Abstract
A novel ordered mesoporous carbon containing basic nitrogen functional groups was synthesized by ammonia-tailoring at a temperature of 1173 K and was applied for reactive dye adsorption. The basic nitrogen-containing functional groups incorporated into the carbon surface could enhance the dispersive interactions between the carbon and dye molecules due to the electron-donating effect as well as the electrostatic interactions between the carbon surface and the anions of the dyes. It was found that this novel functionalized ordered mesoporous carbon could increase the adsorption capacity of reactive red 2 at 298 K by around 40 % and 100 % as compared with the unmodified carbon and a commercial activated carbon, respectively. The Freundlich isotherm showed better correlation with the experimental adsorption data of ammonia-tailored samples than the Langmuir isotherm due to the increased surface heterogeneity induced by the nitrogen-containing functional groups. Adsorption of reactive red 2 was an endothermic process as the adsorption capacity increased with increasing temperature. Low desorption efficiency revealed that the adsorption of reactive red 2 on the modified CMK-3 was extremely favorable, tending to be weakly reversible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The textile industry which uses dyes to color their products and also consumes substantial volume of water is one of the largest industrial producers of wastewaters (Faria et al. 2004). Effluents from spent dyebaths and dye rinse operations containing unfixed dyes are highly colored and their disposal into receiving waters causes damage to the environment as they may significantly affect the photosynthetic activity of aquatic life by cutting off sunlight penetration. Furthermore, most of the dyes are toxic and even carcinogenic, posing a serious hazard to aquatic living organisms. Even the presence of very low concentrations of dyes (less than 1 mg/L) in the effluent is highly visible and is considered undesirable (Senthilkumaar et al. 2006; Asouhidou et al. 2009; Ip et al. 2009).
There are over 100,000 types of dyes commercially available with more than 7×105 tons produced annually (Pearce et al. 2003). Currently, azo-reactive dyes are one of the most widely used classes of dyes in the textile industry and represent 20–30 % of the total dye market owing to their favorable characteristics of bright color, water-fast and simple application techniques with low energy consumption (Al-Degs et al. 2008; El-Barghouthi et al. 2007; Asouhidou et al. 2009). Azo-reactive dyes are characterized by the existence of nitrogen-nitrogen double bonds (–N=N–) and the bright color of their aqueous solutions which is due to these azo bonds and associated chromospheres. They form a covalent chemical bond with the substrate in the course of the dyeing process, which is much more resistant to unusual conditions of use than the physico-chemical bond between other classes of dyes and cellulose (Vijayaraghavan et al. 2009). Due to their strong interaction with many surfaces of synthetic and natural fabrics, reactive dyes are highly used for coloring a wide range of materials including wool, cotton, nylon, silk, and modified acrylics (El-Barghouthi et al. 2007). However, they are the most problematic dyes as large fractions of reactive dyes (10–50 %) are wasted during the dyeing process (up to 0.6–0.8 g dye/dm3 can be detected in dyestuff effluent) as a result of competition of hydroxyl ions present in the dye bath (Vijayaraghavan et al. 2009) and conventional wastewater treatments were found to be inefficient for complete elimination of many reactive dyes (Ip et al. 2009; El-Barghouthi et al. 2007; Al-Degs et al. 2008). Efficient color removal from wastewater is of great importance and has attracted increased research and technological interest in recent years. Amongst the numerous techniques proposed for dye removal (Pokhrel and Viraraghavan 2004; Robinson et al. 2001; Slokar and Le Marechal 1998; Delee et al. 1998; Banat et al. 1996; Cooper 1993), adsorption has already received considerable attention for wastewater treatment due to its ability to remove different types of dye and produce high quality treated water, simplicity of design, ease of operation, insensitivity to toxic pollutants and not resulting in the formation of harmful substances (Crini 2006; Ip et al. 2009). Activated carbon is the most investigated adsorbent to remove reactive dyes from wastewater (Nakagawa et al. 2004; Pereira et al. 2003; Al-Degs et al. 2000, 2008; Orfao et al. 2006; Faria et al. 2004; Vijayaraghavan et al. 2009), however, its microporous nature seriously restrains its applications as large molecules or macromolecules can not easily penetrate into the micropores (<2 nm diameter) and adsorbed onto them (Tanthapanichakoon et al. 2005). Mesoporous (2–50 nm) carbons with open pore structure and large pore size would be advantageous in bulky molecular adsorption. Recently, there has been an increasing interest in adsorption of reactive dyes on mesoporous carbons. Mesoporous activated carbons prepared from bamboo (Ip et al. 2009) and waste tires (Tanthapanichakoon et al. 2005) achieved higher adsorption capacity of reactive dyes than commercial activated carbons. However, the large amount of micropores and disordered pore size distribution of the carbons makes it difficult in understanding the adsorption mechanism on the carbons. More recently, efficient adsorption of bulky dyes with ordered mesoporous carbons has been reported (Zhuang et al. 2009; Asouhidou et al. 2009).
Recently, we successfully introduced basic nitrogen functional groups into ordered mesoporous carbon CMK-3 by ammonia treatment at high temperatures to obtain a novel functionalized adsorbent which still retains the ordered pore structure. The ammonia-treated carbon surface was stable and could enhance the adsorption capacity of a reactive dye (reactive red 2) by up to 40 % and 100 % as compared with the unmodified CMK-3 and a commercial activated carbon, respectively. Various tailoring conditions were studied to observe the surface and structure evolution of carbon in the modification process. The most beneficial modification conditions under which the adsorbent possesses the most favorable adsorption capacities of reactive red 2 were identified. It was found that ammonia treatment could not only remove a great amount of oxygen-containing functional groups on the carbon surface, but also stabilize the free carbon sites against subsequent oxidation. The tailoring temperature played a critical role in the modification process and thus had a significant effect on the adsorption capacity of the modified samples. Variations in contact time and ammonia gas flowrate resulted in little change in the textural properties and surface chemistry of the modified samples. Treatment at the temperature of 1173 K for 40 min with an ammonia flowrate of 60 cm3/min was the best modification condition tested for reactive red 2 adsorption.
In the present study, more work was done to characterize the surface properties of the carbon materials containing nitrogen functional groups and the adsorption mechanism of reactive red 2 on the modified carbon. Detailed adsorption study was carried out including the effect of solution pH and adsorption temperature. Desorption of dye from the dye-loaded modified mesoporous carbon was also studied in order to further understand the adsorption mechanism.
2 Experimental
2.1 Synthesis of mesoporous carbon CMK-3
The CMK-3 samples were fabricated by a nanocasting process using SBA-15 silica as template. The SBA-15 template was synthesized following the synthesis procedure reported by Jun et al. (2000). The molar composition of the final gel was: 1 TEOS: 0.0165 P123: 5.755 HCl: 191.61 H2O and the aging time at 373 K was extended to 24 hrs. The CMK-3 samples were synthesized following the procedures reported by Jun et al. (2000) with minor modifications, which is briefly described below. One gram of SBA-15 was added to a solution containing 1.25 g of sucrose, 0.14 g of H2SO4 and 5 g of H2O. The mixture was placed in a drying oven at 373 K for 6 hrs and then at 433 K for 12 hrs. The silica sample with partially polymerized and carbonized sucrose was treated again at 373 and 433 K in the same way after the addition of 0.8 g of sucrose, 0.09 g of H2SO4 and 5 g of H2O. The resultant mixture was then pyrolyzed at 1173 K under vacuum and then washed in 1 M NaOH solution (50 vol % ethanol–50 vol % H2O) twice at 373 K to dissolve the silica matrix. Finally, the template-free carbon product thus obtained was filtered, washed with water and ethanol, and dried at 378 K overnight.
2.2 Modification of mesoporous carbon CMK-3
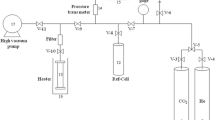
The tailoring of CMK-3 to incorporate nitrogen-containing functional groups was conducted in a home-made thermal treatment system as schematically shown in Fig. 1.
An amount of 0.3 g CMK-3 was loaded in a quartz reactor and outgassed at a temperature of 573 K for 3 hours under vacuum with nitrogen flowing through. Then, the furnace was heated to 1173 K in nitrogen atmosphere before the gas was switched to anhydrous ammonia (min. purity 99.99 %). The flow rates of nitrogen and ammonia were both set at 60 cm3/min. The sample was treated with ammonia for 40 min and then purged with nitrogen for 15 min before cooling down to room temperature in nitrogen gas. The modified sample was then washed thoroughly using water until the effluent pH was neutral and dried at 378 K overnight.
2.3 Characterization
N2 adsorption-desorption isotherms were measured at 77 K on a Quantachrome Autosorb 1. Samples were outgassed at 573 K for at least 3 hours before the adsorption measurements. The specific surface area was calculated from nitrogen adsorption data in the relative pressure range from 0.05 to 0.2 using the BET (Brunauer-Emmett-Teller) equation. The total pore volume was estimated from the amount of nitrogen adsorbed at a relative pressure of about 0.99. The pore size distribution curves were obtained by the BJH (Barrett-Joyner-Halenda) method from the desorption branch.
Elemental analysis of elements C, N, H was performed using Vario EL III Elemental Analyzer. XPS spectra of elements C, N, O of the samples were recorded on a Physical Electronics PHI 5600 XPS spectrophotometer with a monochromatic Al Ka X-ray source.
Zeta potential of carbon sorbents was measured at 298 K using a Zeta Potential Analyzer Brookhaven ZetaPlus. 0.01 g carbon sample was added into 100 ml of 0.01 M NaCl solution under stirring. The pH value of the mixture was then adjusted to a value between 2 and 11 using 0.1 M HCl or 0.1 M NaOH solutions and the mixture was stirred for 24 h. After this, around 2 ml sorbent suspension was drawn for analysis. Five zeta potential (ZP) readings were taken and the average ZP at a certain pH value was plotted against pH over the range from 2 to 11. The isoelectric point (IEP) was taken as the point where the electrokinetic potential was zero.
The acidity constant Ka and the isoelectric point (IEP) of reactive red 2 was determined using the alkalimetric titration method. 50 ml of 300 mg/L dye solution was transferred into each of two clean beakers: one was used for acid titration while the other was used for base titration. The solution was completely mixed using an electromagnetic stirrer and then the initial pH value was determined. After this, 0.1 M HCl/NaOH standard solution was titrated into the solution under continuous stirring. The volume of acid/base added and the corresponding equilibrium pH value were recorded. The buffer intensity was calculated according to Eq. (1) (Pankow 1991). Then plot the volume of acid/base consumption versus pH and buffer intensity versus pH.
where β is the buffer intensity, C A and C B are the concentrations of acid and base in the solution, the d(C B −C A ) (eq/L) represents the incremental change in the eq/L of net strong base in the solution, and dpH represents the resulting change in pH value.
2.4 Adsorption experiments
The molecular structure and 3D representation of reactive red 2 were shown in Fig. 2. The adsorption isotherms of reactive red 2 on carbon samples were measured using a batch adsorption method. Adsorption experiments on a commercial activated carbon (Norit ROW0.8 extrudate) and untreated CMK-3 were also conducted in order to evaluate the enhancement of the modified carbons. The effects of several variables (pH, dye concentration and temperature) were studied. The pH value of the solution was adjusted using NaOH or HCl solutions. Typically, 0.05 g of carbon with particle size smaller than 106 μm was brought into contact with 50 ml of reactive red 2 solution at different initial concentrations (ranging from 300–2100 mg/L) in Erlenmeyer flasks and the mixture was shaken in a thermostat shaking bath (Memmert Type WNB14 equipped with a temperature controller) with a speed of 128 rpm for 16 days which is sufficiently long to reach equilibrium. The temperature of the system was kept at preset temperatures during the whole adsorption process. The solution was then filtrated through a 0.45 μm membrane filter. The initial and equilibrium dye concentrations were determined by measuring the absorbance of reactive red 2 with a UV/VIS Spectrophotometer (Lambda 20, Perkin Elmer) at 538 nm. The equilibrium adsorption capacities (q e ) was determined by Eq. (2)
where q e is the adsorbed phase concentration at equilibrium, C 0 and C e are the liquid-phase concentrations of dye (mg/L) at initial and equilibrium, respectively. V is the volume of the solution (L) and W is the mass of dry adsorbent used (g).
2.5 Desorption experiments
In the desorption experiment, 0.3 g freshly ammonia-tailored CMK-3 sample was first mixed with 300 ml of 2100 mg/L reactive red 2 in an Erlenmeyer flask and shaken at 298 K until equilibrium was achieved. Then, the dye-loaded carbon was recovered by filtration and dried at 323 K under vacuum for 12 h. The dye concentration in the liquid phase was measured in order to calculate the dye adsorbed onto the adsorbent. The dye-loaded adsorbent was allowed to contact deionized water with the solid (mg)/liquid (ml) ratio of 1:2 in an Erlenmeyer flask and shaken at 298 K for 5 days. The amount of dye desorbed was calculated from the concentration of the desorbed dye in the liquid phase. The adsorbent was then recovered and dried at 323 K under vacuum again for the next run of desorption. Three runs of consecutive desorption experiments were conducted. The percentage of the dye desorbed from the adsorbent was calculated from Eq. (3):

3 Results and discussion
3.1 Structural properties and surface chemistry of carbon samples
Figure 3 showed the N2 adsorption/desorption isotherms and pore size distributions of activated carbon, CMK-3 and ammonia-tailored CMK-3, while Table 1 summarized the BET specific surface area, total pore volume and mesopore size of the samples. It is seen that the commercial activated carbon exhibites microporous structure having a large portion of micropore and only a small amount of mesopores. The synthesized CMK-3 sample shows a typical isotherm of mesoporous structure with uniform mesopore size centered at 3.5 nm. After ammonia tailoring, the mesopores were enlarged a little bit while the ordered mesoporous structure were kept intact as confirmed by the similar N2 isotherms as well as the TEM images and XRD patterns of the modified carbon (Figs. 4 and 5).
It was surmised that some surface oxygen-containing groups on activated carbons are not thermally stable and might decompose to CO2 and CO upon heating in an inert environment, creating highly reactive edge sites (Suarez et al. 1999). These reactive edge sites containing unpaired electrons were an important fraction of the sites that subsequently reacted with NH3 radicals, resulting in the formation of new nitrogen-containing surface functional groups on the carbon surface. Elemental analysis data listed in Tables 2 and 3 reveal that there is little nitrogen present in the activated carbon and original CMK-3 samples. Interestingly, after ammonia treatment, there is a substantial increase in the nitrogen content and a significant reduction in H/N ratio of the modified sample. The XPS analysis identifies that four types of nitrogen functional groups (Fig. 6 and Table 4), i.e., pyridine, aromatic amines, quaternary nitrogen and protonated amide (Mangun et al. 2001; Jansen and van Bekkum 1995), were created on the surface of CMK-3 after ammonia tailoring and pyridine-like nitrogen and aromatic amines were the dominant functional groups formed on the surface.
In a word, basic nitrogen functional groups were successfully incorporated into the surface of ordered mesoporous carbon CMK-3 by ammonia treatment at a high temperature of 1173 K without destroying the ordered pore structure. Pyridine-like nitrogen and aromatic amines were the dominant functional groups formed on the carbon surface. Ammonia treatment could not only remove a great amount of oxygen-containing functional groups on the carbon surface, but also stabilized the free carbon sites against subsequent oxidation.
3.2 Zeta-potential and pHIEP
The surface charge of carbons is determined by the nature of surface groups and solution pH, which could be characterized by the isoelectric point (IEP). pHIEP of carbon particles is the pH at which carbon surface carries no net electrical charge and could be found by plotting the average zeta potential (ZP) against the pH value. The point where the curves cross the X-axis, at zero voltage, is taken as the isoelectric point. Figure 7 presented the zeta-potential distribution of activated carbon, CMK-3 and modified CMK-3 samples over the pH range from 2 to 11. It was found that the surface of the carbon particles were positively charged at pH values below the pHIEP and negatively charged at pH values above the pHIEP. The activated carbon had the lowest pHIEP of around 2.8. CMK-3 exhibited a higher pHIEP value of about 5.1, which was shifted to 6.6 after ammonia treatment. The fact that the thermal treatment in ammonia shifted the pHIEP of CMK-3 to higher pH values by around 1.5 units was considered to be the consequence of the change in surface functional groups after ammonia tailoring. The basic surface functional groups created by nitrogen-incorporation rendered the carbon surface more basic, creating a surface that was more positively charged at low pH.
The net charge on a dye molecule is also affected by the pH of their surrounding environment. The pHIEP value of a dye molecule could be estimated with the help of MarvinSketch 5.2, a software developed by ChemAxon Ltd, and experimentally confirmed by an alkalimetric titration method (Al-Degs et al. 2008; Wang et al. 1998). The alkalimetric titration method is mainly used to determine the pKa of a dye molecule by titrating dye solutions with suitable acid or base solutions (Al-Degs et al. 2008; Wang et al. 1998). The pKa values of dyes are measured by plotting the equilibrium pH against buffer intensity of dyes (MpH−1). Figure 8(a) showed the overall acid/base consumption versus pH for a reactive red 2 dye solution and Fig. 8(b) illustrated the variations in buffer intensity of reactive red 2 calculated from the titration curve against pH. The pKa value is obtained from the maximum buffer intensity when the concentration of ionized and neutral dye species are equal while the point with the lowest buffer intensity below pKa is believed to be very close to the pHIEP of the dye molecule (Pankow 1991). The results showed that the dye solution had a peak buffer intensity at around pH=6 and the IEP of reactive red 2 is close to pH=4.41 which was estimated by MarvinSketch 5.2.
3.3 Equilibrium adsorption isotherms
3.3.1 Langmuir and Freundlich equilibrium isotherm models
Reactive red 2 adsorption equilibrium isotherms on activated carbon, CMK-3 and ammonia-tailored CMK-3 at 298 K under unadjusted pH conditions were presented in Fig. 9. The amount adsorbed dramatically increased at lower final solution concentration suggesting a high affinity between the dye molecule and the carbonaceous adsorbent surface and then reached a plateau at higher equilibrium solution concentration reflecting the saturated adsorption (Zhuang et al. 2009). The activated carbon gave the lowest uptake value as a result of its microporous nature. Ordered mesoporous carbon CMK-3 improved the adsorption capacity by almost 40 % and the ammonia-tailored CMK-3 further enhanced the adsorption by doubling the uptake value of activated carbon.
The correlation of the experimental adsorption data with Langmuire and Freundlich equations was undertaken to gain an understanding of the adsorption behavior and the heterogeneity of the adsorbent surface (Al-Degs et al. 2008). The Langmuir isotherm is the simplest and still the most useful equilibrium equation for adsorption, which was proposed by Langmuir (Do 1998) and can be represented as Eq. (4)
where q e and C e are the adsorbate equilibrium concentrations in the solid and liquid phases, q s is the maximum (saturation) adsorption capacity, b is the affinity constant. This model is based on several assumptions that include: (1) surface is homogeneous; (2) adsorption is at fixed number of definite, localized sites; (3) all sites are equivalent; (4) each site can accommodate only one adsorabte molecule; (5) no interaction between adsorbed molecules, even on adjacent sites.
The Freundlich isotherm considers the surface heterogeneity and was named after Freundich (Do 1998). The Freundlich isotherm is originally an empirical equation but later fundamentally formulated based on a local Langmuir isotherm and an exponentially decaying energy distribution and can be expressed as Eq. (5).
where q e and C e are the adsorbate equilibrium concentrations in the solid and liquid phases, K and n are the Freundlich constants. The isotherm is favorable when n>1, linear when n=1 and unfavorable when n<1.
The fitting results of Langmuir and Freundlich isotherms were plotted in Fig. 9 and the parameters were summarized in Table 5. Both models can represent the experimental data very well while the Freundlich isotherm showed better correlation with the experimental adsorption data in the low concentration region compared to the Langmuir model, particularly for the ammonia-tailored sample. This could be attributed to the increase in the surface heterogeneity introduced by nitrogen-containing functional groups formed on the carbon surface during ammonia heat treatment.
3.3.2 Effect of solution pH values
As confirmed by the zeta potential experiments, CMK-3 and ammonia-tailored CMK-3 samples were materials with amphoteric character. Therefore, depending on the solution pH, their surfaces might be positively or negatively charged, which would have a direct influence on the uptake of reactive dye molecules from water in terms of the electrostatic interactions. At pH<pHIEP, the carbon surface became positively charged, favoring the adsorption of anionic species due to electrostatic attraction. At pH>pHIEP, the carbon surface became negatively charged, favoring the adsorption of cationic species.
The effect of solution pH on reactive red 2 removal from solution by CMK-3 and modified CMK-3 was studied under identical conditions (Figs. 10 and 11). It was found that the adsorption capacity of reactive red 2 on CMK-3 decreased when the solution pH was raised from 3 to 6 and further decreased when the solution pH was raised to 8. However, ammonia-tailored CMK-3 behaved differently. The adsorption capacity of reactive red 2 on the modified carbon increased when the solution pH was raised from 3 to 6 but a significant reduction of the uptake was noticed when the solution pH was raised from 6 to 8. This could be explained by the shift of pHIEP rendered by the nitrogen-incorporation. At solution pH<4.4, adsorption of reactive red 2 on the carbon samples was expected to be unfavorable as both the adsorbent and dye molecules were positively charged under such a strong acidic condition. At solution pH=4.4–5.1, adsorption of reactive red 2 on the carbon samples was favorable as the carbon surface was positively charged and the dye molecules were either neutral or partially negatively charged. At solution pH=5.1–6.6, adsorption of reactive red 2 on CMK-3 was expected to be unfavorable as the carbon surface was negatively charged and the repulsive forces between the dye molecules and the carbon surface had a greater effect than that at solution pH=3, resulting in the decrease of the adsorption capacity. However, adsorption of reactive red 2 on ammonia-tailored CMK-3 was more favorable at the solution pH=5.1–6.6 as the ammonia-tailored carbon surface was positively charged as a consequence of the basic nitrogen functional groups incorporation. Therefore, a significant increase in the adsorption capacity of reactive red 2 on the modified sample was observed when solution pH was raised from 3 to 6. At solution pH=6.6–8, a large reduction in dye adsorption at the highly basic conditions was expected because of the electrostatic repulsion between the negatively charged carbon surface and the dye molecules. It seemed that the solution pH had no drastic effect on the adsorption of the reactive dye as the uptake value of the dye remained high under different solution pH conditions.
3.3.3 Effect of adsorption temperature
Adsorption experiments were conducted at three different temperatures (298, 313, 333 K) in order to study the effect of adsorption temperature on the sorption capacity of reactive red 2 on activated carbon, CMK-3 and ammonia-tailored CMK-3 samples. As shown in Figs. 12, 13 and 14, adsorption of reactive red 2 on the three carbon samples all increased with increasing temperature, indicating that adsorption of this dye on the three adsorbents was an endothermic process. This might be attributed to the enhanced mobility and penetration of reactive dye molecules within the sorbent porous structure overcoming the activation energy barrier (Asouhidou et al. 2009) and/or an increase in dimerization of reactive dyes in solution (Al-Degs et al. 2008).
The isosteric heat of adsorption is a critical parameter for the design of adsorptive separation processes as it determines the extents of adsorbent temperature changes within the adsorber during the adsorption and desorption steps (Sircar et al. 1999). The isosteric heat of adsorption for reactive red 2 can be conveniently calculated from the Clausius-Clapeyron equation (Eq. (6)) with the assumption of an isotherm (Wang et al. 2004):
where C is the adsorbate equilibrium concentration in the liquid phase at a given specific surface loading q, T is temperature, ΔH is the heat of adsorption, R is the gas constant. Since ΔH is generally a very weak function of T (Sircar et al. 1999), a plot of lnC against 1/T at constant q yields a linear line with a slope equal to ΔH/R. In this way, ΔH can be easily obtained.
Fitting the experimental data with Langmuir and Freundlich isotherms found that Freundlich isotherm gave much better correlation with the experimental adsorption data at higher temperature, indicating that the carbon surface were more energetically heterogeneous at higher temperature. Therefore, the isosteric heats of adsorption for reactive red 2 adsorption on the carbon surfaces were calculated from the Clausius-Clapeyron equation applied to the Freundlich isotherm.
Figure 15 shows the heat of adsorption of RR2 on activated carbon, CMK-3 and ammonia-tailored CMK-3 plotted against the surface loading. The positive value of the calculated isosteric heat of adsorption is consistent with the experimental results that the adsorption of RR2 on the carbon surfaces is endothermic. The isosteric heat of adsorption on both activated carbon and CMK-3 decreases with increasing adsorbate loading, indicating stronger influence of adsorbent heterogeneity over lateral interactions between the adsorbed molecules (Sircar and Cao 2002). While the increase of heat of adsorption of RR2 on the ammonia-tailored CMK-3 with adsorbate loading might be attributed to the stronger lateral interactions between the adsorbed molecules which is important only at higher adsorbate loadings (Sircar and Cao 2002). It has been discussed that the experimentally observed constancy of the isosteric heat of adsorption of a pure gas over a region of adsorbate coverage is caused by the compensatory effects of lateral interactions and adsorbent heterogeneity on the isosteric heat (Sircar and Cao 2002). However, Sircar and Cao (Sircar and Cao 2002; Sircar 2005) argued that the isosteric heat of adsorption can be strong and complex functions of adsorbate loadings when the adsorbent is energetically heterogeneous as many different gas-solid adsorption systems having different adsorbate sizes and polarities and different adsorbent pore size distibutions and surface chemistries exhibit that behavior.
Physical adsorption and chemisorption can be classified, to a certain extent, by the magnitude of enthalpy change. It is accepted that bonding strengths of <84 kJ/mol are typically physical adsorption type bonds. Chemisorption bond strengths can range from 84 to 420 kJ/mol (Al-Degs et al. 2008). According to this classification, it seems that the adsorption of RR2 on all the carbon samples is a physical adsorption as all the values of heat of adsorption calculated are less than 84 kJ/mol. But it should be mentioned that the enthalpy ranges quoted above are derived from gaseous adsorption studies and may not strictly apply for adsorption from solution. Similar findings have been reported by Al-Degs et al. (2008) when they studied the adsorption behavior of reactive dyes on the activated carbon. Some researchers have suggested that sorption from aqueous solution onto activated carbon is by a different adsorption mechanism, which may not be described as either physical adsorption or chemisorption (Al-Degs et al. 2008).
The endothermic adsorption of reactive dye on carbon samples appeared to be uncommon behavior as adsorption processes (either from gas or liquid phase) were expected to be exothermic due to the heat released after bond formation between solute and adsorbent (Al-Degs et al. 2008). However, several authors have reported endothermic adsorption of reactive dyes on different types of adsorbents (Al-Degs et al. 2008; Asouhidou et al. 2009).
3.3.4 Adsorption mechanism
The adsorption of organic pollutants from aqueous solutions by carbons depends on the nature of the adsorbate and the surface characteristics of the adsorbent. Two parallel mechanisms were considered to understand the adsorption of organic solutes on carbons, one was associated to electrostatic forces and the other to dispersive interactions which refers to the interactions between the delocalized π electrons of the Lewis basic sites in the basal planes of the carbon and the free electrons of the dye molecules present in the aromatic rings and multiple bonds (Orfao et al. 2006; Radovic et al. 1997).
Acidic oxygenated groups present on the carbon surface are detrimental to the adsorption of reactive dyes. As these groups are electron-withdrawing groups, they reduce the electron density on the surface of the carbon and thus decrease the adsorption potential for the dye molecules. In addition, the oxygen-containing functional groups are usually negatively charged in solution, which will result in an increase in the repulsive electrostatic interactions between the anions of the dyes and the carbon surface. However, the presence of basic nitrogen-containing functional groups on the carbon surface can increase the reactivity of the aromatic rings due to the electron-donating effect and, in the mean time, the ionized positively charged functional groups can enhance the interactions between the anions of the dyes and the carbon surface, which accounts for the significant improvement of reactive red 2 adsorption by ammonia-tailored CMK-3. Since the surface areas of three adsorbents are different (Table 1), it might be better to compare the adsorption capacity based on the surface area rather than the mass of adsorbent. In this standard, the adsorption capacity of reactive red 2 on ammonia-tailored CMK-3 is 11 % higher than that on activated carbon and 9 % higher than the as-synthesized CMK-3. The maximum adsorption capacities and Henry constants are 548 mg/g, 10.9 dm3/g for ammonia-tailored CMK-3, 365, 6.13 for CMK-3, and 285, 2.06 for activated carbon. The much higher Henry constant of ammonia-tailored CMK-3 suggests a stronger adsorption.
Solution pH also plays a role in the adsorption of reactive dyes on carbon samples with amphoteric character as the carbon surfaces might be positively or negatively charged depending on the pH of the solution. At pH<pHIEP, the carbon surface becomes positively charged, favoring the adsorption of anionic reactive dye due to electrostatic attraction. Conversely, at pH>pHIEP, the carbon surface is negatively charged, reducing the adsorption of reactive dyes.
3.3.5 Desorption
To investigate the extent of desorption of reactive dyes from the ammonia-tailored carbon, desorption experiments were conducted by contacting the dye-loaded carbon with deionized water. It was found that the desorption efficiency was low as only a small amount of dye (around 20 %) could be desorbed from the carbon by three runs of desorption process (Fig. 16). Moreover, about 95 % of the weakly adsorbed dye was desorbed in the first desorption cycle. The very small amount of dye desorbed in the second and third desorption cycle was negligible. It seemed that adsorption of reactive red 2 on the modified CMK-3 was extremely favorable, tending to be weakly reversible. More acidic and alkaline conditions and other solvent regeneration methods using ethanol (Tanthapanichakoon et al. 2005) or surfactant (Asouhidou et al. 2009; Purkait et al. 2005) as solvent would be attempted to see if more dye molecules could be desorbed.
4 Conclusions
Ammonia-tailoring at high temperature was an effective way to modify the surface chemistry of ordered mesoporous carbon CMK-3 by removing a large amount of oxygen-containing functional groups on the carbon surface and simultaneously incorporating basic nitrogen-containing functional groups into the carbon surface without undermining the ordered pore structure. Pyridine-like nitrogen and aromatic amines were the dominant functional groups formed on the carbon surface. These basic surface functional groups rendered the carbon “more basic”, shifting the pHIEP of CMK-3 from 5.1 to 6.6. This novel functionalized ordered mesoporous carbon could enhance the adsorption capacity of reactive red 2 at 298 K by around 40 % and 100 % as compared with unmodified CMK-3 and commercial activated carbon, respectively. The Freundlich isotherm showed better correlation with the experimental adsorption data than the Langmuir isotherm, particularly for the ammonia-tailored sample. This could be attributed to the increase in the surface heterogeneity induced by the nitrogen-containing functional groups.
Two parallel adsorption mechanisms, electrostatic interactions and dispersive interactions, were proposed to explain the adsorption of reactive red 2 on carbon samples. Basic nitrogen-containing functional groups created on the carbon surface could enhance the dispersive interactions due to the electron-donating effect and the electrostatic attraction between the anions of the dyes and the carbon surface. Although the solution pH had a direct influence on the uptake of reactive dyes from water by the electrostatic interactions, the dispersive force mechanism appeared to predominate in the adsorption process.
Adsorption of reactive red 2 in the three adsorbents was an endothermic process and the adsorbent-adsorbate interactions might involve a more complex interplay which needs to be further studied. The low desorption efficiency indicated that adsorption of reactive red 2 on the modified CMK-3 was extremely favorable, tending to be weakly reversible.
References
Al-Degs, Y., Khraisheh, M.A.M., Allen, S.J., Ahmad, M.N.: Effect of carbon surface chemistry on the removal of reactive dyes from textile effluent. Water Res. 34, 927–935 (2000)
Al-Degs, Y.S., El-Barghouthi, M.I., El-Sheikh, A.H., Walker, G.M.: Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 77, 16–23 (2008)
Asouhidou, D.D., Triantafylidis, K.S., Lazaridis, N.K., Matis, K.A., Kim, S.S., Pinnavaia, T.J.: Sorption of reactive dyes from aqueous solutions by ordered hexagonal and disordered mesoporous carbons. Microporous Mesoporous Mater. 117, 257–267 (2009)
Banat, I.M., Nigam, P., Singh, D., Marchant, R.: Microbial decolorization of textile-dye-containing effluents: a review. Bioresour. Technol. 58, 217–227 (1996)
Cooper, P.: Removing color from dyehouse waste-waters—a critical review of technology available. J. Soc. Dyers Colour. 109, 97–100 (1993)
Crini, G.: Non-conventional low-cost adsorbents for dye removal: a review. Bioresour. Technol. 97, 1061–1085 (2006)
Delee, W., O’Neill, C., Hawkes, F.R., Pinheiro, H.M.: Anaerobic treatment of textile effluents: a review. J. Chem. Technol. Biotechnol. 73, 323–335 (1998)
Do, D.D.: Adsorption Analysis: Equilibria and Kinetics, pp. 13–56. Imperial College Press, London (1998)
El-Barghouthi, M.I., El-Sheikh, A.H., Al-Degs, Y.S., Walker, G.M.: Adsorption behavior of anionic reactive dyes on H-type activated carbon: competitive adsorption and desorption. Stud. Sep. Sci. Technol. 42, 2195–2220 (2007)
Faria, P.C.C., Orfao, J.J.M., Pereira, M.F.R.: Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 38, 2043–2052 (2004)
Ip, A.W.M., Barford, J.P., Mckay, G.: Reactive black dye adsorption/desorption onto different adsorbents: effect of salt, surface chemistry, pore size and surface area. J. Colloid Interface Sci. 337, 32–38 (2009)
Jansen, R.J.J., van Bekkum, H.: XPS of nitrogen-containing functional groups on activated carbon. Carbon 33, 1021–1027 (1995)
Jun, S., Joo, S.H., Ryoo, R., Kruk, M., Jaroniec, M., Liu, Z., Ohsuna, T., Terasaki, O.: Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J. Am. Chem. Soc. 122, 10712–10713 (2000)
Mangun, C.L., Benak, K.R., Economy, J., Foster, K.L.: Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 39, 1809–1820 (2001)
Nakagawa, K., Namba, A., Mukai, S.R., Tamon, H., Ariyadejwanich, P., Tanthapanichakoon, W.: Adsorption of phenol and reactive dye from aqueous solution on activated carbons derived from solid wastes. Water Res. 38, 1791–1798 (2004)
Orfao, J.J.M., Silva, A.I.M., Pereira, J.C.V., Barata, S.A., Fonseca, I.M., Faria, P.C.C., Pereira, M.F.R.: Adsorption of a reactive dye on chemically modified activated carbons—influence of pH. J. Colloid Interface Sci. 296, 480–489 (2006)
Pankow, J.F.: Aquatic Chemistry Concepts, pp. 143–148. Lewis, Chelsea (1991)
Pearce, C.I., Lloyd, J.R., Guthrie, J.T.: The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigments 58, 179–196 (2003)
Pereira, M.F.R., Soares, S.F., Orfao, J.J.M., Figueiredo, J.L.: Adsorption of dyes on activated carbons: influence of surface chemical groups. Carbon 41, 811–821 (2003)
Pokhrel, D., Viraraghavan, T.: Treatment of pulp and paper mill wastewater—a review. Sci. Total Environ. 333, 37–58 (2004)
Purkait, M.K., DasGupta, S., De, S.: Adsorption of eosin dye on activated carbon and its surfactant based desorption. J. Environ. Manag. 76, 135–142 (2005)
Radovic, L.R., Silva, I.F., Ume, J.I., Menendez, J.A., Leon y Leon, C.A., Scaroni, A.W.: An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon 35, 1339–1348 (1997)
Robinson, T., McMullan, G., Marchant, R., Nigam, P.: Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 77, 247–255 (2001)
Senthilkumaar, S., Kalaamani, P., Porkodi, K., Varadarajan, P.R., Subburaam, C.V.: Adsorption of dissolved reactive red dye from aqueous phase onto activated carbon prepared from agricultural waste. Bioresour. Technol. 97, 1618–1625 (2006)
Sircar, S.: Heat of adsorption on heterogeneous adsorbents. Appl. Surf. Sci. 252, 647–653 (2005)
Sircar, S., Cao, D.V.: Heat of adsorption. Chem. Eng. Technol. 25, 945–948 (2002)
Sircar, S., Mohr, R., Ristic, C., Rao, M.B.: Isosteric heat of adsorption: theory and experiment. J. Phys. Chem. B 103, 6539–6546 (1999)
Slokar, Y.M., Le Marechal, A.M.: Methods of decoloration of textile wastewaters. Dyes Pigments 37, 335–356 (1998)
Suarez, D., Menendez, J.A., Fuente, E., Montes-Moran, M.A: Contribution of pyrone-type structures to carbon basicity: an ab initio study. Langmuir 15, 3897–3904 (1999)
Tanthapanichakoon, W., Ariyadejwanich, P., Japthong, P., Nakagawa, K., Mukai, S.R., Tamon, H.: Adsorption-desorption characteristics of phenol and reactive dyes from aqueous solution on mesoporous activated carbon prepared from waste tires. Water Res. 39, 1347–1353 (2005)
Vijayaraghavan, K., Won, S.W., Yun, Y.S.: Treatment of complex remazol dye effluent using sawdust- and coal-based activated carbons. J. Hazard. Mater. 167, 790–796 (2009)
Wang, J.M., Huang, C.P., Allen, H.E., Cha, D.K., Kim, D.W.: Adsorption characteristics of dye onto sludge particulates. J. Colloid Interface Sci. 208, 518–528 (1998)
Wang, K., Qiao, S.Z., Hu, X.J.: Study of isosteric heat of adsorption and activation energy for surface diffusion of gases on activated carbon using equilibrium and kinetics information. Sep. Purif. Technol. 34, 165–176 (2004)
Zhuang, X., Wan, Y., Feng, C.M., Shen, Y., Zhao, D.Y.: Highly efficient adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons. Chem. Mater. 21, 706–716 (2009)
Acknowledgements
Financial support from the Research Grants Council of Hong Kong (CERG, Project No. 617305) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, C., Hu, X. Functionalized ordered mesoporous carbon for the adsorption of reactive dyes. Adsorption 18, 337–348 (2012). https://doi.org/10.1007/s10450-012-9410-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-012-9410-6