Abstract
Fibrin gel has proven a valuable scaffold for tissue engineering. Complex geometries can be produced by injection molding; it offers effective cell seeding and can be produced autologous. In order to evaluate its suitability for respiratory tissue engineering, we examined proliferation, functionality, and differentiation of respiratory epithelial cells on fibrin gel in comparison to culture on collagen-coated, microporous membranes. Respiratory epithelial cells formed a confluent layer by day 4, and proliferation showed no significant difference with respect to surface. Measurement of the transepithelial electrical resistance reflected the development of a confluent epithelial cell layer and the subsequent initiation of adequate ion-transfer processes. Appearance of ciliae could be detected at similar time points, and ciliary beating could be observed for cells on both surfaces. Histology and immunohistochemistry of cells grown on fibrin gel revealed the onset of adequate differentiation. As no significant differences in respiratory epithelial cells’ proliferation, function, and differentiation could be observed between cells grown on fibrin gel compared to cells on a collagen-coated, microporous surface, we concluded that fibrin gel might prove a suitable scaffold for respiratory tissue engineering and merits further investigation to overcome the limitations associated with scaffolds currently in use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A partial resection of the trachea or bronchi might be required for the treatment of bronchotracheal lesions and injuries including post-intubation stenosis, tumor, and iatrogenic trauma. Following bronchial resection and tracheal resection under 50% of the tracheal length, direct anastomosis is preferred for reconstruction.4 Where primary reconstruction is impossible, the defect must be covered with autologous, allogenic, xenogenic, or artificial materials. Autologous material is preferred, as less inflammatory reaction can be expected. Reconstruction of the trachea and main bronchi has been tried using different tissues,10 e.g., small intestine, skin, or aorta. Recently, Zhao et al. as well as Matloub and Yu were successful in creating autologous tracheal and bronchial replacement. Matloub and Yu12 stabilized a skin flap by means of a metal braiding. Zhao et al.19 produced a multilayered prosthesis from a skin flap, platysma, and a muscle flap, which also included a metal braiding as support. Results were promising, but the process puts a high burden on patients requiring multiple surgical interventions and the harvest of donor tissue from one or multiple locations.
Tissue engineering might offer an alternative to overcome these limitations and might improve the situation of donor organ shortage in allogenic transplantation. Tracheal tissue engineering has already become successful with the first human implantation of a tissue-engineered bronchial replacement by Macchiarini et al.11 A decellularized tracheal homograft was used as scaffold in this experiment which was harvested from a regular organ donor. Consequently, using decellularized matrices is an approach that might compete with organs needed for transplantation. In addition, the graft’s geometry and shape cannot be tailored to the patient, neither in allogenic, nor in xenogenic material.
Fibrin gel is a biomaterial that can be produced as autologous scaffold material from as few as 50 mL of patient’s own blood. Completely autologous production lowers the possibilities of an inflammatory reaction due to a foreign body reaction or foreign antigenetic epitopes as well as the possibility of disease transmission. Injection molding can be used to create complex geometries and cell-seeding can be performed very effectively8 using this technique, while these processes represent significant obstacles for most scaffold materials.
Fibrin gel’s use is limited by its mechanical weakness that requires in vitro culture of tissue-engineered constructs to provide a mechanically stable engineered tissue backbone. It might also be reinforced by textile mesh structures. We demonstrated successfully the fabrication of tubular structures like vascular grafts for implantation in the arterial circulation.9 The translation of such techniques might make fibrin-based scaffolds an ideal candidate in respiratory tissue engineering.
Within this pilot study, the suitability of fibrin gels as scaffold material for respiratory tissue engineering has been evaluated with regard to the proliferation, function, and differentiation of respiratory epithelium in comparison to the standard culture conditions. Respiratory epithelium is generally cultured on collagen-coated surfaces. Collagen-coated standard tissue culture surfaces can be used for cell expansion, while adequate physiologic functions such as mucus secretion and formation of beating ciliae can be observed in air-lift culture conditions on collagen-coated, microporous meshes.17 The use of collagen in the engineering of three-dimensional (3D) tissues is more restricted, as it cannot be autologously produced in significant amounts and the production of 3D structures is difficult. Fibrin gel might thus represent an alternative that can be produced autologously and processed easily.
Materials and Methods
Isolation and Expansion of Respiratory Epithelial Cells
Three to four segments of ovine trachea were harvested from a sheep euthanized for other purposes at the animal facilities in the University Hospital Aachen. Cell harvesting was approved by the local ethical committee. Cells were isolated according to a protocol published by Widdicombe et al.17 In brief, the trachea was opened along its dorsal surface, and the mucosal layer incised longitudinally. Strips of mucosa could be readily removed and placed into a solution of protease XIV (Sigma, Germany) at 0.4 mg/mL The strips were left for enzymatic digestion at 4 °C overnight. After removal of the strips, cells were centrifuged, dispersed in a 1:1 mixture of DMEM (Gibco, USA) and Ham’s F12 medium (Gibco, USA) with 5% fetal bovine serum (PAA, Austria) and plated at 2 × 104 cells/cm2. After 24 h, culture media was changed to Gray’s medium (a 1:1 mixture of DMEM and LHC-9 (Invitrogen, USA) supplemented with 1.5 μg/mL of bovine serum albumin (Sigma, Germany)). Subsequently, media were exchanged every 48 h. Cells were passaged upon 80% confluency using 0.5 mg/mL Trypsin/0.22 mg/mL EDTA-solution (PAA, Austria) to detach the cells. Experiments were conducted using cells from the second passage.
Fabrication of Fibrin Gel
Commercial fibrinogen was prepared as previously described.5 No autologous fibrinogen was used to avoid potential confusion of study results with slight differences in gel composition due to differences in blood constituents. In brief, lyophilized human fibrinogen (plasminogen free; Sigma, Germany) was dissolved in purified water at a concentration of 25 mg/mL and dialyzed using a dialysis membrane (Novodirect, Germany) with a cut-off of 6000–8000 MW overnight against Trizma buffered saline (TBS) prepared from 4.91 g/L Trizma HCl, 0.72 g/L Trizma Base, 9.00 g/L NaCl, and 0.23 g/L KCl in double-distilled water at a pH of 7.4. The fibrinogen concentration after sterile filtration was estimated by measuring absorbance at 280 nm using a spectrophotometer (Tecan infinity reader, Tecan, Switzerland).
Fibrin gels with a concentration of 5 mg fibrinogen/mL of gel were then fabricated from the solution by adding TBS, Calcium chloride (50 mmol/L), and Thrombin (40 U/mL). Gels were left at room temperature for 45 min to allow complete polymerization. Subsequently, a solution of TBS with 1.6 μL/mL of tranexamic acid as protease inhibitor was added, and gels were left at 37 °C overnight. For assessment of proliferation and differentiation, gels with a volume of 500 μL were cast into 24-well plates. For assessment of physiologic function, 100 μL of gel were polymerized on top of Transwell™ permeable supports (pore size 0.2 μm, polyethylene; Corning, USA). The TBS/tranexamic acid solution was removed directly before cell seeding.
Collagen Coating
A collagen solution was prepared dissolving 40 μg/mL of collagen from human placenta (Sigma, Germany) in double-distilled water. Following sterile filtration, 24-well plates were incubated overnight with this solution at a concentration of 20 μg of collagen/cm2 at 37 °C. Transwell™ supports were incubated with this solution using the same conditions. One hour before cell seeding, the collagen solution was removed from either the well plate or the Transwell™ supports and the surfaces were allowed to dry at room temperature.
Assessment of Proliferation
Cells from the second passage were harvested and dispersed in Gray’s medium at 7.5 × 104 cells/mL. Cells were seeded at 2 × 104 cells/cm2 in well plates (NUNC, Denmark) coated with either collagen or fibrin gel as described above. Cell culture medium was changed every 24 h. Every 24 h, samples (n = 3) from each group were washed with 1 mL of PBS and subsequently detached using 1 mL of 0.5 mg/mL Trypsin/0.22 mg/mL EDTA-solution (PAA, Austria). Detachment of all the cells was confirmed microscopically and cells counted using a Casy Cell Counter (Roche, Switzerland).
Assessment of Physiologic Function
Cells from the second passage were harvested and dispersed in Gray’s medium at 7.5 × 104 cells/mL. Cells were seeded at 2 × 104 cells/cm2 onto Transwell™ permeable supports (pore size 0.2 μm, polyethylene; Corning, USA) coated with either collagen or fibrin gel as described above. Cell culture medium was changed every 24 h. Every 24 h, transepithelial electrical resistance (TEER) was measured using a proprietary electrode setup (see Fig. 1) connected to a multimeter (Voltcraft, Germany) and recorded as percentage of the baseline level. Fresh samples were analyzed using routine bright field and oil immersion microscopy (AxioImager D1; Carl Zeiss GmbH, Germany) to display ciliary motion every 48 h. Images and movies were acquired using a digital color camera (AxioCam MRc; Carl Zeiss GmbH, Germany). Cells were kept on Transwell inserts for up to 30 days.
Assessment of Differentiation
Cells from the second passage were harvested and dispersed in Gray’s medium at 7.5 × 104 cells/mL. Cells were seeded at 2 × 104 cells/cm2 in well plates (NUNC, Denmark) coated with fibrin gel as described above. Cell culture medium was changed every 24 h. Cells were kept in culture for up to 21 days, and the samples (n = 3) were taken every 7 days for histologic and immunohistochemical analyses. Carnoy’s-fixed, paraffin-embedded native ovine trachea, and cultured constructs were sectioned at 3-μm thickness. Sections were subsequently stained by standard periodic acid Shiff reaction (PAS) protocol, for analysis of general epithelial morphology and development as well as selective staining of goblet cells. Sections were analyzed by routine bright field light microscopy (AxioImager; Carl Zeiss GmbH, Germany).
For immunohistochemical analysis, non-specific sites on Carnoy’s-fixed, paraffin-embedded construct sections were blocked, and the cells permeabilized with 5% normal goat serum (NGS; Sigma) in 0.1% Triton-PBS. Sections were incubated for 1 h at 37 °C with 1:100 rabbit polyclonal anti-pan-cytokeratin antibody (Acris, Germany). The sections were then incubated for 1 h at room temperature with fluorescein-conjugated goat–anti-rabbit secondary antibodies (1:400; Molecular Probes, The Netherlands). Cells were counterstained with DAPI nucleic acid stain (Molecular Probes). Native ovine aortic valve tissue samples served as positive controls. As negative controls, the samples were incubated in diluent and the secondary antibody only. A solution of 1 g bovine serum albumin (Sigma, Germany) and 0.1 g sodium azide (Sigma, Germany) in 100 mL of Phosphate buffered Saline (PAA, Germany) was used as primary antibody diluent, while 2% of normal goat serum (Millipore, Germany) was added to this solution for the secondary antibody diluent. The samples were viewed using a microscope equipped for epi-illumination (AxioImager; Carl Zeiss GmbH, Germany).
Statistics
Continuous variables are expressed as mean ± SD or median and interquartile range in parenthesis unless stated otherwise. Frequency comparison was done using the χ2-test. Statistical tests were two-tailed, and a p value < 0.05 was considered statistically significant. Data analysis was performed using commercially available software (SAS enterprise guide version 4, SAS Institute Inc., NC, USA & Microsoft Excel 2007, The Microsoft Corporation, USA).
Results
Assessment of Proliferation
Initial seeding of the cells to the different surfaces could be achieved as confirmed by light microscopy after 1 day. For each time point, detachment of cells could be performed with Trypsin-solution. The t-test revealed no significant differences in cell numbers between the two coatings at days 1, 2, and 4 (see Fig. 2). At day 3, significantly more cells were counted in the collagen group, but this was observed for this measurement only and is due to a very low deviation of the measured values.
In the collagen group, a significant (p < 0.05 for a two-tailed t-test) increase in counted cells could be observed comparing any of the different measurements, as reflected in Fig. 3. No significant changes in cell number could be observed during the first 3 days for the fibrin gel group. The highest proliferation could be observed between the third and the fourth day for each of the groups. During this interval, cell numbers quadrupled growing on fibrin gel or collagen-coated surfaces. At day 4, cells also reached confluency for both groups and the experiment was discontinued.
Assessment of Physiologic Function
Initial seeding of the cells on different surfaces could be achieved as confirmed by light microscopy after 24 h. After 4 days of culture, the seeded cells completely covered the culture area (see Fig. 4) of each surface.
Appearance of ciliae could be detected after 8 days on a collagen-coated surface and after 10 days on fibrin gel (see Fig. 5). After 16 days of culture, ciliary beating could be observed for cells on both surfaces, but no coordinated, directed beating could be observed during the whole culture period (see movies 1 and 2).
Light microscopy reveals the first appearance of ciliae at day 8 on collagen and day 10 on fibrin gel. (a) Collagen (8 days) and (b) fibrin gel (10 days). This image shows a high magnification of few respiratory epithelial cells (cell bodies selectively marked by solid arrows) with multiple ciliae on each cell (selectively marked by dashed arrows)
The TEER shows a marked increase during the first 6 days with an almost 4-fold increase in resistance for cells cultured on fibrin gel and a 2-fold increase for cells cultured on collagen (see Fig. 6). During the following 4 days, the TEER declines to a level slightly above baseline for cells on each surface.
Transepithelial electrical resistance of respiratory cells grown on fibrin gel or a collagen-coated permeable support, respectively. An increase in resistance can be shown after 6 days, which is more pronounced for cells cultured on fibrin gel. Resistance drops to a level slightly above baseline after 8–12 days
Assessment of Differentiation
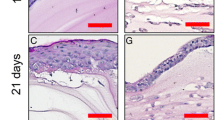
Figure 7 shows histologic and immunohistochemical stainings of fibrin gels coated with respiratory epithelial cells and cultured for up to 21 days in immersed conditions. The PAS staining reveals the development of a multilayered epithelium within 21 days with different cell morphologies for basal and apical cells. After 7 days of culture, goblet cell-like differentiation can be observed, and ciliation of epithelial cells occurs after 14 days, both persisting for the whole culture period. On day 21, cells of the apical layer have a larger cell body, while cells in the basal layer retain their original cytoplasm/nucleus ratio. Signs of mitotic activity are observable in the basal layer at all time points. In comparison to the native trachea, cell number in the epithelium is lower, and cells in the apical layer are rounder. From a global point of view, the epithelium is more ordered on the native tissue.
Histologic and immunohistochemical analyses of respiratory epithelium cultured on fibrin gel. (a–d) PAS staining of a native trachea (a) and tissue-engineered respiratory epithelium after 7 (b), 14 (c), and 21 (d) days of in vitro culture. Scale bar: 50 μm. The development of a multilayered epithelium within 21 days with different cell morphologies for basal and apical cells is visible. After 7 days of culture, goblet cell-like differentiation (solid arrows) can be observed, and ciliation of epithelial cells occurs after 14 days (dotted arrows), both persisting for the whole culture period. On day 21, cells of the apical layer have a larger cell body, while cells in the basal layer retain their original cytoplasm/nucleus ratio. Signs of mitotic activity are observable in the basal layer at all time points. In comparison to the native trachea, cell number in the epithelium is lower, and cells in the apical layer are rounder. From a global point of view, the epithelium is more ordered on the native tissue. (e–h) Anti-Pan-Cytokeratin staining of a native trachea (e) and tissue-engineered respiratory epithelium after 7 (f), 14 (g), and 21 (h) days of in vitro culture. Scale bar: 20 μm. Strong staining for all epithelial cells of the cultured constructs can be observed. The distribution of fluorescence is more uniform than in native tissue, where basal cells show a higher signal level than apical cells, and fluorescence is more localized; the highest signal level occurs in the perinuclear area. (i–l) Negative controls for Anti-Pan-Cytokeratin staining of a native trachea (i) and tissue-engineered respiratory epithelium after 7 (j), 14 (k), and 21 (l) days of in vitro culture. Scale bar: 20 μm
The immunohistochemical staining against pan-cytokeratin demonstrates strong staining for all epithelial cells of the cultured constructs. The distribution of fluorescence is more uniform than in native tissue, where basal cells show a higher signal level than apical cells and fluorescence is more localized; the highest signal level occurs in the perinuclear area.
Discussion
Tissue engineering might offer an alternative to overcome the current limitations of broncho-tracheal replacements. Choosing an adequate scaffold for tracheal tissue engineering is a crucial step that influences availability, cell growth, and differentiation as well as possible inflammatory reactions after implantation. Synthetic and biological polymers as well as decellularized matrices were investigated as scaffold materials in the past.
Macchiarini et al.11 used a human decellularized donor trachea for the successful tissue engineering of a main bronchus. Decellularized matrices offer the exact dimensions and geometries of the original organ. The preserved extracellular matrix might also provide stimuli for cell differentiation as well as mechanical resistance, though matrix proteins are usually affected by the decellularization process to a certain extent.2 If human decellularized matrices are to be used to a greater extent in bronchotracheal tissue engineering, then donor organ scarcity will also become a relevant problem as is true for tracheal transplants already.
Synthetic and biological polymers are widely used in tissue engineering as they are readily available and can be manufactured into complex geometries using proper manufacturing techniques.13,15 Still, achieving adequate geometry and dimensions as well as mechanical stability is an obstacle for the use of polymers as scaffold material.16
Fibrin hydrogel is used extensively for tissue-engineering applications, as it can be cast into complex geometries by an injection-molding technique and can be easily produced from the patient’s own blood.1,6,14 The cell-seeding process is also very effective because cells can be embedded into fibrin gel during the molding process, and subsequent cell coating of the surface has also been successfully performed. A carefully designed molding process can ensure the integrity of the gel and cells embedded,5 and adding a protease inhibitor such as aprotinin or tranexamic acid to the culture media will avoid degradation of the gel during in vitro culture. Mechanical instability is the major downside of fibrin hydrogels: they do not provide adequate mechanical strength for direct implantation. Thus, adequate in vitro cultivation before implantation becomes mandatory, unless fibrin gel is reinforced by incorporation of support materials.
Until the advent of tissue engineering, collagen-coated, flat surfaces have been the standard for successfully culturing respiratory epithelial cells. As mentioned above, synthetic polymers and decellularized matrices have been used for respiratory tissue engineering thus far; hydrogels have only played a minor role in this field. Collagen hydrogel is a scaffold material with similar mechanical properties as fibrin gel. Its main drawbacks are the missing autologous availability, as collagen cannot be produced in significant amounts from a patient, and there are more difficulties in creating 3D structures.
When more complex structures such as the trachea or even the tracheobronchial bifurcation are concerned, injection molding is one of the few methods applicable to scaffold production, which can reliably create structures that are adaptable to the patient. For enhanced mechanical strength, fibrin gel might also be reinforced by textile structures incorporated during the molding process.
Fibrin gel’s suitability for respiratory tissue engineering has not been shown before. We hypothesized that respiratory epithelium’s proliferation is at least equal to fibrin gel compared to collagen-coated surfaces, and that signs of physiologic epithelial function do not arise later. Also, we speculated that markers of epithelial differentiation could be observed for cells growing on fibrin gel as was already shown for cells on collagen-coated surfaces by other groups.17
Our experiments were conducted to provide a comparison of respiratory epithelium’s behavior on fibrin gel to that on collagen-coated surfaces as a pilot study for fibrin gel’s application in respiratory tissue engineering. The injection-molding technique applied in this study can be directly translated to the molding of 3D structures and using Transwell™ inserts, we relied on an industry-standard air-lift culture model for comparison. Thus, our study design is directly applicable to future study on respiratory tissue engineering.
Comparison of cell proliferation showed no relevant differences in cell numbers after 4 days of immersed culture. Still, cells on collagen-coated surfaces began to proliferate earlier, but no significant differences in cell numbers could be observed after days 1 and 2 compared with the fibrin gel group. After 3 days of culture, cells on a collagen-coated surface showed a significantly higher number than on fibrin gel, but this difference was small in comparison to the marked proliferation between the third and the fourth days on both surfaces. This quadrupling of cell numbers is unexpected and is possibly a result of the washing step that was introduced in the protocol before enzymatic detachment of the cells from the surface. This might have already removed some of the cells. On day 4, the progressing differentiation of the cells might have inhibited the initial detachment during the washing process. Alternatively, there might have been seeded cells that went to necrosis or apoptosis for a few days. Thus, there might have been an overlap between proliferation and necrosis/apoptosis, explaining this behavior.
These results indicate that respiratory epithelial cells proliferate adequately on fibrin gel. Adequate cell proliferation is crucial for successful tissue engineering of an airway, as cells can be seeded on the inner surface of a tubular structure as monolayer at best. For the development of a stratified epithelium, proliferation is mandatory. Still, inadequately high proliferation might be the result of inflammatory processes as response to a scaffold material that might inhibit differentiation of the seeded cells. Our results show comparable proliferation for respiratory cells on collagen-coated surfaces and fibrin gel.
Functionality of the respiratory epithelial cells was assessed using two different approaches: recording TEER, and analysis of ciliary motion. TEER is a marker of epithelial integrity and adequate barrier function. When respiratory epithelial cells are brought into air-lift culture models, cells are separated from each other following seeding. In this situation, the TEER is defined by the membrane separating the two chambers of an air-lift culture model. In the case of this study, this is either the collagen-coated Transwell™ membrane or the fibrin gel-coated membrane. TEER steeply rises when respiratory epithelial cells form a confluent layer and establish tight junctions.3 Consequently, it drops to a level slightly higher than the baseline, which is generally attributed to the proper initiation of ion-transfer processes. Thus, TEER is a marker of two important epithelial functions.
Compared to the results of Coleman et al., who recorded an increase of TEER after 6 days, we observed an earlier rise of TEER at even 4 days. The increase is about as marked as described previously: we recorded a doubling for collagen-coated surfaces and a quadrupling for fibrin gel, while Coleman et al. saw a threefold increase in TEER. The drop in TEER is also comparable and leads to a steady state little above baseline after 10 days. The time shift of the increase as compared to the results of Coleman et al.3 might be due to differences in cell culture media that enhance the differentiation of respiratory epithelial cells.
Transepithelial electrical resistance of the seeded respiratory epithelium developed similarly for cells on both surfaces. The kinetics of rise and drop were very similar. It might be concluded that differentiation processes regarding epithelial barrier function and ion transport follow the same time frame for cells on either surface. The difference in the increase of the value might be due to a more pronounced formation of tight junctions of cells on fibrin gels.
Physiologic function was also compared by recording the first appearance of ciliae and the first movement of ciliae. Ciliary motion is crucial to the proper function of respiratory epithelium, and inherited defects as seen in primary ciliary dyskinesia lead to severe illness.7 Development of ciliae and ciliary function followed a similar time frame for cells on both surfaces. The first appearance of ciliae could be recorded 2 days earlier for cells seeded on a collagen-coated surface. Ciliary motion started after 16 days of cell culture for the cells seeded on either surface. As respiratory epithelial cells take approximately 4 weeks for full differentiation in vitro, the observed time frame for the appearance of ciliae and the first movement of ciliae is reasonable to expect full differentiation in vitro.18 During the whole culture period, no directed ciliary motion could be observed, which might be attributed to missing biomechanical stimulation of the culture. For future research, it is planned to conduct studies in flow chambers that support air-lift culture and allow for selective modification of the air-flow profile over the respiratory epithelium, while ensuring adequate nutrient supply.
Differentiation of seeded respiratory epithelial cells was also assessed using histologic and immunohistochemical methods. Immunohistochemical staining demonstrates the epithelial differentiation with high levels of cytokeratin being detectable throughout the engineered epithelium. PAS light microscopy displays a multilayered and hierarchical structure of the engineered epithelium, which is a sign of cell differentiation. Respiratory differentiation could be clearly demonstrated by the appearance of PAS-positive, goblet-like cells as well as ciliae on the apical cells.
Comparison with the native trachea reveals significant differences: the epithelial layer is less structured, and apical cells are rounder and display less ciliae. Goblet cells are also more numerous in the native trachea. The cytokeratin staining also shows differences regarding the distribution of the fluorescence sign: in native trachea, it is mostly present in the basal cells, concentrated in the perinuclear space, while in our engineered epithelium, it is more evenly distributed. These observations underline that the engineered epithelial construct must be judged a less mature tissue.
Finding ways to improve differentiation of respiratory epithelium in vitro will be important for the advance of respiratory tissue engineering. To this end, the study of biomechanical influences on ciliary epithelium in vitro as outlined above will play an important role as well as further advance the biochemical clues for differentiation in the form of optimized culture media.
Based on the comparison of the growth and differentiation of respiratory epithelial cells on fibrin gel and collagen-coated surfaces, similar proliferation rates could be demonstrated on either surface. Signs of physiologic epithelial function—TEER, appearance of ciliae, and first ciliary motion—develop at similar time points. Furthermore, respiratory epithelium cultured on fibrin gel takes a multilayered morphology with signs of adequate differentiation. As scaffold materials currently applied in respiratory tissue engineering are subject to important limitations, we conclude that fibrin gel is a scaffold material that might be applied to overcome these limitations, and thus it merits further investigation.
References
Ahmed, T. A, E. V. Dare, M. T. Hincke. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng. B Rev. 14:199–215, 2008 [Epub ahead of print].
Bader, A., T. Schilling, O. E. Teebken, G. Brandes, T. Herden, G. Steinhoff, and A. Haverich. Tissue engineering of heart valves—human endothelial cell seeding of detergent acellularized porcine valves. Eur. J. Cardiothorac. Surg. 14(3):279–284, 1998.
Coleman, D. L., I. K. Tuet, and J. H. Widdicombe. Electrical properties of dog tracheal epithelial cells grown in monolayer culture. Am. J. Physiol. 246:C335–C359, 1984.
Ferguson, D. I., I. J. Wild, and O. H. Wangensteen. Experimental resection of the trachea. Surgery 28:597–619, 1950.
Flanagan, T. C., C. Cornelissen, S. Koch, B. Tschoeke, J. S. Sachweh, T. Schmitz-Rode, and S. Jockenhoevel. The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 28:3388, 2007.
Galler, K. M., A. C. Cavender, U. Koeklue, L. J. Suggs, G. Schmalz, and R. N. D’Souza. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 6(2):191–200, 2011.
Hildebrandt, F., T. Benzing, and N. Katsanis. Ciliopathies. N. Engl. J. Med. 364(16):1533–1543, 2011.
Jockenhoevel, S., K. Chalabi, J. S. Sachweh, H. V. Groesdonk, L. Demircan, M. Grossmann, G. Zund, and B. J. Messmer. Tissue engineering: complete autologous valve conduit—a new moulding technique. Thorac. Cardiovasc. Surg. 49(5):287–290, 2001.
Koch, S., T. C. Flanagan, J. S. Sachweh, F. Tanios, H. Schnoering, T. Deichmann, V. Ellä, M. Kellomäki, N. Gronloh, T. Gries, R. Tolba, T. Schmitz-Rode, and S. Jockenhoevel. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 31(17):4731–4739, 2010.
Kucera, K. A., A. E. Doss, S. S. Dunn, L. A. Clemson, and J. B. Zwischenberger. Tracheal replacements: part 1. ASAIO J. 53:497–505, 2007.
Macchiarini, P., P. Jungebluth, T. Go, M. A. Asnaghi, L. E. Rees, T. A. Cogan, A. Dodson, J. Martorell, S. Bellini, P. P. Parnigotto, S. C. Dickinson, A. P. Hollander, S. Mantero, M. T. Conconi, and M. A. Birchall. Clinical transplantation of a tissue-engineered airway. Lancet 372:2023–2030, 2008.
Matloub, H. S., and P. Yu. Engineering a composite neotrachea in a rat model. Plast. Reconstr. Surg. 117(1):123–128, 2006.
Mol, A., C. V. Bouten, F. P. Baaijens, G. Zünd, M. I. Turina, and S. P. Hoerstrup. Review article: tissue engineering of semilunar heart valves: current status and future developments. J. Heart Valve Dis. 13(2):272–280, 2004.
Park, S. H., B. H. Choi, S. R. Park, and B. H. Min. Chondrogenesis of rabbit mesenchymal stemcells in fibrin/hyaluronan composite scaffold in vitro. Tissue Eng. A 17(9-10):1277–1286, 2011.
Sodian, R., M. Loebe, A. Hein, D. P. Martin, S. P. Hoerstrup, E. V. Potapov, H. Hausmann, T. Lueth, and R. Hetzer. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO J. 48(1):12–16, 2002.
Stock, U. A., J. P. Vacanti, J. E. Mayer, Jr, and T. Wahlers. Tissue engineering of heart valves—current aspects. Thorac. Cardiovasc. Surg. 50(3):184–193, 2002.
Widdicombe, J. H., A. S. Lorne, L. M. Joby, and E. F. Walter. Expansion of cultures of human tracheal epithelium with maintenance of differentiated structure and function. Biotechniques 39:249–255, 2005.
Yamaya, M., W. E. Finkbeiner, S. Y. Chun, and J. H. Widdicombe. Differentiated structure and function of cultures from human tracheal epithelium. Am. J. Physiol. 262:L713–L724, 1992.
Zhao, F., Y. Zhang, S. Liu, and J. Yu. Artificial trachea reconstruction with two-stage approach using memory-alloy mesh. Chin. Med. J. (Engl) 116(12):1949–1951, 2003.
Acknowledgments
This study was supported by a grant from the START program of the Medical Faculty of the RWTH Aachen University.
Conflict of interest
The authors declare that they have no competing financial or other interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer West oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 15588 kb)
Supplementary material 2 (AVI 29199 kb)
Rights and permissions
About this article
Cite this article
Cornelissen, C.G., Dietrich, M., Krüger, S. et al. Fibrin Gel as Alternative Scaffold for Respiratory Tissue Engineering. Ann Biomed Eng 40, 679–687 (2012). https://doi.org/10.1007/s10439-011-0437-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0437-8