slag-derived lithium silicates in the different \(P_{{{\text{CO}}_{2} }}\) studied, n values of rapid reaction stage are > 1, and < 1 for the diffusion-control stage. This means that the formation rate and growth of the carbonate external shell controls the rapid reaction stage, the first step of the whole CO2 capture process, while the diffusion processes control the second stage. The reaction rate K values of the rapid reaction stage are in general higher than those of K values in the diffusion-control stage. This is in agreement with previous studies and means that the limiting step of the total process is the CO2 sorption controlled by diffusion processes (Qi et al. 2013; Zhang et al. 2014). In S1-Li4SiO4, K values of rapid reaction stage are up to eight orders of magnitude greater than the diffusion-control stage with \(P_{{{\text{CO}}_{2} }}\) of 0.05 and 0.10. When the CO2 partial pressure increased to 0.15 and 0.20 the K values of the diffusion-control stage also increased. Whilst for S2-Li4SiO4, the K values of rapid reaction stage are also greater than K values for the diffusion-control stage, but only in three or six orders of magnitude. In addition, it is noticeable that the K values of the rapid reaction and diffusion-control stages, obtained for both slag-derived lithium silicates, are at least one order of magnitude higher than those reported for Li4SiO4, in 10 vol% of CO2 (Zhang et al. 2014) and similar to those obtained in pure CO2 flow (Qi et al. 2013).
3.5 Cyclic performance
Finally, the slag-derived lithium silicates were tested in twenty cycles of CO2 sorption-desorption in order to evaluate their regeneration properties and thermal stability. The cyclic performance in \(P_{{{\text{CO}}_{2} }}\) of 0.2 and 0.05 was analyzed at 600 and 650 °C for S2-Li4SiO4 and S1-Li4SiO4, respectively, temperatures at which the highest CO2 capture capacities were obtained. For the desorption step, the flow was switched to N2 and the temperature was raised to 750 °C and maintained for 20 min. Figure 11a shows the results obtained for S1-Li4SiO4, in \(P_{{{\text{CO}}_{2} }}\) of 0.2 an uptake of 110.1 mgCO2/g sorbent was reached for the first cycle, and after 20 cycles, the CO2 capture decreased to 77.32 mgCO2/g sorbent (1.76 mmolCO2/g sorbent). When the \(P_{{{\text{CO}}_{2} }}\) diminished to 0.05, the CO2 adsorption was 121.5 mgCO2/g sorbent in cycle number one and, in cycle fourteen, it decreased by 20% stabilizing in 96 mgCO2/g sorbent (2.18 mmolCO2/g sorbent). S2-Li4SiO4 showed less thermal stability and after 20 cycles the CO2 capture diminished by 32% in \(P_{{{\text{CO}}_{2} }}\) = 0.2 and almost 40% in \(P_{{{\text{CO}}_{2} }}\) = 0.05.
The morphological changes in both materials after multicycle analysis were analyzed using backscattering electron micrographs, presented in Fig. 12. Before CO2 capture, the slag derived lithium silicates consisted of dense polyhedral particles with a compact and non-porous surface, with small sizes ranging from 5 to 18 µm. Some agglomerates could be observed with a non-uniform size distribution, of 35–70 µm in S1-Li4SiO4 and of around 40 µm in S2-Li4SiO4 (Fig. 12a, b). However, during 20 cycles of CO2 sorption-desorption (sorption in \(P_{{{\text{CO}}_{2} }}\) = 0.05) the sintering process occurred in both materials due to the high value of the regeneration temperature (750 °C). Thus, the agglomerates of S1-Li4SiO4 and S2-Li4SiO4 particles became larger after 20 cycles as it was previously reported for other lithium-based sorbents (Fig. 12c, d). The increase of agglomerates size was greater in S2-Li4SiO4 than in S1-Li4SiO4 particles, from 40 to 214 µm (5 times their size) and from 71 to 160 µm, respectively. This increase makes more difficult the CO2 diffusion in the sorption step and also in the desorption process, which has a negative effect in the regeneration of the materials during cyclic tests, similar to that observed in other lithium-based ceramics (Chen et al. 2016; Rodríguez-Mosqueda and Pfeiffer 2010; Wang et al. 2016; Xiang et al. 2015).
4 Conclusions
In this work, lithium orthosilicate adsorbents were successfully prepared using two different iron and steel slags as silica sources. In addition, the prepared materials were characterized and tested as high temperature CO2 adsorbents in CO2 partial pressures between 0.20 and 0.05. Both slag-derived lithium silicates presented better CO2 capture capacities, at least thrice higher than the one of pure Li4SiO4. The most promising slag-derived sorbent prepared in this work was S1-Li4SiO4, which had the highest CO2 capture, 134 mgCO2/g sorbent at 650 °C with a \(P_{{{\text{CO}}_{2} }}\) of 0.05, a CO2 uptake higher than that of pure Li4SiO4 and other lithium based materials prepared with fly ash and tested with low CO2 concentrations (\(P_{{{\text{CO}}_{2} }}\) = 0.05–0.20). In both slag-derived silicates, the increase in the CO2 partial pressure from 0.1 to 0.2 enhanced the CO2 uptake and the reaction rate of the CO2 adsorption process. The presence of calcium phases and small amounts of Mg, Fe and Al in the slag-derived lithium silicates improved the CO2 uptake as well as the kinetic behavior. The kinetic parameters, calculated according to the Avrami–Erofeev model, showed that the formation and growing of the carbonate external shell controls the rapid reaction stage in both materials. The reaction rate K of rapid reaction stage was at least four orders of magnitude higher than the K values of the diffusion control stage, i.e., the diffusion control stage was the limiting step of the total CO2 capture process. The cyclic tests indicated that the S1-Li4SiO4 sorbent had a good thermal stability and high CO2 capture capacity after 20 cycles (2.18 mmol CO2 per g of material), despite the low CO2 concentration. All these results showed that the slag derived silicates are promising materials to be used in CO2 capture processes operated at high temperatures (T ≥ 600 °C) with low CO2 partial pressures (0.05 ≤ \(P_{{{\text{CO}}_{2} }}\) ≤ 0.20), such as sorption enhanced reforming and in the looping of a post-combustion CO2 capture into natural gas combined cycle (NGCC) plants. Therefore, future research will be focused on the structural and textural modifications of the slags derived silicates, using other gases in the mixture (such as NOx, SOx, CO, O2 and water vapor), in order to guarantee a good cyclic stability.
References
Albo Sánchez, J.: Carbon dioxide capture processes, technology and environmental implications. 1–345 (2015)
Alcérreca-Corte, I., Fregoso-israel, E., Pfeiffer, H.: CO2 absorption on Na2ZrO3: a kinetic analysis of the chemisorption and diffusion processes. J. Phys. Chem. C 112, 6520–6525 (2008)
Amorim, S.M., Domenico, M.D., Dantas, T.L.P., José, H.J., Moreira, R.F.P.M.: Lithium orthosilicate for CO2 capture with high regeneration capacity: kinetic study and modeling of carbonation and decarbonation reactions. Chem. Eng. J. 283, 388–396 (2016). https://doi.org/10.1016/j.cej.2015.07.083
Avalos-Rendón, T., Casa-Madrid, J., Pfeiffer, H.: Thermochemical capture of carbon dioxide on lithium aluminates (LiAlO2 and Li5AlO4): a new option for the CO2 absorption. J. Phys. Chem. A. 113, 6919–6923 (2009). https://doi.org/10.1021/jp902501v
Bejarano Peña, W.: Captura de CO2 con silicatos de litio sintetizados a partir de escorias metalúrgicas de la industria del hierro. http://132.248.9.195/ptd2018/noviembre/0782891/Index.html (2018). Accessed 16 Oct 2019
Castillo Villa, A., Salinas Gutiérrez, J., Navarro Gómez, C.J., Aquino De los Rios, Rentería Villalobos, G.S., Cortés, M., Palacios, L., López Ortiz, A., Collins-Martínez, V.: Kinetic study of the CO2 desorption process by carbonated Na2ZrO3 solid absorbent. Int. J. Hydrogen Energy. (2015). https://doi.org/10.1016/j.ijhydene.2015.08.036
Chen, X., Xiong, Z., Qin, Y., Gong, B., Tian, C., Zhao, Y., Zhang, J., Zheng, C.: High-temperature CO2 sorption by Ca-doped Li4SiO4 sorbents. Int. J. Hydrogen Energy. 41, 13077–13085 (2016). https://doi.org/10.1016/j.ijhydene.2016.05.267
Chowdhury, M.B.I., Quddus, M.R., deLasa, H.I.: CO2 capture with a novel solid fluidizable sorbent: thermodynamics and temperature programmed carbonation–decarbonation. Chem. Eng. J. 232(7), 139–148 (2013). https://doi.org/10.1016/j.cej.2013.07.044
Chung, F.H.: Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 7, 519–525 (1974)
Dou, B., Wang, C., Song, Y., Chen, H., Jiang, B., Yang, M., Xu, Y.: Solid sorbents for in-situ CO2 removal during sorption-enhanced steam reforming process: a review. Renew. Sustain. Energy Rev. 53, 536–546 (2016). https://doi.org/10.1016/j.rser.2015.08.068
Duan, Y., Luebke, D., Pennline, H.: Efficient theoretical screening of solid sorbents for CO2 capture applications*. Int. J. Clean Coal Energy 1, 1–11 (2012)
Duan, Y., Pfeiffer, H., Li, B., Romero-Ibarra, I.C., Sorescu, D.C., Luebke, D.R., Halley, J.W.: CO2 capture properties of lithium silicates with different ratios of Li2O/SiO2: an ab initio thermodynamic and experimental approach. Phys. Chem. Chem. Phys. 15, 13538–13558 (2013). https://doi.org/10.1039/c3cp51659h
Durán-Muñoz, F., Romero-Ibarra, I.C., Pfeiffer, H.: Analysis of the CO2 chemisorption reaction mechanism in lithium oxosilicate (Li8SiO6): a new option for high-temperature CO2 capture. J. Mater. Chem. A. 1, 3919 (2013). https://doi.org/10.1039/c3ta00421j
EUROSLAG: possition paper on the status of ferrous slag complying with the waste framework directive 2008/98/CE and the REACH regulation. https://www.euroslag.com/research-library-downloads (2008)
Gao, N., Ma, K., Ding, T., Cai, J., Tian, Y., Li, X.: Enhanced carbon dioxide adsorption performance and kinetic study of K and Al co-doped Li4SiO4. Chin. Chem. Lett. 29, 482–484 (2017). https://doi.org/10.1016/j.cclet.2017.07.031
Garcia, S., Sanchez, E., Stewart, A.J., Maroto-Valer, M.M.: Process integration of post-combustion CO2 capture with Li4SiO4/Li2CO3 looping in a NGCC plant. Energy Procedia. 114(2016), 2611–2617 (2017). https://doi.org/10.1016/j.egypro.2017.03.1421
Gauer, C., Heschel, W.: Doped lithium orthosilicate for absorption of carbon dioxide. J. Mater. Sci. 41, 2405–2409 (2006). https://doi.org/10.1007/s10853-006-7070-1
Hu, Y., Liu, W., Yang, Y., Qu, M., Li, H.: CO2 capture by Li4SiO4 sorbents and their applications: current developments and new trends. Chem. Eng. J. 359, 604–625 (2019). https://doi.org/10.1016/j.cej.2018.11.128
IPCC: Climate change 2014: mitigation of climate change (2014)
Izquierdo, M.T., Gasquet, V., Sansom, E., Ojeda, M., Garcia, S., Maroto-Valer, M.M.: Lithium-based sorbents for high temperature CO2 capture: effect of precursor materials and synthesis method. Fuel 230, 45–51 (2018). https://doi.org/10.1016/j.fuel.2018.05.041
Kaniwa, S., Yoshino, M., Niwa, E., Hashimoto, T.: Evaluation of reaction kinetics of CO2 and Li4SiO4 by thermogravimetry under various CO2 partial pressures. Mater. Res. Bull. 97, 56–60 (2018). https://doi.org/10.1016/j.materresbull.2017.08.045
Kato, M., Nakagawa, K., Essaki, K., Maezawa, Y., Kogo, R., Hagiwara, Y.: Novel CO2 absorbents using lithium-containing oxide. Int. J. Appl. Ceram. Technol. 2, 467–475 (2005)
López Ortiz, A., Escobedo Bretado, M.A., Guzmán Velderrain, V., Meléndez Zaragoza, M., Salinas Gutiérrez, J., Lardizábal Gutiérrez, D., Collins-Martínez, V.: Experimental and modeling kinetic study of the CO2 absorption by Li4SiO4. Int. J. Hydrogen Energy 39, 16656–16666 (2014). https://doi.org/10.1016/j.ijhydene.2014.05.015
Lowell, S., Shields, J.E., Thomas, M.A., Thommes, M.: Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density. Particle Technology Series. Kluwer Academic Publishers, London (2004)
Martínez-dlCruz, L., Pfeiffer, H.: Microstructural thermal evolution of the Na2CO3 phase produced during a Na2ZrO3–CO2 chemisorption process. J. Phys. Chem. C 116, 9675–9680 (2012)
Mercado-Borrayo, B.M., Schouwenaars, R., González-Chávez, J.L., Ramírez-Zamora, R.M.: Multi-analytical assessment of iron and steel slag characteristics to estimate the removal of metalloids from contaminated water. J. Environ. Sci. Health Part A. 48, 887–895 (2013). https://doi.org/10.1080/10934529.2013.761492
Monica, P., Maurizia, S., Sandra, V.: CO2 capture at high temperature and low concentration on Li4SiO4 based sorbents. Chem. Eng. Trans. 32, 1279–1284 (2013). https://doi.org/10.3303/CET1332214
Ochoa-Fernández, E., Rusten, H.K., Jakobsen, H.A., Rønning, M., Holmen, A., Chen, D.: Sorption enhanced hydrogen production by steam methane reforming using Li2ZrO3 as sorbent: sorption kinetics and reactor simulation. Catal. Today 106, 41–46 (2005). https://doi.org/10.1016/j.cattod.2005.07.146
Olivares-Marín, M., Maroto-Valer, M.: Development of adsorbents for CO2 capture from waste materials: a review. Greenh. Gases Sci. Technol. 2, 20–35 (2012). https://doi.org/10.1002/ghg
Olivares-Marín, M., Drage, T.C., Maroto-Valer, M.M.: Novel lithium-based sorbents from fly ashes for CO2 capture at high temperatures. Int. J. Greenh. Gas Control. 4, 623–629 (2010). https://doi.org/10.1016/j.ijggc.2009.12.015
Ortiz-Landeros, J., Gómez-Yáñez, C., Palacios-Romero, L.M., Lima, E., Pfeiffer, H.: Structural and thermochemical chemisorption of CO2 on Li(4 + x)(Si(1-x)Al(x))O4 and Li(4-x)(Si(1-x)V(x))O4 solid solutions. J. Phys. Chem. A. 116, 3163–3171 (2012). https://doi.org/10.1021/jp3006298
Qi, Z., Daying, H., Yang, L., Qian, Y., Zibin, Z.: A kinetic behaviors and reaction mechanisms on Li4SiO4 analysis of CO2 sorption/desorption. AIChE J. 59, 901–911 (2013). https://doi.org/10.1002/aic.13861
Rodríguez-Mosqueda, R., Pfeiffer, H.: Thermokinetic analysis of the CO2 chemisorption on Li4SiO4 by using different gas flow rates and particle sizes. J. Phys. Chem. A. 114, 4535–4541 (2010). https://doi.org/10.1021/jp911491t
Romero-Ibarra, I.C., Ortiz-Landeros, J., Pfeiffer, H.: Microstructural and CO2 chemisorption analyses of Li4SiO4: effect of surface modification by the ball milling process. Thermochim. Acta 567, 118–124 (2013). https://doi.org/10.1016/j.tca.2012.11.018
Sanna, A., Ramli, I., Maroto-Valer, M.: M.: Development of sodium/lithium/fly ash sorbents for high temperature post-combustion CO2 capture. Appl. Energy. 156, 197–206 (2015). https://doi.org/10.1016/j.apenergy.2015.07.008
Seggiani, M., Puccini, M., Vitolo, S.: High-temperature and low concentration CO2 sorption on Li4SiO4 based sorbents: study of the used silica and doping method effects. Int. J. Greenh. Gas Control. 5, 741–748 (2011). https://doi.org/10.1016/j.ijggc.2011.03.003
Seggiani, M., Puccini, M., Vitolo, S.: Alkali promoted lithium orthosilicate for CO2 capture at high temperature and low concentration. Int. J. Greenh. Gas Control. 17, 25–31 (2013). https://doi.org/10.1016/j.ijggc.2013.04.009
U.S. Department of Commerce: National Oceanic and Atmospheric Administration. https://www.esrl.noaa.gov/gmd/ccgg/trends/ (2019)
Wang, K., Guo, X., Zhao, P., Wang, F., Zheng, C.: High temperature capture of CO2 on lithium-based sorbents from rice husk ash. J. Hazard. Mater. 189, 301–307 (2011). https://doi.org/10.1016/j.jhazmat.2011.02.040
Wang, K., Zhou, Z., Zhao, P., Yin, Z., Su, Z., Sun, J.: Synthesis of a highly efficient Li4SiO4 ceramic modified with a gluconic acid-based carbon coating for high-temperature CO2 capture. Appl. Energy. 183, 1418–1427 (2016). https://doi.org/10.1016/j.apenergy.2016.09.105
Wang, K., Li, W., Yin, Z., Zhou, Z., Zhao, P.: High-capacity Li4SiO4-based CO2 sorbents via a facile hydration–NaCl doping technique. Energy Fuels 31, 6257–6265 (2017a). https://doi.org/10.1021/acs.energyfuels.6b03453
Wang, K., Yin, Z., Zhao, P., Zhou, Z., Su, Z., Sun, J.: Development of metallic element-stabilized Li4SiO4 sorbents for cyclic CO2 capture. Int. J. Hydrogen Energy. 42, 4224–4232 (2017b). https://doi.org/10.1016/j.ijhydene.2016.10.058 b .
Wang, H., Zhang, J., Wang, G., Wang, Q., Song, T.: High-temperature capture of CO2 by Li4SiO4 prepared with blast furnace slag and kinetic analysis. J. Therm. Anal. Calorim. 133, 981–989 (2018). https://doi.org/10.1007/s10973-018-7167-1
WorldSteel: Steel facts. https://www.worldsteel.org/about-steel/steel-facts.html (2016)
Xiang, M., Zhang, Y., Hong, M., Liu, S., Zhang, Y., Liu, H., Gu, C.: CO2 absorption properties of Ti- and Na-doped porous Li4SiO4 prepared by a sol–gel process. J. Mater. Sci. 50, 4698–4706 (2015). https://doi.org/10.1007/s10853-015-9020-2
Yancheshmeh, M.S., Radfarnia, H.R., Iliuta, M.C.: High temperature CO2 sorbents and their application for hydrogen production by sorption enhanced steam reforming process. Chem. Eng. J. (2015). https://doi.org/10.1016/j.cej.2015.06.060
Zhang, S., Zhang, Q., Wang, H., Ni, Y., Zhu, Z.: Absorption behaviors study on doped Li4SiO4 under a humidified atmosphere with low CO2 concentration. Int. J. Hydrogen Energy. 39, 17913–17920 (2014). https://doi.org/10.1016/j.ijhydene.2014.07.011
Zhang, Y., Gao, Y., Pfeiffer, H., Louis, B., Sun, L., O’Hare, D., Wang, Q.: Recent advances in lithium containing ceramic based sorbents for high-temperature CO2 capture. J. Mater. Chem. A. 7, 7962–8005 (2019). https://doi.org/10.1039/c8ta08932a
Zhao, M., Fan, H., Yan, F., Song, Y., He, X., Memon, M.Z., Bhatia, S.K., Ji, G.: Kinetic analysis for cyclic CO2 capture using lithium orthosilicate sorbents derived from different silicon precursors. Dalton Trans. 47, 9038–9050 (2018). https://doi.org/10.1039/c8dt01617h
Acknowledgements
The project was financially supported by DGAPA Grant IT101519. Brenda-Cecilia Alcántar-Vázquez thanks to DGAPA-UNAM for the postdoctoral financial support. Authors gratefully acknowledge the support of XRD Laboratory of the Geology Institute at UNAM, member of National Laboratory of Mineralogy and Geochemistry of Mexico, in the materials characterization, especially to Dr. T. Pi-Puig. Finally, thanks to M. C. Leticia García Montes de Oca for technical assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alcántar-Vázquez, BC., Ramírez-Zamora, RM. Lithium silicates synthetized from iron and steel slags as high temperature CO2 adsorbent materials. Adsorption 26, 687–699 (2020). https://doi.org/10.1007/s10450-019-00198-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00198-z