Abstract
In vitro mechanical testing of orthopedic implants provides information regarding their mechanical performance under simulated biomechanical conditions. Current in vitro component stability testing methods for reverse shoulder implants are based on anatomical shoulder designs, which do not capture the dynamic nature of these loads. With glenoid component loosening as one of the most prevalent modes of failure in reverse shoulder replacements, it is important to establish a testing protocol with a more realistic loading regime. This paper introduces a novel method of mechanically testing reverse shoulder implants, using more realistic load magnitudes and vectors, than is currently practiced. Using a custom made jig setup within an Instron mechanical testing system, it is possible to simulate the change in magnitude and direction of the joint load during arm abduction. This method is a step towards a more realistic testing protocol for measuring reverse shoulder implant stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
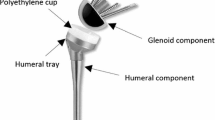
Reverse shoulder arthroplasty (RSA) is commonly used to treat glenohumeral arthritis associated with severe rotator cuff deficiency, otherwise known as rotator cuff tear arthropathy. In contrast to anatomical shoulder reconstructions, RSA reverses the natural anatomy of the “ball and socket” glenohumeral joint. The articulation consists of a metal hemisphere attached to the glenoid and a polyethylene cup attached to a stem that is implanted into the humerus. This reversed articulation design produces two desired effects. Firstly, it provides some constraint and stability, preventing the shoulder from proximal subluxation when the deltoid muscle contracts; and secondly medialises the center of rotation of the joint which increases the moment arm of the deltoid, hence improving the function of this muscle as well as minimising the excessive torques which could otherwise lead to early loosening.3
Whilst RSA has been reported to produce pain relief, greater mobility and generally good patient outcomes,5,6,14,19 loosening of the glenoid component is one of the most prevalent modes of failure.7,12,17,18 This may be due to difficulties in achieving sufficient stability of the uncemented glenoid component.9 It has been shown that design variables such as screw length, screw diameter, insertion angle of the screws as well as offset can affect the relative motion between the implant (baseplate of the glenoid component) and underlying bone.8,9 The aim of stable fixed implants is to create a mechano-biologically desirable environment to promote osseo-integration between the implant and bone.
Stability of different RSA glenoid baseplates has been assessed using in vitro testing methods.4,8 These studies have employed testing standards10 which are originally designed for anatomical shoulder implants. The loading recommended in these standards is divided into shear and compressive force components and based on a proportion of body weight. However, they do not consider the dynamics of the joint, nor do they necessarily reflect the magnitude of the load experienced within a reverse shoulder.
More recently, detailed analysis of the effect and contribution of different muscle groups around the shoulder in both intact and post-operative conditions has demonstrated and quantified the changes in shear and compressive forces that occur during the arc of motion of the reverse shoulder replacement.1 The cadaveric study used a sophisticated anatomical setup to obtain this information, which would be a difficult task to reproduce for RSA testing.
Mathematical models, based on accurate anatomical geometries and kinematic muscle input data from the literature (and electromyographic activity), have also been developed to calculate joint reaction forces in reverse shoulders.11,16 These studies indicate that the forces across the joint are complex and that as the arm goes through a full range of abduction there is a change in shear and compression force components.
The purpose of this paper is to introduce a simplified mechanical testing protocol to reproduce the pattern of compressive and shear forces occurring in the RSA as previously reported, which can be used to test RSA glenoid component loosening. We hypothesize that using such a setup will provide more realistic testing regime. This technique will allow analysis of the RSA while mimicking the movement of the shoulder through an arc of motion and applying a varying balance of shear and compressive forces similar to those thought to occur in vivo.
Materials and Methods
Specimen Preparation
Solid foam scapula specimens (www.sawbones.com) were prepared prior to testing. Bone prominences such as the acromion and the scapular spines were trimmed and scapulae potted upright into polyurethane containers with polymethyl-methacrylate (PMMA). The glenoid baseplate (Aequalis Reversed Shoulder System, Tornier Inc.) of the reverse shoulder arthroplasty (RSA) was implanted on the reamed glenoid surface and the glenosphere (36 mm) secured to the baseplate as specified in the surgical technique.13
Experimental Setup
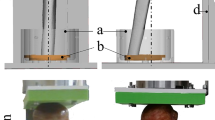
The prepared specimen block was fixed to the base of an electro-mechanical tension–torsion Instron testing system (E10000, Instron Corporation, Norwood, MA, USA), with the acromion process facing downwards (Fig. 1).
A torsion arm was connected to the Instron biaxial load cell (10 kN/100 Nm) which in turn was connected to the Instron actuator. This arm had a linear bearing block sitting on it which was free to slide and positioned by the user. The distance from the center of the load cell to the center of this bearing block (i.e., the moment arm) was set to 195 mm on the torsion arm. This ensured that the required compressive force across the implant was achievable based on the Instron torque rating. Figure 2 is a side view profile of the experimental setup. The humeral component was eccentrically attached to the linear bearing block and this was positioned such that it articulated with the glenosphere Fig. 3. The eccentric offset of the humeral component on the bearing block was set to 40 mm Fig. 4. This offset was tested iteratively until the required ratio of shear to compression loading across the implant was obtained for an abduction–adduction motion.
Close-up schematic of the glenosphere-humeral component articulation. The specimen block (consisting of the scapula, baseplate and glenosphere) is fixed onto the base of the Instron system; the linear bearing block slides freely on the torsion arm; and offsetting the humeral component on the linear bearing block introduces the eccentric loading
By altering the actuator displacement (vertically) the humeral component could be moved to different positions on the glenosphere; whilst rotating the actuator (or varying the torque) increased or decreased the shear and compressive loading at the implant. The actuator was cycled up and down by ±15 mm at a constant displacement rate of 10 mm/s which caused the humeral component to move to the anterior and superior limits of the glenosphere. At the same time torque was cycled up and down from 29 to 47 Nm (Fig. 5). This torque generated a compressive force at the implant of between 150 and 240 N which was equivalent to Ackland et al.1 An offset of 40 mm produced a superior shear load of 130–190 N which closely matched the loads reported in the literature.1,16
Abduction Angles
The Metaphyseal neck angle is dependent on the stem of choice. In this study, the Aequalis RSA humeral stem (Tornier SA, Montbonnot, France) was used which has a metaphyseal neck angle of 155°. This angle affects the defined angles between different articulating implant components. The Glenohumeral angle is defined as the angle between the humeral shaft and the RSA glenosphere (Fig. 6); and the arm abduction angle (or humerothoracic angle) is the angle between the humeral shaft and the longitudinal axis of the thorax.
Schematic of the experimental setup (left) showing the fixed specimen (orange) with the glenosphere in articulation with the polyethylene liner (cup) of the humeral component, with the linear bearing block overlayed (blue); and active forces on the reconstructed joint (right). The humeral stem is not used in the experimental setup. This is purely for schematic/explanatory purposes
While both the glenohumeral angle and the arm abduction angle increase as the arm is abducted, they do so at two different rates. This is because the scapula also rotates with abduction. Poppen and Walker15 found that from 0° to 30° abduction, there exists only humeral motion, but beyond 30° abduction, a 2:1 ratio of scapula to humeral motion exists. This relationship is shown in Table 1. While this ratio was determined in normal shoulders, the true ratio may differ in patients following RSA.
The experimental setup was such that the loading cycle started at the lowest and moved to the highest possible arm abduction angle without impingement or abutment of the polyethylene component against the glenoid bone. This was approximately 45° of arm abduction (30° glenohumeral angle) and 100° of arm abduction (65° glenohumeral angle), respectively. Table 1 provides a general outline of the relationship between abduction and glenohumeral angles.
Data and Image Acquisition
Displacement and torque data was acquired from the Instron machine at an acquisition rate of 10 Hz. Images of the implant articulation were collected using a synchronized digital camera (JAI CB-200-GE), and were used to monitor, record and measure the joint angle. Images were opened in Irfanview 4.32 and angle measurements made between the glenosphere and the polyethylene liner. This angle was then converted to the glenohumeral angle based on the metaphyseal neck angle of the Tornier Aequalis implant, which is 155°.
Results
Figure 7 shows the implant compressive and shear forces generated by our testing system compared to those published by Ackland et al.1 As outlined by Ackland, this reflects the forces experienced by the joint throughout abduction when all the cuff muscles (subscapularis, supraspinatus, infraspinatus and teres minor) are absent, as is the case for a reverse shoulder.
Compressive and Shear forces expressed by Ackland et al.,1 forces set out by the ASTM standard and forces obtained from the new experimental setup (black)
The pattern of increasing compressive force and decreasing superior shear force with increasing abduction angle, and the point of cross-over of the curves at approximately 65°, correlates well. Also, Fig. 7 shows the significant difference between forces determined by Ackland for a reverse shoulder and that recommended by ASTM standards.10
Figure 8 shows the resultant glenohumeral joint force, defined as the vector summation of compressive and shear forces, from Ackland et al. 1 and Terrier et al.,16 compared to our in vitro testing data. Again the magnitude between these two previous publications and our data correlates. Figure 8 also shows the resultant force based on the compressive and shear forces as outlined in the ASTM standard for shoulder testing.
Discussion
The main objective of this study was to develop an in vitro dynamic testing method which recreated the loads in a RSA. By using an Instron tension/torsion material testing system we were able to recreate the compression and shear loading for a RSA component through a range of abduction, as predicted by Ackland1 and Terrier.16 Although Ackland and Terrier used two different methods of estimating the joint forces (experimental and computational, respectively), both the trends and magnitudes were nearly identical to our resultant force vectors. To our knowledge this is the first study to recreate the compression and shear loading over a range of abduction angles for a RSA.
Previous ASTM Standard testing protocols10 are based on forces determined for anatomical shoulder arthroplasties and these forces are clearly different to those of a RSA with the ASTM Standard force approximately 250% higher than that calculated for a RSA according to Ackland and Terrier. Also, ASTM standard testing protocols apply a fixed abduction angle and hence do not reproduce in any way the dynamic physiological loading experienced by an implant in vivo.16
Whilst this testing methodology is a major advancement in terms of recreating the realistic loading conditions at the joint, there were some limitations. Firstly, this testing regime recreates the loading conditions of a RSA between 45° and 100° of abduction only. Although the lower end of the abduction arc of motion is not simulated, these magnitudes are much lower than those experienced in the 45°–100° range where the values are higher and more likely to result in implant failure. Also, this testing protocol was based on manufactured sawbones with minimal anatomic variance. It is possible that in cadaveric specimens, with greater geometric variation, the implants may be positioned differently and therefore may affect the loads. It is the intention of the authors to investigate this further. Another limitation of this study was the loads used for this setup. Since it is very difficult to make in vivo measurements of a reverse shoulder joint, we have reproduced loads which were predicted by experimental and computational means. It’s possible that there may be a discrepancy between these values and those occurring in vivo. However, at this stage, these values are the most realistic loading patterns available in the literature.
The current testing regime simulates RSA with a complete deficiency of the rotator cuff muscles. Our future aim is to further develop the testing protocol to simulate RSA with a deficiency of the supraspinatus only, as discussed in Terrier et al.16 This will provide the two different clinical scenarios.
This new testing regime has been developed to measure glenoid stability in RSA. By using these more realistic forces, we will be better able to assess the biomechanics of these implants. Stability can be been measured by displacement transducers,8 dial gauges2 and optical means. The authors intend to further this work by using this experimental setup and optical measurement techniques to test reverse shoulder implants in cadaveric specimens.
References
Ackland, D. C., S. Roshan-Zamir, M. Richardson, and M. G. Pandy. Muscle and joint-contact loading at the glenohumeral joint after reverse total shoulder arthroplasty. J. Orthop. Res. 29:1850–1858, 2011. doi:10.002/jor.21437 (Epub 2011 May 12).
Anglin, C., U. P. Wyss, R. W. Nyffeler, and C. Gerber. Loosening performance of cemented glenoid prosthesis design pairs. Clin. Biomech. (Bristol, Avon) 16:144–150, 2001.
Boileau, P., D. J. Watkinson, A. M. Hatzidakis, and F. Balg. Grammont reverse prosthesis: design, rationale, and biomechanics. J. Shoulder Elbow Surg. 14:147S–161S, 2005.
Codsi, M. J., and J. P. Iannotti. The effect of screw position on the initial fixation of a reverse total shoulder prosthesis in a glenoid with a cavitary bone defect. J. Shoulder Elbow Surg. 17:479–486, 2008; (Epub 2008 Feb 20).
Cuff, D., D. Pupello, N. Virani, J. Levy, and M. Frankle. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J. Bone Joint Surg. Am. 90:1244–1251, 2008. doi:10.2106/JBJS.G.00775.
Frankle, M., J. C. Levy, D. Pupello, S. Siegal, A. Saleem, M. Mighell, and M. Vasey. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J. Bone Jt. Surg Am. 88:178–190, 2006.
Gallo, R. A., S. C. Gamradt, C. J. Mattern, F. A. Cordasco, E. V. Craig, D. M. Dines, and R. F. Warren. Instability after reverse total shoulder replacement. J. Shoulder Elbow Surg. 20:584–590, 2011. doi:10.1016/j.jse.2010.08.028; (Epub 10 Dec 16).
Harman, M., M. Frankle, M. Vasey, and S. Banks. Initial glenoid component fixation in “reverse” total shoulder arthroplasty: a biomechanical evaluation. J. Shoulder Elbow Surg. 14:162S–167S, 2005.
Hopkins, A. R., U. N. Hansen, A. M. Bull, R. Emery, and A. A. Amis. Fixation of the reversed shoulder prosthesis. J. Shoulder Elbow Surg. 17:974–980, 2008. doi:10.1016/j.jse.2008.04.012; (Epub 08 Aug 28).
International ASTM. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. In: F2028-08. West Conshohocken: ASTM International, 2008.
Kontaxis, A., and G. R. Johnson. The biomechanics of reverse anatomy shoulder replacement—a modelling study. Clin. Biomech. (Bristol, Avon) 24:254–260, 2009.
Lam, F., D. N. Bhatia, S. B. Mostofi, K. van Rooyen, and J. F. de Beer. Biomechanical considerations of the normal and rotator cuff deficient shoulders and the reverse shoulder prosthesis. Current Orthopaedics 21:40–46, 2007.
Tornier Surgical Implants. Surgical Technique: Shoulder Prosthesis—Aequalis-Reversed. http://depts.washington.edu/shoulder/Surgery/TornierAequalisReversedTechnique.pdf.
Mulieri, P., P. Dunning, S. Klein, D. Pupello, and M. Frankle. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J. Bone Joint Surg. Am. 92:2544–2556, 2010. doi:10.106/JBJS.I.00912.
Poppen, N. K., and P. S. Walker. Normal and abnormal motion of the shoulder. J. Bone Joint Surg. Am. 58:195–201, 1976.
Terrier, A., A. Reist, F. Merlini, and A. Farron. Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. J. Bone Joint Surg. Br. 90:751–756, 2008.
Walch, G., A. A. Young, P. Boileau, M. Loew, D. Gazielly, and D. Mole. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: results of a multicenter study with more than five years of follow-up. J. Bone Joint Surg. Am. 94:145–150, 2012. doi:10.2106/JBJS.J.00699.
Werner, C. M., P. A. Steinmann, M. Gilbart, and C. Gerber. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the delta iii reverse-ball-and-socket total shoulder prosthesis. J. Bone Joint Surg. Am. 87:1476–1486, 2005.
Young, A. A., M. M. Smith, G. Bacle, C. Moraga, and G. Walch. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J. Bone Joint Surg. Am. 93:1915–1923, 2011. doi:10.2106/JBJS.J.00300.
Acknowledgments
We would like to acknowledge Tornier Australia for providing implants and funding for this research.
Conflict of interest
Implants and funds for this study were supported by research funds donated by Tornier Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. Torry oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Dabirrahmani, D., Bokor, D. & Appleyard, R. A Novel Dynamic Mechanical Testing Technique for Reverse Shoulder Replacements. Ann Biomed Eng 42, 727–732 (2014). https://doi.org/10.1007/s10439-013-0942-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0942-z