Abstract
Small-diameter tissue-engineered vascular grafts are urgently needed for clinic arterial substitute. To simulate the structures and functions of natural blood vessels, we designed a novel triple-layer poly(ε-caprolactone) (PCL) fibrous vascular graft by combining E-jet 3D printing and electrospinning techniques. The resultant vascular graft consisted of an interior layer comprising 3D-printed highly aligned strong fibers, a middle layer made by electrospun densely fibers, and an exterior structure composed of mixed fibers fabricated by co-electrospraying. The biocompatible triple-layer graft was used for in vivo implantation, and results demonstrated that the longitudinally-aligned fibers within the lumen of the graft could enhance the proliferation and migration of endothelial cells, while maintained good mechanical properties. The exterior layer provided a pathway that encouraged cells to migrate into the scaffold after implantation. This experimental graft overcame the limitations of conventionally electrospun vascular grafts of inadequate porosity and lowly cell penetration. The unique structure of the triple-layer vascular graft promoted cell growth and infiltration in vivo, thus provided an encouraging substitute for in situ tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases are considered to be a problem on a global scale that result in a high probability of deaths annually.29 Demand for small vessels is growing for clinical vascular surgeries.17,21 Tissue engineering has developed as a vital domain of the regenerative medicine and a critical way of biomedical regeneration.2 As observed in situ, tissues are embedded in highly aligned basement membranes, surrounded by a complex formation of extracellular matrix (ECM) proteins comprising well-organized functional fibers.14 For instance, the tunica intima of vessels is coated with an anti-thrombogenic layer of longitudinally-orientated endothelial cells,4 the alignment and orientation of the structural features of which effect the functions of cells through interactions with vascular tissues.9 Therefore, an essential factor in tissue engineering is the construction of an organized scaffold by which the behavior of cells can be controlled.
Ideal tissue-engineered vessels have structures and functions comparable to native blood vessels, which could guide the regeneration of new vascular tissues.24 Previous studies have revealed that multi-layered grafts architecturally mimic native vascular walls and are mechanically matched to the native arteries could be an effective approach.10 Therefore, to fabricate a functional scaffold that faithfully mimics the structures of natural extracellular matrix, which could direct cell migrations and control cell activities, is the major challenge.18 The interspace of scaffolds is also a critical factor when regenerating functional tissues. Porosity and pore interconnectivity of scaffolds are essential for cell ingrowth and associated activities.12,22
Electrospinning has drawn a great deal of interest for the vascular grafts production, for electrospun scaffolds constructed in this way having a unique micro-to-nanoscale topography.7 However, electrospun biomaterials often have tiny pores (diameter < 6 μm) and low porosity (< 80%),20 which would inhibit cell adhesion, infiltration and tissue regeneration.13 Electro-hydrodynamic jet (E-jet) 3D printing is a novel printing technique in which a wide range of biomaterials can be utilized in the fabrication of scaffolds whose geometric features can be more precisely controlled.19,26 In E-jet 3D printing, a fine jet of polymer solution or melt is created, much narrower than the diameter imposed by the limitations imposed by the physical nozzle, using an electric field so as to produce micro- to nanoscale features.27 Consequently, this is a valuable printing technique for constructing precise 3D structures at fine scales through controlling the deposition of the tiny stream of fluid to an extreme level of fidelity.26,28
This study develops a novel triple-layered vascular graft by combining E-jet 3D printing with electrospinning. The graft can simulate the structures of native blood vessels and functions of the surface of a vascular lumen and that of the tunica media. This vascular graft could induce the vascular tissues regeneration, which are suitable for clinical arterial replacements.
Materials and Methods
Materials
Polyethylene glycol (PEG, molecular weight is 3.0 × 105 Da) powder, dimethylsulfoxide (DMSO), tetrahydrofuran (THF), N,N-dimethylformamide (DMF), sodium heparin, and acetate were obtained from Aladdin Chemicals (China). Pellets of poly(ε-caprolactone) (PCL, molecular weight is 1.2 × 105 Da) were bought from Daigang (China). Hematoxylin and eosin (H&E), vinculin, phalloidin and DAPI staining reagents, and paraformaldehyde were purchased from Biotopped Science (China).
Vascular Grafts Fabrication
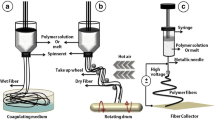
The triple-layered vascular grafts were fabricated via three steps. Firstly, the middle layer, made of electrospun longitudinally-oriented fine PCL fibers (Fig. 1a), was produced by using a customized collector as per our previous report.25 The electrospinning device included two DC power suppliers, a liquid supplying system, and a grounded collector composed of two paralleled aluminum strips (Fig. 1b). PCL pellets were dissolved in a 1:1 mixture of DMF/THF, and stirred at 40 °C for 1 day. Then the solution (6% w/v) was supplied to a blunt-ended needle (27G, ID: 0.21 mm, OD: 0.41 mm) with a flow velocity of 1.0 mL/h. This nozzle was applied with a positive voltage of + 13 kV, and the collector was applied with a negative voltage of − 1.5 kV. The distance between the needle and the collector was 12 cm, and a jet would be ejected from the needle due to applied strong electric field which resulted in a fiber bridging the two strips. The electrostatic repulsion between neighboring fibers would align them between the strips (Fig. 1b). The thickness of the middle layer formed by the aligned fibers was approximately 100 μm.
The fabrication process of triple-layered vascular grafts. (a) Two paralleled aluminum strips were utilized for collecting electrospun fibers; (b) aligned fibers were collected by the strips; (c) the E-jet 3D printing process; (d) the printed interior layer made of highly-aligned filaments; (e) the co-electrospun exterior layer formed by randomly mixed fibers was wrapped over the bilayer film; (f) the final triple-layer tubular graft: an interior layer composed of highly-aligned strong PCL fibers, a compactly packed middle layer, and an exterior layer comprising two types of random fibers.
The internal layer was constructed using our customized E-jet 3D printing system,19 which included a high-voltage DC power source, a 3D precision stage, and a solution feeding system (Fig. 1c). After applying a suitable voltage (2.6–3.0 kV) to the nozzle, size controllable filaments were created as a consequence of varied forces being applied to the printing solution when it was ejected. Different voltages would form different fiber diameters, and the narrowest fiber was created using a voltage of 2.7 kV. By varying the electric field and the path of plotting, different structures could be printed.19 In this case, the distance between the needle and substrate was kept at 2 mm, and the 3D printing process was monitored by an optical observation system. Highly-aligned PCL fibers were printed directly onto the middle layer so as to construct the internal layer (Fig. 1d). Then, the bilayer film was wrapped over a mandrel collector (radius: 2 mm).
The exterior layer, composing hybrid PCL and PEG fibers, was fabricated by co-electrospinning (Fig. 1e). PEG solution (10% w/v) was prepared by dissolving in 90% ethanol with 12 h stir. PCL and PEG solutions were fed into separate syringes loaded on pumps with a 27G or 18G (ID: 0.9 mm, OD: 1.26 mm) blunt-ended needle, respectively. The distances between the needle tips and the collector were 12 cm for PCL and 15 cm for PEG, respectively. Tubular grafts were fabricated using a 2 mm diameter spinning tribolet collector, rotating at 400 r/min. The co-electrospinning was performed with a flow rate of 1.0 mL/h for PCL solution, either 0 or 1.5 mL/h for PEG solution. The hybrid triple-layer graft was prepared using the bilayer film wrapped collector as mentioned above (Fig. 1f), and a pure PCL (0 mL/h for PEG solution) monolayer tubular graft was fabricated as a control, using a new collector. Then, all grafts were put in a vacuum dryer for 2 days.
Characterization of Vascular Grafts
The grafts was rinsed in phosphate-buffered saline (PBS) for 1 day at 37°C to dissolve the PEG components, and a Fourier transform infra-red (FTIR, WQF-410, Thermo) assessment was performed to characterize the PEG dissolution.25 Then, the samples were sputter-coated with gold for 50 s, and the surface morphology was imaged using SEM (JSM-6700F, JEOL, Japan). To measure the porosity, specimens were dried and weighed, then immersed in ethanol for 3 h. After that, took the samples out, and removed any excess ethanol on it. Finally, re-weighed the samples.8 The porosity was calculated using the following equations:
where Ve is the volume of ethanol inside the sample; Vs represents the volume of the sample; Ww and Wd are the wet and dry weights of the sample, respectively; De is the density of ethanol, and Ds represents the density of the scaffold materials.
Hemocompatibility Evaluation
The grafts were immersed in sodium chloride solution (0.9%) for half an hour at 37 °C before use. Ten milliliter fresh blood from healthy Sprague–Dawley (SD) rats was diluted with 1 mL NaCl solution. The use of animals was permitted by the Animal Care and Use Committee of Hunan University. Fifty microliter of diluted blood was added to each sample and incubated for 1 h.3 Then, samples were centrifuged for 10 min at 2 × 103 r/min. The absorbance of collected supernatant was measured at 545 nm by a micro-plate reader (Benchmark). Fifty microliter of diluted blood was added to 2 mL of distilled water or NaCl solution, which was used as a positive or negative control. Hemolysis of samples was calculated using the following equation:
where AS is the absorption value of the sample, AN and AP are the absorption value of the negative and positive control, respectively.
Mechanical Testing
Mechanical properties of grafts were tested by a tensile tester (HY-0230, Hengyi). First, tubular grafts (ID: 2 mm; length: 15 mm) were immersed in PBS for 1 day at 37 °C. Then, specimens were clamped to the tensile tester by a 100 N load cell. A stress was applied at a speed of 5 mm/min until rupture. The stress/strain of the grafts along its diameter were measured, and the results were recorded by the machine. The burst pressures of grafts were calculated using following equation,5 and an abdominal aorta from SD rats was used as the control.
where Pb is the burst pressure of the sample, σb is the tensile strength, T and D are the thickness and the diameter of grafts, respectively.
Heparin Immobilization
Sodium heparin was dissolved in 0.2 M acetate buffer at pH 4.6 with the solution concentration of 0.5 mg/mL. Triple-layer vascular grafts were first washed by deionized water to remove the PEG component. Then, the scaffolds were placed into heparin solution and agitated for 1 day. Later, samples were rinsed three times with deionized water, then dried in a vacuum at room temperature overnight. Surface hydrophilic/hydrophobic characteristics of the grafts were assessed by WCA analysis (DSA100, Kruss).
Fresh blood from SD rats (about 250 g) was centrifuged for 10 min, then, the platelet-rich plasma (PRP) supernatant was collected. Specimens were cut to form sheets, rinsed with PBS and then placed into a 24-well cell culture plate. Five hundred microliter of PRP were added to each well for platelet adhesion studies, then incubated at 37 °C for 1 h with gentle agitation. The PRP was removed from the scaffolds which were then rinsed by PBS to remove unattached platelets. The samples were immersed in 2.5 wt% glutaraldehyde for 2 h at 4 °C, rinsed with PBS and then dehydrated through an increasing ethanol gradient. The samples were sputtered with gold, and the adhered platelets were observed using SEM.
Cell Adhesion and Proliferation
The scaffolds were cut to form sheets, immersed in 75% ethanol for 2 h, then exposed to ultraviolet light for 2 h following 3 rinses in PBS. Human umbilical vein endothelial cells (HUVECs, Shanghai Institutes for Biological Sciences (SIBS)) were cultured on the sheets and tissue culture polystyrene (TCP) both of which were placed in a 12-well culture plate at a density of 5.0 × 104 cells per well, and maintained in a cell incubator under 5% CO2 at 37 °C, the culture medium contained 90% low-glucose Dulbecco’s modified Eagle’s medium (Sigma-Aldrich), 10% fetal bovine serum (Sigma-Aldrich), and 1% antibiotic–antimycotic (×100) (Sigma-Aldrich). The cell morphology was assessed using SEM at days 1, 2 and 3. Samples were rinsed by PBS, then cells were fixed using 2% glutaraldehyde, later stained with DAPI, vinculin, phalloidin and H&E. All samples were observed by a fluorescent microscope (TE-2000, Nikon).
Implantation in Animals
Tubular grafts (ID: 2 mm; length: 15 mm) were sterilized, then implanted into the SD rats (about 250 g) by replacing a segment of the abdominal artery. The rats were anesthetized by an intraperitoneal injection of chloral hydrate (300 mg/kg). The grafts were sterilized and then implanted with 3–5 interrupted stitches using 9–0 monofilament nylon sutures (Yuan Hong, China). No anticoagulant drugs were used. After 4, 8 and 12 weeks, samples were explanted, and the rats were sacrificed by injection with an overdose of chloral hydrate. The explanted grafts were fixed with 4% paraformaldehyde and dehydrated in 30% sucrose solution. The mechanical testing of explanted samples after 12 weeks was performed. Later, the specimens were embedded in optimal cutting temperature compound (OCT), then sliced into 6 μm-thick sections by a freezing microtome (CM1950, Leica). Stained samples were observed by a fluorescence microscope (SMZ1000, Nikon; objective 40×, eyepiece 10×) and a confocal microscope (PerkinElmer, green laser), and the distance of cell penetration into the grafts was measured. DAPI and phalloidine were excited at 359 and 545 nm, and their emission was set at 461 and 570 nm respectively.
Statistics Analysis
Each experiment was carried out in five replicates at least and all results were presented as mean ± standard deviation. A one-way analysis of variance (ANOVA) with Tukey honestly significant difference post hoc test using SPSS software (USA). A value of p < 0.05 was considered significant.
Results
Characteristics of Vascular Grafts
The triple-layer scaffolds (Fig. 1f) were constructed from a 3D-printed internal layer composed of highly-aligned, strong PCL fibers, an electrospun densely middle layer, and a co-electrospun exterior layer comprising mixed fibers (PCL/PEG). The inner diameter of the tubular grafts is 2 mm, and the cross section consists of three layers (Figs. 2b and 2c).
SEM images of fabricated vascular grafts. (a) Megascopic view; (b)–(f) images of the triple-layer grafts: (b) cross-section, (c) high-magnification view of (b), (d) lumen of highly aligned internal layer, (e) compactly middle layer, (f) the exterior layer before PEG was dissolved; (g) the lumen of monolayer grafts (control); (h) the exterior layer of the triple-layer grafts after dissolution of PEG; (i) high-magnification view of image in (g); (j) high-magnification view of image in (h).
The 3D-printed PCL fibers maintain a particular orientation that forms a highly-aligned structure as the internal layer (Fig. 2d), providing the graft strong mechanical support. PEG was removed from the composites by dissolving in PBS (Fig. S1A). Before dissolution, the middle and exterior layers comprised longitudinally-oriented PCL nanofibers (Fig. 2e) and randomly-oriented mixed (PCL/PEG) microfibers, respectively (Fig. 2f). The external layer following removal of PEG is shown in Figs. 2h and 2j, the pore size of which became significantly larger than that of control (Figs. 2g and 2i). The PEG removal yielded more loosely packed fibers (Figs. 2h and 2j) and an increase in porosity of approximately 16.2%, increasing from 75.3 to 91.5% comparing triple-layer and monolayer grafts, respectively (Table 1). Moreover, the large pores between fibers are greatly interconnected after the PEG dissolution (Figs. 2h and 2j). Such interconnected macro-pores promote the cell infiltration and orientation.
Hemocompatibility of Fabricated Grafts
A test of hemolysis was used to show the hemocompatibility of fabricated grafts.30 The hemocompatibility observed was approximately 0.65% (Table 1). The hemolysis values of grafts are much lower than 5%, which is the safe limit for blood-contacting materials according to ISO10993 part 4. The results demonstrate that our tubular grafts were safe when used in vascular tissue engineering.
Mechanical Properties
The tensile mechanical properties of varied vascular grafts in the radial direction were measured (Fig. 3), and abdominal aortas from SD rats were used as the control. There was significant difference in mechanical testing of the grafts and control, and the triple-layer graft exhibited added toughness. The E-jet 3D-printed interior layer composed of highly-aligned strong PCL fibers resulted in an improved mechanical performance. The tensile strength and burst pressure of the triple-layer grafts were both higher than that of rat abdominal aortas (Table 2). The results demonstrate that the triple-layer scaffold could provide adequate support for cells organization and vascular tissue formation, in addition to provide sufficient strength to counterbalance the hemodynamic stress in vivo.
Heparin Functionalization
The SEM images of heparinized vascular grafts showed that the number of adhered platelets on the heparin functionalized scaffolds decreased significantly, possibly due to the charge repulsion as platelets were also negatively charged (Fig. 4). Comparing the morphology, the platelets adhered to the non-heparinized scaffolds were dendritic with extruding pseudopodia but round with fewer pseudopodia on the heparinized scaffolds, which suggested a less activated state. The results demonstrate that the adhesion of platelet was hindered greatly on the heparin functionalized vascular grafts.
Cell Growth
To characterize cells adhered on varied vascular grafts, H&E, DAPI, vinculin and phalloidin staining, and SEM were used. The morphologies of HUVECs on the lumen of triple-layer and monolayer grafts, which consisted of aligned and nonaligned fibers, were observed. Cells on the aligned scaffolds tended to grow along the fibers, and were in a spindle-shaped morphology after 1 day of culture (Fig. 5d). Moreover, cells formed into spindle or triangular-shaped morphologies, indicating that the internal layer represented a suitable cell growing surface (Figs. 6C and 6c). The aligned scaffolds were also beneficial for the adhesion of cells (Fig. S2), with some HUVECs even showing star-like morphologies. Fewer cells were observed on nonaligned scaffolds (Figs. 5a and 6b) than that on the aligned scaffolds (Figs. 5d and 6c).
Cell proliferation, distribution and morphology of HUVECs cultured on the interior surface of monolayer and triple-layer vascular grafts up to 3 days. Blank tissue culture polystyrenes (TCPs) were used as controls. Light microscope images of HUVECs cultured on TCPs (A, D, G), interior surface of monolayer (B, E, H) and triple-layer (C, F, I) grafts, which were stained by H&E. Confocal microscope images of HUVECs cultured on TCP (a, d, g), internal surface of monolayer (b, e, h) and triple-layer (c, f, i) grafts, which were stained by DAPI and phalloidin. Scale bar = 100 μm.
At day 2, more cells bridged between the fibers which would form a cell layer on the two fibrous scaffolds (Figs. 5b and 5e). HUVECs adhered well and elongated along aligned fibers (Fig. 6f), and spread faster than that on the controls (Figs. 6d and 6e). After 3 days of culture, there was a significant increase of cells. The lumens of the triple-layer fibrous scaffolds were fully covered by HUVECs, and cells settled directionally from top to bottom, with the aligned fibers (Figs. 6I and 6i). The HUVECs grew well on the interior surface of the triple-layer grafts, showing great proliferation, spreading and interaction. The results demonstrated that the E-jet 3D printed aligned fibers of the grafts are likely to guide the growth of HUVECs in vivo, with a longitudinal alignment of the lumen, thus result in a rapid endothelialization.
Vascular Grafts Implantation
The implanted vascular grafts were explanted from rats after 4, 8 and 12 weeks. To evaluate the cell migration, specimens were embedded, sliced into 7 μm sections, then stained by H&E and DAPI (Fig. 7). The results show that after 4 weeks, cells appeared mainly on the interfaces of grafts (Figs. 7a–7d). However, cells spread deeper into the scaffolds after 8 weeks (Figs. 7e–7h). Due to the high porosity, the exterior layer of triple-layer grafts exhibited much greater cellular infiltration, with more cells penetrated into the scaffolds (Figs. 7g and 7h), which was significantly better than that of controls (Figs. 7e and 7f). After 12 weeks, cells were distributed throughout the entire structure of both graft types (Figs. 7i–7l), and the triple-layer grafts achieved a superior endothelialization (Figs. 7k and 7l), which resulted in a slightly improved mechanic performance (Fig. 3). The above results demonstrated that our triple-layer grafts promoted the migration of cells in vivo than the monolayer grafts due to the layered structure and greater sponginess following the dissolution of PEG. Furthermore, the 3D printed highly-aligned interior layer improved cell penetration, which was similar to the in vitro results.
Discussion
Ideal tissue engineering vessels have appropriate biomechanical performance and excellent biocompatibility, which can facilitate the vascular tissue formation. This study combined E-jet 3D printing and electrospinning to produce a novel triple-layer vascular graft, the interior layer composed of highly-aligned strong PCL fibers, the densely middle layer made by electrospun fibers, and the exterior layer comprised mixed PCL/PEG fibers. Furthermore, the grafts were heparinized to obtain anti-thrombogenic abilities. The exterior layer provided a passageway through which cells could migrate into the scaffolds in vivo, while the longitudinally-aligned lumen afforded the graft with the essential mechanical support and promoted the growing, spreading, and migration of endothelial cells. This structure endows the triple-layer grafts with suitable mechanical performance, better cell penetration and endothelialization in vivo, which are important for vascular tissue engineering.15,23
Porosity and pore interconnectivity of scaffolds affect the cell infiltration significantly, which can be controlled by alerting the size and density of fibers.6 Our triple-layer vascular grafts are much spongier compared to normal electrospun scaffolds, especially in the exterior layer as PEG would dissolved quickly in vivo. Larger pores were created between the fibers, which resulted in a loose structure suitable for cell penetration.1 The porosity of the triple-layer grafts was greater than that of the control by ~ 16%. The increase in porosity was mainly due to a density reduction of the external fibers, while the average size of fibers was similar to that of the monolayer grafts. The triple-layer scaffolds described here also provided an additional critical feature: pores of implanted grafts formed an interconnected three-dimensional structure, which is vital for cell ingrowth and vascularization in vivo.
To suit the in vivo stressful environment, the mechanical properties of tubular grafts are important. High porosity usually results in a deterioration of the scaffold strength. Nevertheless, due to the 3D-printed aligned internal layer, our extremely porous triple-layer grafts possessed superior yield strength, elasticity, and toughness compared to the monolayer grafts comprising random fibers. Furthermore, the grafts did not undergo distortion or breakage, following 12 weeks in vivo implantation. The mechanical performance of our grafts can also be altered by controlling the intimal-to-external-layer-thickness ratio or related parameters. The above characteristics make our triple-layer grafts valuable for tissue engineering applications when mechanical strength is an essential bio-functionality. The grafts could endure kink and compression in vivo, hold adequate tensile and shear strength to resist fraying and tearing, and preserve sufficient circumferential strength to withstand arterial pressures.
Poor endothelialization of the vascular grafts is a critical issue that would result in restenosis after implantation. Researchers demonstrated that scaffold structure had significant effect on cell proliferation and spread in addition to extracellular matrix regeneration.16 For the triple-layer grafts, the longitudinally-aligned intimal and middle layers could promote cellular proliferation. Furthermore, the 3D printed PCL filaments are able to enhance the adhesion of cells to the lumen. To evaluate the benefits of the longitudinally-aligned structure and high porosity of scaffolds, the in vivo cell penetration and tissue formation of the triple-layer grafts was compared with that of monolayer grafts. Experimental results demonstrate that cell infiltration was significantly enhanced in the triple-layer vascular grafts. The extremely porous external-layer of the grafts served as an efficient path allowing migration of cells inside the scaffolds. Greater cell infiltration into the triple-layer grafts was observed than that in the controls. Cells distributed homogeneously in the grafts after in vivo implantation of 12 weeks, which could enhance the maturation and formation of vascular tissues.11 The results elucidate that the lumen of the grafts modulated cell growth and tissue reform in vivo, finally generated an equivalent of new endothelium. Our triple-layer vascular grafts are advantageous for vascular tissue reconstructions.
In conclusion, this study fabricated a novel triple-layer vascular graft by combining E-jet 3D printing and electrospinning techniques. The graft consists of an interior layer comprising 3D printed highly aligned strong fibers, a densely middle layer made by electrospinning, and an exterior layer composed of hybrid fibers by co-electrospinning. This graft was assessed by in vivo and histological tests as a potential vascular tissue replacement. Our triple-layer vascular grafts are mechanically stronger and possess superior porosity compared to usual electrospun monolayer grafts, due to its unique structure. The graft lumen constructed of longitudinally-aligned fibers enhanced the cell growth and spread, while worked as an ideal mechanical support. The highly porous exterior layer improved the cell penetration and tissue ingrowth. Our triple-layer vascular grafts have a potential to overcome the limits of normal electrospun monolayer grafts, thus provide an encouraging substitute for in situ tissue engineering.
References
Baker, B. M., A. O. Gee, R. B. Metter, A. S. Nathan, R. L. Marklein, J. A. Burdick, and R. L. Mauck. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 29:2348–2358, 2008.
Campbell, J. J., A. Husmann, R. D. Hume, C. J. Watson, and R. E. Cameron. Development of three-dimensional collagen scaffolds with controlled architecture for cell migration studies using breast cancer cell lines. Biomaterials 114:34–43, 2017.
Caracciolo, P. C., M. I. Rial-Hermida, F. Montini-Ballarin, G. A. Abraham, A. Concheiro, and C. Alvarez-Lorenzo. Surface-modified bioresorbable electrospun scaffolds for improving hemocompatibility of vascular grafts. Mater Sci Eng C 75:1115–1127, 2017.
Chan-Park, M. B., J. Y. Shen, Y. Cao, Y. Xiong, Y. Liu, S. Rayatpisheh, G. C.-W. Kang, and H. P. Greisler. Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels. J Biomed Mater Res A 88:1104–1121, 2009.
Dwivedi, N., V. Kumar, A. Shrivastava, and R. Nareliya. Burst pressure assessment of pressure vessel using finite element analysis: a review. J Press Vessel Technol ASME 135:044502–044505, 2013.
Eichhorn, S. J., and W. W. Sampson. Statistical geometry of pores and statistics of porous nanofibrous assemblies. J R Soc Interface 2:309–318, 2005.
Ekaputra, A. K., G. D. Prestwich, S. M. Cool, and D. W. Hutmacher. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules 9:2097–2103, 2008.
Garg, K., N. A. Pullen, C. A. Oskeritzian, J. J. Ryan, and G. L. Bowlin. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 34:4439–4451, 2013.
Ghanian, M. H., Z. Farzaneh, J. Barzin, M. Zandi, M. Kazemi-Ashtiani, M. Alikhani, M. Ehsani, and H. Baharvand. Nanotopographical control of human embryonic stem cell differentiation into definitive endoderm. J Biomed Mater Res A 103:3539–3553, 2015.
Han, F., X. Jia, D. Dai, X. Yang, J. Zhao, Y. Zhao, Y. Fan, and X. Yuan. Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 34:7302–7313, 2013.
Horst, M., V. Milleret, S. Nötzli, S. Madduri, T. Sulser, R. Gobet, and D. Eberli. Increased porosity of electrospun hybrid scaffolds improved bladder tissue regeneration. J Biomed Mater Res A 102:2116–2124, 2014.
Hu, J., D. Kai, H. Ye, L. Tian, X. Ding, S. Ramakrishna, and X. J. Loh. Electrospinning of poly(glycerol sebacate)-based nanofibers for nerve tissue engineering. Mater Sci Eng C 70:1089–1094, 2017.
Hu, C., C. Tercero, S. Ikeda, M. Nakajima, H. Tajima, Y. Shen, T. Fukuda, and F. Arai. Biodegradable porous sheet-like scaffolds for soft-tissue engineering using a combined particulate leaching of salt particles and magnetic sugar particles. J Biosci Bioeng 116:126–131, 2013.
Kim, H. N., A. Jiao, N. S. Hwang, M. S. Kim, D. H. Kang, D.-H. Kim, and K.-Y. Suh. Nanotopography-guided tissue engineering and regenerative medicine. Adv Drug Deliv Rev 65:536–558, 2013.
Koobatian, M. T., S. Row, R. J. Smith, Jr, C. Koenigsknecht, S. T. Andreadis, and D. D. Swartz. Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials 76:344–358, 2016.
Lee, J., A. A. Abdeen, K. L. Wycislo, T. M. Fan, and K. A. Kilian. Interfacial geometry dictates cancer cell tumorigenicity. Nat Mater 15:856–862, 2016.
Li, Y., K. Jiang, J. Feng, J. Liu, R. Huang, Z. Chen, J. Yang, Z. Dai, Y. Chen, N. Wang, W. Zhang, W. Zheng, G. Yang, and X. Jiang. Construction of small-diameter vascular graft by shape-memory and self-rolling bacterial cellulose membrane. Adv Healthc Mater 6:1601343–1601352, 2017.
Li, D., T. Wu, N. He, J. Wang, W. Chen, L. He, C. Huang, H. A. Ei-Hamshary, S. S. Al-Deyab, and Q. Ke. Three-dimensional polycaprolactone scaffold via needleless electrospinning promotes cell proliferation and infiltration. Colloids Surf B 121:432–443, 2014.
Liu, T., R. Huang, J. Zhong, Y. Yang, Z. Tan, and W. Tan. Control of cell proliferation in E-jet 3D-printed scaffolds for tissue engineering applications: the influence of the cell alignment angle. J Mater Chem B 5:3728–3738, 2017.
Loh, X. J., P. Peh, S. Liao, C. Sng, and J. Li. Controlled drug release from biodegradable thermoresponsive physical hydrogel nanofibers. J Control Release 143:175–182, 2010.
Mozaffarian, D., E. J. Benjamin, A. S. Go, D. K. Arnett, M. J. Blaha, M. Cushman, F. S. De, J. P. Després, H. J. Fullerton, and V. J. Howard. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 131:e29–322, 2015.
Peng, X. F., H. Y. Mi, X. Jing, P. Yu, J. P. Qu, and B. Y. Chen. Preparation of highly porous interconnected poly(lactic acid) scaffolds based on a novel dynamic elongational flow procedure. Mater Des 101:285–293, 2016.
Pu, J., F. Yuan, S. Li, and K. Komvopoulos. Electrospun bilayer fibrous scaffolds for enhanced cell infiltration and vascularization in vivo. Acta Biomater 13:131–141, 2015.
Seifu, D. G., A. Purnama, K. Mequanint, and D. Mantovani. Small-diameter vascular tissue engineering. Nat Rev Cardiol 10:410–421, 2013.
Tan, Z., H. Wang, X. Gao, T. Liu, and Y. Tan. Composite vascular grafts with high cell infiltration by co-electrospinning. Mater Sci Eng C 67:369–377, 2016.
Visser, J., F. P. W. Melchels, J. E. Jeon, E. M. van Bussel, L. S. Kimpton, H. M. Byrne, W. J. A. Dhert, P. D. Dalton, D. W. Hutmacher, and J. Malda. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun 6:6933, 2015.
Wei, C., and J. Dong. Direct fabrication of high-resolution three-dimensional polymeric scaffolds using electrohydrodynamic hot jet plotting. J Micromech Microeng 23:025017, 2013.
Wei, C., and J. Dong. Hybrid hierarchical fabrication of three-dimensional scaffolds. J Manuf Process 16:257–263, 2014.
World Health Organization. World health statistics 2017: monitoring health for the SDGs, sustainable development goals. Geneva. Licence: CC BY-NC-SA 3.0 IGO, 2017.
Zhu, T., K. Yu, M. A. Bhutto, X. Guo, W. Shen, J. Wang, W. Chen, H. El-Hamshary, S. S. Al-Deyab, and X. Mo. Synthesis of RGD-peptide modified poly(ester-urethane) urea electrospun nanofibers as a potential application for vascular tissue engineering. Chem Eng J 351:177–190, 2017.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 31600782), Shenzhen Science and Technology Innovation Committee (No. JCYJ20170818112151323), and Scientific Research Foundation of Hunan University (No. 53112102).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kent Leach oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, R., Gao, X., Wang, J. et al. Triple-Layer Vascular Grafts Fabricated by Combined E-Jet 3D Printing and Electrospinning. Ann Biomed Eng 46, 1254–1266 (2018). https://doi.org/10.1007/s10439-018-2065-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2065-z