Abstract
A vaccine that induces broadly neutralizing antibodies (bnAbs) against the human immunodeficiency virus (HIV) would be instrumental in controlling the disease. The membrane proximal external region (MPER) peptide is an appealing antigen candidate since it is conserved and is the target of several human bnAbs, such as 2F5. We previously found that liposomes containing cobalt porphyrin-phospholipid (CoPoP) can bind to a his-tagged MPER peptide, resulting in biomimetic antigen presentation on a lipid bilayer. The present study generated various his-tagged, synthetic MPER fragments, which were bound to liposomes containing CoPoP and a synthetic monophosphoryl lipid A (MPLA) and assessed for immunogenicity in mice. MPER peptides with amino acids stretches originating from the membrane insertion point that were at least 25 amino acids in length, had greater 2F5 reactivity and induced stronger antibody responses, compared to shorter ones. Immunization with the lipid-presented MPER elicited stronger antibody responses compared to Alum and Montanide adjuvants, which could recognize recombinant gp41 and gp140 proteins that contained MPER sequences. The induced antibodies neutralized a tier 1A virus that is sensitive to neutralizing antibodies (W61D(TCLA)0.71), but not another tier 1A nor a tier 2 strain. Co-formulation of the MPER peptide with an unrelated malaria protein antigen (Pfs25) that is effectively adjuvanted with liposomes containing CoPoP and MPLA resulted in elicitation of higher MPER antibody levels, but did not improve neutralization, possibly due to interference with proper peptide presentation in the membrane. Murine hybridomas were generated that produced MPER antibodies, but they were non-neutralizing. These results do not show that bnAbs could be generated with MPER peptides and CoPoP liposomes, but do not rule out this possibility with additional improvements to the approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly 40 million people worldwide are living with the human immunodeficiency virus (HIV) and 35 million have died from the disease to date. Developing a prophylactic vaccine which generates broadly neutralizing antibody directed against HIV has been an intense research topic for decades.6,19 A vaccine that targets the short membrane proximal external region (MPER) has a number of advantages, including potential ease of manufacturing, cross-clade conservation and targeting by several human broadly neutralizing antibodies (bnAbs), such as 2F5, 4E10 and 10E8.3,9,10,30 Passive antibody transfer studies have shown protection in a number of model systems.11,17 This suggests that developing vaccines that produce high and durable MPER antibody levels may protect against HIV. In general, better understanding of the mechanism of antibody neutralization and developing more efficient immunogens with improved vaccine formulations, are strategies being targeted to generate an efficacious HIV vaccine.4,23,27
Nanoparticle formulations have been utilized for vaccine design and immunotherapy.1 Considering that MPER is proximal to the lipid membrane,15 presenting the MPER antigen in such a context may more accurately replicate the presentation of the epitopes and improve immunogenicity. Membrane interactions are known to impact MPER epitope conformation.21 Therefore, liposomal MPER formulation strategies have been proposed.2 A number of studies have characterized MPER vaccine responses when presented in liposomes containing monophosphoryl lipid A (MPLA).12,31 Neutralizing antibody responses have been observed in some cases,18 although bnAbs have remained elusive. A longer MPER region showed improved protection over shorter regions containing only the N-terminus (2F5 epitope) or the C-terminus (4E10 epitope). Although these vaccines generated a serum response that recognized gp140, they failed to exhibit inhibition of viral entry.28,29
Since it is was discovered that porphyrin-lipid (PoP) is able to form liposome-like bilayers,16 additional modifications by metal chelation in the porphyrin macrocycle has extended their application.24,26 We reported that through insertion of cobalt into the PoP molecule, the obtained cobalt-porphyrin-lipid (CoPoP) was able to self-assemble into a liposome bilayer structure and that this was promising for MPER immunization.25 Due to the chelation between histidine and cobalt(III), through simple aqueous incubation, his-tagged polypeptides are able to stably bind the bilayer of CoPoP liposome, even under stringent chemical and biological conditions. This provides a platform for liposome-based particle vaccine formulation that is potentially simpler than alternative constructs such as lipopeptide-based or covalent conjugation approaches. Recombinant proteins presented in CoPoP liposome bilayer have been shown to induce production of high levels of functional antibodies for malaria transmission blocking antigens.14
We sought to evaluate various MPER based constructs when presented in CoPoP/MPLA liposomes. In this current study, his-tagged MPER-derived antigens with different lengths were synthesized and utilized to evaluate and optimize the feasibility of a vaccine based on immunogenic CoPoP liposomes.
Methods
Materials were obtained from Sigma unless otherwise noted. MPER peptides were obtained from GenScript. The purity and identity were assessed by HPLC and MS (Supplementary Table 1). HIV HXBc2 gp41 protein was purchased from eEnzyme. The following reagents were obtained through the NIH AIDS reagent program: HIV JRFL gp140CF protein (from Dr. Barton F. Haynes and Hua-Xin Liao), gp41 monoclonal antibody 2F5 (from Polymun), gp120 monoclonal antibody IgG1 b12 (from Dr. Dennis Burton and Carlos Barbas), and gp120 monoclonal antibody F105 (from Dr. Marshall Posner and Dr. Lisa Cavacini). Synthetic MPLA (3D-(6-acyl) PHAD, referred to as MPLA) was purchased from Avanti Polar Lipids (#699855P). 1,2-Dipalmitoyl-sn-glycero-3-phophocholine (DPPC; Cat #LP-R4-057) and 1-Palmitoyl-2-lyso-sn-glycero-3-phosphocholine (lyso-lipid; Cat #LP-R4-078) were purchased from Corden Phama. Cholesterol was obtained from Wilshire Technologies (PhytoChol). MONTANIDE™ ISA720 was obtained from SEPPIC Inc. Alhydrogel® 2% (aluminium hydroxide gel; Cat #A1090BS) was purchased from Accurate Chemical and Scientific Corporation.
Generation of PoP Liposomes
PoP (lacking cobalt) and CoPoP were synthesized as previously described,25 but at 2 g scale. PoP was synthesized by esterifying C16 lyso-phosphatidylcholine with pyropheophorbide-a followed by purification with a silica column. CoPoP was then generated by stirring PoP with 30-fold excess of cobalt nitrate hexahydrate in methanol for 30 h, protected from light. Excess cobalt was removed by extraction with a water/chloroform/methanol system. The organic layer was collected and the solvent was removed by rotary evaporation. The obtained product was freeze-dried in 20% water in tert-butanol.
For PoP and CoPoP liposome preparation, all components were mixed and dissolved in 1 mL pre-heated ethanol at 60 °C for 10 min. 4 mL of pre-heated PBS was added and the mixture was incubated at 60 °C for another 10 min. A lipid extruder was used to for extrusion with stacked 200 nm, 100 nm and 80 nm polycarbonate membranes, at 60 °C. After 15 extrusion passes, the obtained liposome suspension was dialyzed in PBS at 4 °C to remove ethanol. The liposome formulation for PoP/MPLA liposomes was [DPPC: Cholesterol: PoP: MPLA] in a mass ratio of [2:1:0.5:1] (corresponding to a molar ratio of [43:41:7:9]). CoPoP/MPLA liposomes used CoPoP instead of PoP. The CoPoP alone liposomes omitted MPLA. The obtained liposome solution was stored at 4 °C.
MPER Peptide Binding to CoPoP Liposomes
MPER antigen was incubated with CoPoP/MPLA liposomes or PoP liposomes at 4 °C overnight (1:4 mass ratio of peptide to CoPoP or PoP). The obtained mixture was transferred to a 1.5 mL microcentrifugal filtration tube with a 100 kDa filter membrane (PALL Cat #OD100C34) to separate the free peptide and liposomes. The 100 kDa filter were pre-rinsed with PBS and centrifuged at 1200 rcf for 5 min. The flow through was removed and samples were placed into the filter. After centrifugation at 1200 rcf for up to 60 min, flow through was collected and peptide concentration were measured by Micro BCA Protein Assay (Thermo Scientific #23235). The antigen without incubating with liposome was used as the 0% binding standards. The binding ratio is (1 − Cbottom/Cstandard) × 100%.
Whole-Liposome Immunoprecipitation
The antibody used for pulldown was bound with protein G magnetic beads (Bioclone Inc. MAG-102) for 20 min at room temperature with shaking. The obtained mixture was washed with 200 μL PBS twice. After removing the washing buffer, liposomes bound with MPER were added to the tubes with the magnetically tagged antibodies. The obtained solution was incubated at 37 °C with shaking for 1 h. After removing the supernatant, the pellets were washed twice with 100 μL PBS. 300 μL PBS containing 1% TX-100 detergent was added and the PoP fluorescence in the supernatant was measured and the number of captured PoP molecules was calculated based on fluorescence values.
Animal Immunization
Animal experiments were conducted in accordance with the policies and approval of the University at Buffalo Institutional Animal Care and Use Committee (IACUC). 6-week-old ICR mice were immunized on days 0, 21, and 42 via intramuscular injection with different types of MPER antigens and adjuvants. Unless otherwise indicated, each dose contained 0.5 μg MPER antigen, 2 μg CoPoP and 4 μg MPLA in 50 µL aqueous solution. The peptide:CoPoP:MPLA mass ratio was fixed to [0.5:2:4]. To prepare the KLH-NMPER, a cysteine-modified NMPER was obtained from Genscript, pre-reduced by tris(2-carboxyethyl)phosphine (TCEP) and then conjugated with maleimide-activated KLH. The concentration of the obtained KLH-NMPER was measured by Bradford method. Complete Freund’s adjuvant, Alum, Montanide ISA720 adjuvants were mixed with antigen directly 1 h before injection. Blood was collected from the submandibular vein and the serum was obtained via centrifuging the blood at 2000×g for 15 min and stored at − 80 °C. Antibody titer was measured by conventional ELISA using HRP-linked anti-mouse secondary (Genscript A00160) and TMB substrate (Southern Biotech). For the ELISA against peptides, nickel plates (Well-Coated Nickel Plate, G-Biosciences #786-749) were coated with his-tagged MPER peptides. For the ELISA against proteins, the regular ELISA plates (Nunc-Immuno MicroWell 96-Well Plates Thermo #442404) were coated with the indicated proteins, including the gp41, gp140CF or Pfs25. Titers were defined as reciprocal serum dilution at which the absorbance exceeded the background by greater than 0.5 absorbance units. Typical background absorption values were 0.2-0.25.
Neutralization of Viral Entry
Neutralizing antibody activity was measured in 96-well culture plates by using Tat-regulated luciferase (Luc) reporter gene expression to quantify reductions in virus infection in TZM-bl cells. TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program (from John Kappes and Xiaoyun Wu). Assays were performed with HIV-1 Env-pseudotyped viruses as described previously.20 Test samples were diluted over a range of 1:20 to 1:43740 in cell culture medium and pre-incubated with virus (~ 150,000 relative light unit equivalents) for 1 h at 37 °C before addition of cells. Following a 48 h incubation, cells were lysed and Luc activity determined using a microtiter plate luminometer and BriteLite Plus Reagent (Perkin Elmer). Neutralization titers are the sample dilution (for serum) or antibody concentration (for monoclonal antibodies) at which relative luminescence units (RLU) were reduced by 50% compared to RLU in virus control wells after subtraction of background RLU in cell control wells.
Monoclonal Antibody Production
Monoclonal antibody production was carried out in collaboration with the Hybridoma Core at Lerner Research Institute at Case Western University. Mice were immunized intramuscularly with the CoPoP/MPLA MPER vaccine at 3 week intervals, for a total of three injections. 1 week after the third injection blood was drawn and the specific antigen titer of the serum measured by ELISA. One week later, spleen cells were isolated from high titer mice and frozen. Spleen cells were then fused with an SP2/0 myeloma cell line using a standard polyethylene glycol (PEG/DMSO) protocol and successfully fused cells were selected using HAT media. Fourteen days later, supernatant was removed and assayed for the specific antigen by ELISA. Cells from positive wells were expanded for cloning. Individual cells producing antibodies were isolated from the mixed culture by the limiting dilution method of cloning.
Results
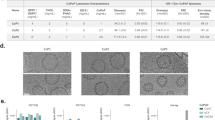
As shown in Fig. 1a, five different MPER sequences, all modified with a hepta-histidine tag at the C-terminus, were generated by conventional peptide synthesis. The nomenclature is the number of included amino acids proximal to the transmembrane portion plus the length of the his-tag (i.e., “X” + 7). The exception to this peptide naming is the “NMPER” construct, which represents a 16 amino acid portion of the N-terminus end of the MPER attached to a linker and then a 7X histidine tag, as this sequence has been used previously for MPER vaccination studies.28 The [30 + 7] and [25 + 7] antigens contain both the 2F5 and 4E10 epitopes, while the NMPER antigen contains the 2F5 epitope, and the [15 + 7] and [20 + 7] constructs included the 4E10 epitope.
MPER peptides used in this study and their binding with CoPoP/MPLA liposomes. (a) The amino acid sequence of the MPER peptides within the context of the HIV envelope protein (env). The membrane proximal external region (MPER) and transmembrane (TM) region are indicated, as are the N- and C-terminal heptad repeats (NHR and CHR) and the cytoplasmic tail (CT). The binding sites of the 2F5 and 4E10 bnAbs are shown. The sequences of the peptides used in this study are named in the boxed area, based on sequence length. (b) Binding of MPER peptides to CoPoP/MPLA or PoP/MPLA liposomes after simple mixing and overnight incubation. (c) Particle size of CoPoP/MPLA liposomes following binding of MPER peptides. Reported as the mean with standard deviation of three samples.
Spontaneous binding occurred between MPER peptides and CoPoP/MPLA liposomes with simple mixing in aqueous conditions, as shown in Fig. 1b. With CoPoP/MPLA liposomes, all MPER antigens achieved more than 70% binding yield; however, using analogous PoP/MPLA liposomes, which are identical but lack cobalt, only roughly 30% of the peptides attached. The average size of CoPoP/MPLA liposomes was 105 nm after 80 nm membrane extrusion. The association resulted in a slight size increase (Fig. 1c). The polydispersity index increased with binding of [15 + 7] and [20 + 7] MPER peptides (Supplementary Fig. 1) which may due to limited solubility of these antigens; however, no visible precipitation was observed in any samples.
The schematic illustration of the liposome is shown in Fig. 2a. The lipidic components including CoPoP and MPLA form a lipid bilayer structure. The his-tag is predicted to bind within the bilayer, since based on simulation, the porphyrin component resides fully within the bilayer.8 This leaves the MPER antigens to be presented on the surface of the liposome in a putatively biomimetic fashion. This is consistent with the observation that MPER-CoPoP liposomes could be recognized by the 2F5 bnAb. As shown in Fig. 2b, 2F5 could bind [25 + 7], [30 + 7] and NMPER peptides bound to CoPoP/MPLA liposomes, but not the [15 + 7] and [20 + 7] peptides that did not contain 2F5 epitope in their sequence. The B12 and F105 antibodies that target gp120 did not show binding to MPER-attached CoPoP/MPLA liposomes, as those antibodies do not target MPER. When peptides were mixed with PoP liposomes, which lack cobalt and do not bind the peptides, no recognition and liposome pulldown by the antibody was observed.
Schematic illustration of the MPER presentation and functionalized liposome reactivity with the 2F5 monoclonal antibody. (a) Schematic illustration of CoPoP and a single leaflet of a bilayer that has bound a his-tagged MPER peptide. The hydrophilic heads of the lipid components are presented with different colors. The polyhistidine sequence of the MPER peptides chelate with CoPoP. (b) MPER-CoPoP immunoprecipitation with various antibodies. Peptides were incubated with CoPoP/MPLA liposomes or PoP/MPLA liposomes, then immunoprecipitated with indicated antibodies. The 2F5 antibody recognized some MPER sequences. Data show the calculated number of captured CoPoP or PoP molecules (mean ± standard deviation for n = 3).
To assess immune responses to these constructs, groups of mice were immunized with various MPER and CoPoP/MPLA formulations, with subsequent serum antibody titers measured by ELISA. As shown in Fig. 3, for the mice immunized with [15 + 7] and [20 + 7] peptide antigens, antibody titers against all different MPER antigens was generally less than 103. The two groups of mice immunized with [25 + 7] and [30 + 7] produced over a log increase in IgG titer, in the range of 60,000-100,000 antibody titer against [25 + 7], [30 + 7] and NMPER peptides coated on the ELISA plate. However, these antibodies did not show recognition to the [15 + 7] and [20 + 7] MPER antigens despite containing overlapping sequence. Mice vaccinated with [30 + 7] MPER and NMPER, but not the [25 + 7] MPER showed significant difference in the IgG antibody titer against the [30 + 7] peptide, compared to mice vaccinated with the shorter [20 + 7] and [15 + 7] MPER peptides. Mice immunized with NMPER also elicited high titers against [30 + 7] and NMPER, but diminished reactivity against the [25 + 7] MPER peptide when compared to the immune responses of the [25 + 7] and [30 + 7] constructs. Mice immunized with [15 + 7] and [20 + 7] antigens elicited weak immune responses, generally lower by one log in titer, against all types of MPER antigens. Mice immunized with PoP/MPLA liposomes and MPER, which does not result in MPER particleization, generated low antibody titer (Supplementary Fig. 2A). The vaccine containing CoPoP but omitting MPLA in the liposome formulation also resulted in a weak immune response (Supplementary Fig. 2B). This shows that both CoPoP and MPLA components are needed for inducing strong antibody responses for his-tagged MPER antigens, and also that the peptide sequence itself influences the level of elicited antibodies.
Antibody titer of mice immunized with different length MPER antigens. Mice were immunized with 0.5 μg different MPER antigens bound to CoPoP/MPLA liposomes containing 4 μg MPLA. For each group of the mice, the antibody titer was measured against 5 different MPER antigens, respectively. Mean ± SD for n = 4 mice per group. Asterisks show select significant differences for antibody reactivity with the longest-length [30 + 7] construct, as determined by one-way ANOVA with post hoc Tukey test: ns, not significant p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005.
The antibodies from mice immunized with some MPER fragments could recognize the recombinant HIV envelope protein (env) that contained the MPER sequence. Figure 4 shows the ELISA against HIV proteins for mice immunized with different MPER antigens. HIV proteins were used which contained MPER sequences; HXBc2 gp41 and JRFL gp140CF. The mice immunized with [30 + 7] or NMPER generated antibody against both gp41 and gp140CF. The antibody titer level roughly corresponded to 1 µg/mL of the 2F5 monoclonal antibody. Mice immunized with [25 + 7] also showed antibody responses against the HIV env proteins, however, the titer was lower than [30 + 7] and NMPER. No significant difference was observed between mice vaccinated with [25 + 7] and [20 + 7] group. This indicates that the N-terminus portion of MPER is more immunogenic than the C-terminus.
Recognition of recombinant env by MPER-immunized post-immune sera. Mice were immunized with 0.5 μg different MPER antigens pre-associated to CoPoP/MPLA immunogenic liposomes. The plates were coated with recombinant HIV protein gp41 and gp140CF respectively. Mean ± SD for n = 4 mice per group. Asterisks show significance as determined by One-way ANOVA with post hoc Tukey test: ns, not significant p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005.
Owing to higher immunogenicity, recognition by the 2F5 antibody, and inclusion of the “LWYIK” cholesterol recognition/interaction amino acid consensus motif,7 subsequent experiments focused on the [30 + 7] MPER antigen, unless otherwise specified. Mice were immunized with same dose and volume of CoPoP/MPLA immunogenic liposome containing 0.5 μg MPER, using three different administration methods: intramuscular (IM), subcutaneous (SC) and Intraperitoneal (IP). Serum responses were compared by ELISA and the IM injection demonstrated higher serum titers against the MPER antigen (Fig. 5a). This may be due to enhanced uptake in antigen presenting cells (APCs) and presentation of the MPER within draining lymph nodes with the IM route.
Comparisons of different immunization methods and adjuvant formulations with the [30 + 7] MPER peptide. (a) Comparison of different immunization administration methods including the intramuscular (IM), subcutaneous (SC) and intraperitoneal (IP) administration using CoPoP/MPLA adjuvant. (b) The antibody titer of the mice immunized IM with indicated adjuvants. All the injection contained 0.5 μg antigen per dose. Asterisks show significance as determined by One-way ANOVA with post hoc Tukey test: ns, not significant p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005.
Immunization of MPER with a couple of other common adjuvants was compared to MPER CoPoP constructs. Mice immunized with the CoPoP/MPLA adjuvant showed a higher antibody titer than Alum and Montanide (Fig. 5b). This is likely due to the hapten-like behavior of peptide antigens, which may not be uptaken by APCs without attachment to a larger particle or protein. Mice immunized with CoPoP generated increased antibody titer than those ones with the combination of KLH conjugated to MPER adjuvanted with complete Freund’s adjuvant (CFA), in both post prime and post boost when the NMPER construct are used (Supplementary Fig. 3).
Pfs25 is a recombinant malaria protein approximately 20 kDa in size that was previously demonstrated to induce strong antibody levels when displayed in the CoPoP system.14 To assess if co-delivery of a peptide and protein antigens could enhance the MPER response, we created CoPoP/MPLA particles containing both Pfs25 and MPER peptides and compared their response to single antigen MPER peptide. Mice immunized with CoPoP/MPLA liposome containing both Pfs25 and MPER showed strong immune response against each respective antigen. As shown in Fig. 6a, mice immunized with CoPoP/MPLA liposome containing MPER and Pfs25 showed more than fivefold increased MPER antibody titer than ones immunized with CoPoP MPER alone. For the CoPoP/MPLA MPER alone groups, mice immunized with 0.5 μg antigen generated 60,000-90,000 antibody titer which was near levels obtained with the 2 μg antigen dose. However, when the injection dose decreased to 0.1 μg, a reduction in resulting antibodies was observed. Mice immunized with CoPoP/MPLA liposome containing 0.02 μg MPER and 0.02 μg Pfs25 (an approximate molar ratio 1:4 for Pfs25 to MPER) combination generated a greater than one log increase in antibody titer compared to 0.02 μg CoPoP/MPLA MPER alone demonstrating the efficient improvement in the immune response through the co-injection with Pfs25.
Immunization with MPER and a non-specific protein. (a) Antibody titers against the [30 + 7] MPER antigen for mice immunized with different dose of [30 + 7] MPER antigen and a non-specific his-tagged protein, Pfs25. The mass ratio between MPER and Pfs25 was 1:1 (molar ratio 1:4 for Pfs25 to MPER). (b) The antibody against gp140CF for the mice immunized with MPER and Pfs25. Each dose contained 0.5 μg MPER antigen and the indicated amount of Pfs25 (molar ratio 1:4, 1:20 or 1:100 for Pfs25 to MPER). (c) IgG antibody subclass ratios against MPER antigen for mice immunized with MPER or MPER + Pfs25 with CoPoP/MPLA liposomes. Mean ± SD for n = 4 mice per group. (d) Neutralization of virus entry for serum from mice immunized with MPER antigens with and without Pfs25. W61D(TCLA)0.71, which is sensitive to broadly neutralizing antibodies targeting MPER, was used in this assay. Asterisks show significance as determined by One-way ANOVA with post hoc Tukey test: ns, not significant p > 0.05; *p < 0.05; **p < 0.01; ***p < 0.005.
The antibodies generated from mice immunized with the both CoPoP MPER and Pfs25 constructs showed higher titer against gp140CF protein. Mice immunized with 0.5 μg CoPoP with MPER and Pfs25 showed a higher antibody titer against gp140CF protein than those immunized with only MPER alone. The enhancement in MPER antibody may stem from enhanced helper T cell support induced by the Pfs25 sequence. However, little difference was observed among different doses of Pfs25 constructs when using the 0.5 μg MPER dose in all regimens. MPER-specific antibody titers were similar when 0.02 µg (molar ratio 1:100 for Pfs25 to MPER) or 0.5 µg (molar ratio 1:4 for Pfs25 to MPER) Pfs25 were co-immunized (Fig. 6b).
Th1 or Th2 responses can induce differences in IgG1 and IgG2 subclass ratios. Certain IgG subclass ratios were assessed, as shown in Fig. 6c. The (IgG2a + IgG2b) vs. IgG1 ratio was 0.86 and 0.74 for mice immunized with MPER or MPER + Pfs25, respectively. This shows minimal change in the Th1 or Th2 bias of the response. The MPER antigen did not increase the production of antibody against Pfs25. Mice immunized with CoPoP constructs containing 0.02 μg Pfs25 and 0.5 μg MPER (approximate molar ratio 1:100 for Pfs25 to MPER) showed the same antibody titer against Pfs25 protein as the mice immunized with CoPoP/MPLA liposome only containing 0.02 µg Pfs25 (Supplementary Fig. 4).
The post-immune sera from mice were collected to assess the inhibition of viral entry by HIV in TZM-bl cells (Fig. 6d). Serum from mice immunized with CoPoP/MPLA liposome containing 0.5 μg MPER antigen showed neutralization of HIV strain W61D(TCLA)0.71(tier 1A) HIV virus, but not MN.3 (tier 1A) nor C3347.c11 (tier 2). No inhibition was observed in the mice immunized with only Pfs25, indicating a lack of non-specific carrier responses and supports specificity of targeting the MPER antigen. Interestingly, despite increasing the MPER antibody titer, the addition of Pfs25 did not show improved neutralization.
We next assessed whether monoclonal antibodies (mAbs) could be generated with the CoPoP/MPLA system and the [30 + 7] MPER peptide. Mice immunized with MPER-CoPoP vaccine elicited anti-MPER antibodies after the 2nd boosting (Fig. 7a). Following the isolation of spleens, the fusion of splenocytes with myeloma cells, the obtained hybridoma was cultured to generate the clones. Totally, twelve positive hits were selected based on the optical density (OD) of the ELISA screen (Fig. 7b). The hybridoma supernatants from the positive hits were assayed against the same three MPER-sensitive viruses used previously, but no neutralizing activity was detected. While disappointing, we are not aware of any mouse monoclonal antibodies that have been developed with broad neutralization. It is worth noting that many of the human MPER bnAbs such as 2F5 and 4E10 are of the human IgG3 subclass.
Generation of monoclonal antibodies. (a) Antibody titer against [30 + 7] MPER antigen for the mice treated with MPER-CoPoP vaccine. Serum was collected 7 days after the 2nd boosting. Then mice are sacrificed and the spleens were harvest for the generation of mAbs. (b) The positive hits for the screen of the hybridoma clone supernatants. ELISA was applied to check the hits. The samples with ELISA optical density (OD) more than 0.15 were assessed for neutralization. No hybridoma supernatants induced substantial viral neutralization of the W61D(TCLA)0.71, MN.3 strains or C3347.c11 HIV strains (data not shown).
Discussion
The utilization of a carrier is an efficient way to improve the immunity of antigens with low immunogenicity such as short synthetic peptides. His-tagged MPER antigens were able to associate specifically with CoPoP liposome, which is consistent with previous work,25 due to chelation between histidine and cobalt in the center of porphyrin. The CoPoP liposome provided a simple method to generate particle-based MPER vaccines. PoP liposomes, which lacked cobalt to induce MPER particleization showed poor immunogenicity when compared to CoPoP. The TLR-4 agonist MPLA, was also an important component to induce strong antibody responses.
The shorter, C-terminus MPER antigens [15 + 7] and [20 + 7], failed to elicit a strong immune response when the CoPoP was used as the adjuvant, consistent with previous work utilizing monophosphoryl lipid A.18,29,31 However, the NMPER construct showed robust responses implying length alone was not the only potential limiting factor. As the latter contained a spacer sequence between the histidines and the antigen, this also may indicate that portions of the targeted peptide sequence could be buried into the bilayer structure.
Besides peptide length, the antigen sequence impacted the immune responses. Although the [20 + 7] and NMPER constructs were similar in length, a strong immune response was only observed in the mice immunized with NMPER. A previously published study also showed the N-terminus of the MPER was more immunogenic; and, C-terminus chemical modification enhanced MPER immunogenicity.31 A full length MPER sequence induced a more robust immune response, than shorter ones containing only the N-terminus (2F5 epitope) or the C-terminus (4E10 epitope) alone.28
Presentation in CoPoP/MPLA constructs has a number of advantages over the other common commercially available adjuvants tested. Compared with the combination of KLH conjugation and CFA, the benefit of CoPoP/MPLA was more substantial in low dose (0.5 μg antigen) conditions where an order of magnitude higher antibody was observed post boost. For the CoPoP/MPLA liposome groups, the mice injected with 5 μg antigen and 0.5 μg antigen did not show much difference in generating antibody. Another consideration for adjuvant choice is the preparation and administration. Conjugating the antigen to KLH required two step chemical synthesis and purification; however, simple aqueous incubation is used in creation of a CoPoP/MPLA liposome containing MPER constructs.
The addition of Pfs25 in MPER vaccine showed enhanced antibody titer against MPER antigen. This result demonstrated that utilization of another non-specific antigen with high immunogenicity could enhance the immune response against all the antigens in the vaccine. The subtype ratio of IgG antibody resulting from MPER-CoPoP revealed a Th1- and Th2- combined immune response elicited in immunized mice. Addition of Pfs25 did not appear to shift the Th bias. Pfs25 may have contributed T cell epitopes that led to an overall enhanced immune response. The convenient binding approach between CoPoP liposome with his-tagged antigens enable it to be associated with several antigens simply by aqueous incubation at the same time. The obtained antibodies showed the recognition of the MPER sequence, but no improved neutralization of HIV. As a possible explanation, antigen conformation interference may have occurred when MPER and Pfs25 were presented in a single CoPoP/MPLA liposome, resulting in a lack of improved neutralizing antibody response. The mechanism of the immune response induced by protein-helper dependent MPER may be different from that induced by independent MPER peptide antigen. Further exploration of this is planned for future studies on utilization of CoPoP/MPLA immunogenic liposomes as a multivalent vaccine carrier.
CoPoP/MPLA with MPER immunogens were able to induce a response that could neutralize the W61D(TCLA)0.71(tier 1A) HIV strain, which is exquisitely sensitive to MPER-specific antibodies; however, neutralization of other viruses failed. Creation of a vaccine based on gp41 sequences remains a challenge. Numerous attempts to use gp41 constructs and MPER specific constructs as vaccines show disappointing results.13 The natural immune response against gp41 during infection is focused against two immunodominant epitopes (termed Cluster I and Cluster II) that tend to not induce neutralizing Abs. This presents difficulty with using full gp41 constructs, supporting a more focused approach. More recently, a structurally informed approach has led to improvement in immunogenic responses.5,22 Because of the potential for broad neutralization, continued immunological study of the MPER region is warranted. Our results in this study continue to support that CoPoP/MPLA liposomes are a useful platform for peptide antigens.
To conclude, the designed MPER antigens were able to associate with CoPoP/MPLA immunogenic liposomes through simple aqueous incubation. CoPoP/MPLA liposomes, containing lipid-presented MPER antigens on the surface, elicited immune response with murine immunizations, with sufficiently lengthy MPER segments. The NMPER peptide was also immunogenic. The immunized mice generated antibodies that recognized the HIV proteins containing MPER sequences, and that neutralized the W61D(TCLA)0.71 HIV strain. The addition of protein helper increased the antibody titer, however, did not improve the neutralization against HIV viral entry. In spite of the failure to generate neutralizing mouse mAbs against HIV, the CoPoP immunogenic liposome system provided advantages of rapid design and testing while using low dose synthetic peptide antigens. Further studies are required to better determine whether other antigens delivered by CoPoP/MPLA liposomes could induce better neutralization.
References
Aikins, M. E., J. Bazzill, and J. J. Moon. Vaccine nanoparticles for protection against HIV infection. Nanomedicine 12:673–682, 2017.
Apellániz, B., and J. L. Nieva. The use of liposomes to shape epitope structure and modulate immunogenic responses of peptide vaccines against HIV MPER. Advances in Protein Chemistry and Structural Biology, Elsevier: New York, 2015, pp. 15–54.
Banerjee, S., H. Shi, M. Banasik, H. Moon, W. Lees, Y. Qin, A. Harley, A. Shepherd, and M. W. Cho. Evaluation of a novel multi-immunogen vaccine strategy for targeting 4E10/10E8 neutralizing epitopes on HIV-1 gp41 membrane proximal external region. Virology 505:113–126, 2017.
Banerjee, S., H. Shi, H. H. Habte, Y. Qin, and M. W. Cho. Modulating immunogenic properties of HIV-1 gp41 membrane-proximal external region by destabilizing six-helix bundle structure. Virology 490:17–26, 2016.
Bomsel, M., D. Tudor, A. S. Drillet, A. Alfsen, Y. Ganor, M. G. Roger, N. Mouz, M. Amacker, A. Chalifour, L. Diomede, G. Devillier, Z. Cong, Q. Wei, H. Gao, C. Qin, G. B. Yang, R. Zurbriggen, L. Lopalco, and S. Fleury. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 34:269–280, 2011.
Burton, D. R., R. Ahmed, D. H. Barouch, S. T. Butera, S. Crotty, A. Godzik, D. E. Kaufmann, M. J. McElrath, M. C. Nussenzweig, B. Pulendran, C. N. Scanlan, W. R. Schief, G. Silvestri, H. Streeck, B. D. Walker, L. M. Walker, A. B. Ward, I. A. Wilson, and R. Wyatt. A blueprint for HIV vaccine discovery. Cell Host Microb. 12:396–407, 2012.
Carravilla, P., A. Cruz, I. Martin-Ugarte, I. R. Oar-Arteta, J. Torralba, B. Apellaniz, J. Pérez-Gil, J. Requejo-Isidro, N. Huarte, and J. L. Nieva. Effects of HIV-1 gp41-derived virucidal peptides on virus-like lipid membranes. Biophys. J. 113:1301–1310, 2017.
Carter, K. A., S. Shao, M. I. Hoopes, D. Luo, B. Ahsan, V. M. Grigoryants, W. Song, H. Huang, G. Zhang, R. K. Pandey, J. Geng, B. A. Pfeifer, C. P. Scholes, J. Ortega, M. Karttunen, and J. F. Lovell. Porphyrin–phospholipid liposomes permeabilized by near-infrared light. Nat. Commun. 5:3546, 2014.
Chakrabarti, B., L. Walker, J. Guenaga, A. Ghobbeh, P. Poignard, D. Burton, and R. Wyatt. Direct antibody access to the HIV-1 membrane-proximal external region positively correlates with neutralization sensitivity. J. Virol. 85:8217–8226, 2011.
Donius, L. R., Y. Cheng, J. Choi, Z.-Y. J. Sun, M. Hanson, M. Zhang, T. M. Gierahn, S. Marquez, M. Uduman, S. H. Kleinstein, D. Irvine, J. C. Love, E. L. Reinherz, and M. Kim. Generation of long-lived bone marrow plasma cells secreting antibodies specific for HIV-1 gp41 MPER in the absence of polyreactivity. J. Virol. 90:8875–8890, 2016.
Florese, R. H., K. K. Van Rompay, K. Aldrich, D. N. Forthal, G. Landucci, M. Mahalanabis, N. Haigwood, D. Venzon, V. S. Kalyanaraman, M. L. Marthas, and M. Robert-Guroff. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J. Immunol. 177:4028–4036, 2006.
Hanson, M. C., W. Abraham, M. P. Crespo, S. H. Chen, H. Liu, G. L. Szeto, M. Kim, E. L. Reinherz, and D. J. Irvine. Liposomal vaccines incorporating molecular adjuvants and intrastructural T-cell help promote the immunogenicity of HIV membrane-proximal external region peptides. Vaccine 33:861–868, 2015.
Ho, J., R. A. Uger, M. B. Zwick, M. A. Luscher, B. H. Barber, and K. S. MacDonald. Conformational constraints imposed on a pan-neutralizing HIV-1 antibody epitope result in increased antigenicity but not neutralizing response. Vaccine 23:1559–1573, 2005.
Huang, W. C., B. Deng, C. Lin, K. A. Carter, J. Geng, A. Razi, X. He, U. Chitgupi, J. Federizon, B. Sun, C. A. Long, J. Ortega, S. Dutta, C. R. King, K. Miura, S. Lee, and J. F. Lovell. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 13:1174–1181, 2018.
Kim, M., Z.-Y. J. Sun, K. D. Rand, X. Shi, L. Song, Y. Cheng, A. F. Fahmy, S. Majumdar, G. Ofek, Y. Yang, P. D. Kwong, J. Wang, J. R. Engen, G. Wagner, and E. L. Reinherz. Antibody mechanics on a membrane-bound HIV segment essential for GP41-targeted viral neutralization. Nat. Struct. Mol. Biol. 18:1235–1243, 2011.
Lovell, J. F., C. S. Jin, E. Huynh, H. Jin, C. Kim, J. L. Rubinstein, W. C. Chan, W. Cao, L. V. Wang, and G. Zheng. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 10:324, 2011.
Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210, 2000.
Matyas, G. R., L. Wieczorek, Z. Beck, C. Ochsenbauer-Jambor, J. C. Kappes, N. L. Michael, V. R. Polonis, and C. R. Alving. Neutralizing antibodies induced by liposomal HIV-1 glycoprotein 41 peptide simultaneously bind to both the 2F5 or 4E10 epitope and lipid epitopes. AIDS 23:2069–2077, 2009.
McCoy, L. E., and D. R. Burton. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol. Rev. 275:11–20, 2017.
Montefiori, D. C. Measuring HIV neutralization in a luciferase reporter gene assay. HIV Protocols, Berlin: Springer, 2009, pp. 395–405.
Montero, M., N. Gulzar, K.-A. Klaric, J. E. Donald, C. Lepik, S. Wu, S. Tsai, J.-P. Julien, A. J. Hessell, S. Wang, S. Lu, D. R. Burton, E. F. Pai, W. F. DeGrado, and J. K. Scott. Neutralizing epitopes in the membrane-proximal external region of HIV-1 gp41 are influenced by the transmembrane domain and the plasma membrane. J. Virol. 86:2930–2941, 2012.
Ofek, G., F. J. Guenaga, W. R. Schief, J. Skinner, D. Baker, R. Wyatt, and P. D. Kwong. Elicitation of structure-specific antibodies by epitope scaffolds. Proc. Natl. Acad. Sci. USA 107:17880–17887, 2010.
Sarkar, A., S. Bale, A. J. Behrens, S. Kumar, S. K. Sharma, N. de Val, J. Pallesen, A. Irimia, D. C. Diwanji, R. L. Stanfield, A. B. Ward, M. Crispin, R. T. Wyatt, and I. A. Wilson. Structure of a cleavage-independent HIV Env recapitulates the glycoprotein architecture of the native cleaved trimer. Nat. Commun. 9:1956, 2018.
Shao, S., T. N. Do, A. Razi, U. Chitgupi, J. Geng, R. J. Alsop, B. G. Dzikovski, M. C. Rheinstädter, J. Ortega, M. Karttunen, J. A. Spernyak, and J. F. Lovell. Design of hydrated porphyrin-phospholipid bilayers with enhanced magnetic resonance contrast. Small 13:1602505, 2017.
Shao, S., J. Geng, H. A. Yi, S. Gogia, S. Neelamegham, A. Jacobs, and J. F. Lovell. Functionalization of cobalt porphyrin–phospholipid bilayers with his-tagged ligands and antigens. Nat. Chem. 7:438, 2015.
Shao, S., V. Rajendiran, and J. F. Lovell. Metalloporphyrin nanoparticles: coordinating diverse theranostic functions. Coord. Chem. Rev. 379:99–120, 2019.
Steichen, J. M., D. W. Kulp, T. Tokatlian, A. Escolano, P. Dosenovic, R. L. Stanfield, L. E. McCoy, G. Ozorowski, X. Hu, O. Kalyuzhniy, B. Briney, T. Schiffner, F. Garces, N. T. Freund, A. D. Gitlin, S. Menis, E. Georgeson, M. Kubitz, Y. Adachi, M. Jones, A. A. Mutafyan, D. S. Yun, C. T. Mayer, A. B. Ward, D. R. Burton, I. A. Wilson, D. J. Irvine, M. C. Nussenzweig, and W. R. Schief. HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity 45:483–496, 2016.
Venditto, V. J., D. S. Watson, M. Motion, D. Montefiori, and F. C. Szoka. Rational design of membrane proximal external region lipopeptides containing chemical modifications for HIV-1 vaccination. Clin. Vaccine Immunol. 20:39–45, 2013.
Venditto, V. J., L. Wieczorek, S. Molnar, F. Teque, G. Landucci, D. S. Watson, D. Forthal, V. R. Polonis, J. A. Levy, and F. C. Szoka. Chemically modified peptides based on the membrane-proximal external region of the HIV-1 envelope induce high-titer, epitope-specific nonneutralizing antibodies in rabbits. Clin. Vaccine Immunol. 21:1086–1093, 2014.
Verkoczy, L., G. Kelsoe, and B. F. Haynes. HIV-1 envelope gp41 broadly neutralizing antibodies: hurdles for vaccine development. PLoS Pathog. 10:1371, 2014.
Watson, D. S., and F. C. Szoka, Jr. Role of lipid structure in the humoral immune response in mice to covalent lipid–peptides from the membrane proximal region of HIV-1 gp41. Vaccine 27:4672–4683, 2009.
Acknowledgments
This work was supported by the National Institutes of Health (R01AI125119, R21AI122964, DP5OD017898, HHSN272201800004C). We would like to thanks Earl Poptic at Cleveland Clinic for hybridoma work. We acknowledge PATH-MVI for providing Pfs25.
Conflict of interest
JF.L and W.H. hold interest in POP Biotechnologies. All other authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Debra T. Auguste oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shao, S., Huang, WC., Lin, C. et al. An Engineered Biomimetic MPER Peptide Vaccine Induces Weakly HIV Neutralizing Antibodies in Mice. Ann Biomed Eng 48, 1991–2001 (2020). https://doi.org/10.1007/s10439-019-02398-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02398-8