Abstract
Clinical studies have shown that drugs delivered intrathecally distribute much faster than can be accounted for by pure molecular diffusion. However, drug transport inside the cerebrospinal fluid (CSF)-filled spinal canal is poorly understood. In this study, comprehensive experimental and computational studies were conducted to quantify the effect of pulsatile CSF flow on the accelerated drug dispersion in the spinal canal. Infusion tests with a radionucleotide and fluorescent dye under stagnant and pulsatile flow conditions were conducted inside an experimental surrogate model of the human spinal canal. The tracer distributions were quantified optically and by single photon emission computed tomography (SPECT). The experimental results show that CSF flow oscillations substantially enhance fluorescent dye and radionucleotide dispersion in the spinal canal experiment. The experimental observations were interpreted by rigorous computer simulations. To demonstrate the clinical significance, the dispersion of intrathecally infused baclofen, an anti-spasticity drug, was predicted by using patient-specific spinal data and CSF flow measurements. The computational predictions are expected to enable the rational design of intrathecal drug therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrathecal (IT) drug delivery involves the administration of medications directly into the spinal fluid, circumventing the blood–brain barrier (BBB). For over 30 years, IT drug delivery has been used for chronic pain management. The treatment typically delivers drugs like morphine via the cerebrospinal fluid (CSF) to specific receptor sites in dorsal regions of the spinal cord.12 , 28 , 30 Clinical studies show that the IT delivery affords perfect pain control using a small fraction of the dose and fewer side effects compared to the other methods such as intravenous or oral administration.21 , 28 However, IT administration of baclofen offers a safe and effective treatment for severe spasticity because of a variety of causes such as multiple sclerosis, cerebral palsy, traumatic and hypoxic brain injury, and spinal cord injury.29 , 31 IT is also considered as a highly promising treatment method to deliver neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF), to treat neurodegenerative disorders like amyotrophic lateral sclerosis (ALS), and Parkinson’s and Huntington’s diseases.2 IT may be a feasible pathway to administer gene therapy via viral vectors to the central nervous system.
One of the main advantages of IT delivery is its ability to bypass the BBB, which limits the access of drugs systematically administered to the central nervous system (CNS). Despite recent advances in methods to overcome the BBB by chimeric molecules, pseudonutrients, and ligand binding proteins,1 , 3 many potentially efficacious agents still cannot reach to the brain parenchyma in therapeutic concentrations. Since the infused drug molecules are confined within the CSF space and can freely exchange between CSF and extracellular fluid of the brain parenchyma, effective therapeutic drug concentrations can be easily achieved in specific regions of the CNS using smaller doses without causing dangerous bioaccumulation in the body. Furthermore, drugs in the CSF encounter minimal protein binding and are not exposed to enzymatic activities relative to the drugs distributed via blood plasma, leading to longer drug half-life in the CSF.26 Thus, IT delivery to overcome the BBB is a significant research target for novel treatment options, such as gene therapy with viral vectors, administration of proteins, or the introduction of functionalized nanoparticles for CNS diseases.
Despite its potential as a very effective drug delivery method for the treatment of CNS diseases, current IT treatment lack quantitative procedures and rest mainly on empirical observations. The current IT practice guidelines14 for IT pain management and chemotheraphy are not intended as standards or precise requirements; their use cannot guarantee reproducible outcomes or desired therapeutic concentrations in specific target regions of brain or spinal cord. Key infusion parameters, such as mode of delivery, dosage, posture, duration, and the site of infusion are determined based on the best available clinical evidence and perspective of the individual clinician, leading to substantial variations in practice choices.39 Factors, such as drug concentration at the desired target, distribution, and pharmacokinetics, to access the efficacy of the drug delivery are unknown. Also, the fundamental transport mechanism and pharmacology that governs the IT drug dispersion within CSF and CNS are still poorly understood. One of the crucial phenomena that is not fully investigated concerns the apparent acceleration of drug dispersion from the infusion site in the lumbar region toward the brain.19
There is also uncertainty as to the causes for wide variations in drug distribution patterns observed in vivo. For example, inadequate distribution of spinal anesthesia after IT infusion results in partial or completely failed neural block, hindering the efficiency of a surgical procedure. Possible factors affecting the inadequate spread of spinal anesthesia include individual anatomical differences, anatomical abnormality, drug solution density, or local anesthetic resistance.9 In addition, variability in individual CSF pulsatile stroke volume, heart rate, and breathing rhythm may also affect IT drug dispersion. Moreover, postural changes had been observed to impact the effectiveness of spinal anesthesia. Different degrees of spinal block were achieved with patient in sitting, supine, or Trendelenburg positions after IT infusion.40 For many therapeutic drugs, their binding kinetics to specific receptors, clearance or final drug fate are unknown. The lack of fundamental understanding of IT drug delivery warrants a rigorous mathematical analysis of drug dispersion mechanism in CNS. Our analysis will be anchored in in vitro experiments and accurate first principle modeling of physiological and mechanistic functions.
Transport of intrathecally infused drugs inside the spinal canal cannot be explained by molecular diffusion alone.19 A significant contribution comes from convective transport associated with the pulsatile CSF flow in the subarachnoid spaces. The CSF bulk convection throughout the CNS has been investigated in detail by the authors previously.23 – 25 , 43 Even though the CSF bulk flow is laminar, oscillatory displacements in pulsatile flow have been known to substantially enhance micromixing of dispersed agents, despite zero net bulk flow.41 , 42 The interaction between the non-uniform CSF flow velocity distribution and the difference in radial drug concentration across the transverse plane of the spinal canal can lead to better molecular dispersion as a function of the flow amplitude and frequency, the spine anatomy, and the molecular properties of the infused drug. In addition, other factors like deformable soft tissue boundaries in the spinal epidural space, fine anatomical structures, and binding reactions may also impact drug dispersion. The hypothesis to demonstrate in this article is whether CSF pulsations are a main factor in the apparent increase in drug dispersion in the spinal canal. The term dispersion used in the article describes the total transport of drug based on the diffusion and convection due to oscillatory bulk flow. Dispersion of non-reacting substances in pulsating or oscillatory flow has been studied in the contexts of blood flow, airway gas mixing, extraction columns, estuary tidal flow, and wave boundary layers.4 , 5 , 7 , 8 , 13 , 15 , 17 , 27 , 37 , 45 , 46 However, few studies deal with drug transport in the CNS as a function of pulsatile CSF flow.42 Stockman has studied the effect of the nerves and trabeculae on the dispersion of solutes in the spinal subarachnoid space and concluded that the added stirring by the fine structures increase the dispersion by five to ten times.42
For this study, we conducted an experimental and computational analysis to investigate the effect of oscillatory flow pulsations on drug dispersion. The experimental apparatus can reproduce the pulsatile flow condition in the CSF with an amplitude and frequency similar to that of the human spinal canal. The species dispersion after an IT bolus injection was studied. Single photon emission computed tomography (SPECT) experiments with a radioactive isotope were conducted to trace the spatiotemporal distribution pattern of the infused species. We also conducted florescence dye experiments to qualitatively study the drug displacement in pulsatile flow. The term displacement in this article means the change in spatial position of the drug in the fluid column by diffusion and convection due to oscillatory bulk flow. In parallel, a three-dimensional (3D) computational model was developed to compute the drug transport under pulsatile flow conditions. We also predicted the dispersion of therapeutic agent in the patient-specific reconstruction of the human CNS. The anatomy of the spinal canal was modeled using image reconstruction software. The time-dependent drug dispersion profiles inside the CNS after IT bolus infusion was investigated. The successful characterization of accelerated drug transport due to CSF pulsations and the accurate patient specific computational models are expected to improve the clinical applications of IT drug administration by providing quantitative results for proper dosing and duration of IT drug infusion necessary to achieve desired therapeutic concentrations in specific target regions of the CNS.
Outline: The article is organized as follows. “Methodology” section will introduce the surrogate spinal canal apparatus, experimental procedures, and computational approach. “Results” section will present experimental and computational results for increased drug dispersion associated with the pulsatile flow dynamics, validation of results, and potential of using computational methods in patient-specific therapy design. “Discussion” section will emphasize outcomes and design consideration of the IT drug delivery. The article closes with conclusions and an outlook into future research.
Methodology
In order to quantify the effect of pulsatile flow dynamics on drug dispersion, a surrogate model of the spinal canal was built to simulate the oscillatory flow and bolus drug administration. Two imaging methods were employed to study the drug dispersion. An optical imaging modality using florescence of tracers was employed to visualize the drug dispersion trends, and the SPECT method is employed to record the amount of dispersion under pulsatile flow and stagnant conditions. In addition, a first principle 3D computational model was developed to predict accurately the species dispersion in pulsatile flow. The validity of the computational model is confirmed by the experimental data.
Spinal Canal Experimental Setup
We designed spinal canal experimental setup to study the transport of intrathecally injected macromolecules in the spinal canal under the pulsatile flow. In order to isolate the effect of pulsatile flow on the drug transport as a key parameter in our study, many anatomical details were deliberately simplified in the proposed design. The schematic of the experimental apparatus is depicted in Fig. 1. The surrogate spinal canal model consists of three main components: (i) a fluid-filled annular canal, (ii) oscillatory flow generator, and (iii) a drug infusion mechanism.
Fluid-filled annular canal: The spinal canal was constructed from a 45-cm-long cylindrical pipette with an inner diameter of 1.5 cm. Inside of the pipette, a 0.78-cm-diameter dowel was suspended to represent the spinal cord, thus creating an annular fluid-filled section to mimic the spinal subarachnoid space of the lumbar section of an adult. The dimensions are chosen so as to approximate anatomical spinal canal dimensions in humans.18 , 34
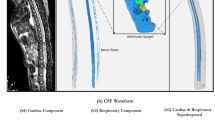
Oscillatory flow generator: A modified peristaltic pump coupled with a collapsible elastic reservoir system was used to induce a pulsatile flow with 1 cm3 oscillatory amplitude, but zero net flow. The pump was modified to create a ramping flow pattern by removing two of three metal rollers that drive the master peristaltic. One end of the surrogate spinal canal model was connected to the peristaltic pump providing a pulsed function to induce flow oscillations; the other end was closed with a deformable membrane. The pump was set at 60 rpm for the pulsatile experiments. The generated pulsatile flow profile displayed a waveform of 1 Hz similar to human spinal CSF fluid flow acquired previously by the phase-contrast MR imaging methods6 as shown in Fig. 2.
Pulsatile waveform generated by modified peristaltic pump compared to the CSF waveform measured with phase-contract MR imaging.6 The solid line (—) and cross (×) represent the normalized volumetric flow amplitudes of the current experiment and in vivo measurements, respectively
IT Infusion mechanism: A 0.03-mm-diameter injection port was created at the center of the spinal canal about 2 mm beyond the boundary of the canal. A programmable syringe pump was used to control the 0.1 mL bolus infusion of tracer over a 30-s period. In addition, a second fluid-filled annular canal with stagnant CSF was built as a control.
Qualitative Dye Experiments
The fluorescent dye experiments, which were performed under dark-room conditions, demonstrate the difference in apparent drug dispersion in a pulsating flow field vs. a stagnant fluid column. Under Ultraviolet (UV) illumination, the fluorescent dye emits visible light which can be imaged optically. The dispersion of a fluorescent dye injected over a 30-s period was measured under pulsatile and stagnant flow conditions. A Canon 500D camera mounted on a tripod was remotely controlled by a computer to take one exposure every 30 s. Using 2-s exposure time allowed the camera to record the advancement of the dye front. The images were processed to estimate the qualitative spatiotemporal advancement of the dye front. We have conducted dye experiments using optical methods to qualitatively study the effect of pulsatile flow on the dispersion. The oscillatory nature of the flow, the progressive tracer dilution diminishing the signal, the inaccuracy in defining the front threshold, and the refraction and reflection encountered at the wall make it very difficult to interpret the precise speed of the dye front. Therefore, dye experiments presented here should not be interpreted with strict precision expectations, but will be addressed by SPECT.
Quantitative Radionuclide Experiments
The SPECT technique was employed to accurately determine the distribution patterns of a radioactive substance under pulsatile and stagnant flow conditions. The same experimental settings and flow conditions were maintained for both pulsatile and stagnant cases. Technetium-99m, a commercially available radionuclide typically used in medical tests, was used in the experiment. A Siemens dual-head e.cam gamma camera was used to dynamically track the radionucleotide distribution. First, a bolus injection of 0.1 mL of 1.47 mC/mL Sodium Pertechnetate Technetium-99m (Na99mTcO4) sterile solution containing radioactive Technetium was injected over a 30-s period. The stagnant and the pulsatile experiments were run in parallel. The images of the radionucleotide distribution were dynamically recorded using a gamma camera with 10-s exposure. The lower threshold of the gamma camera was set such that the displayed low-level gray shaded color can accurately capture the low count density areas, such as dispersion front of the Technetium.
Three-Dimensional Computational Modeling of Spinal Canal
We performed rigorous 3D computational fluid dynamic simulations to compute the effect of pulsatile motion on the mixing and transport of chemical species. Figure 3 shows the 3D computational mesh of the spinal canal. The dimensions of the computational model, pulsatile flow waveform, and infusion parameters are chosen to match the experiments described in “Spinal Canal Experimental Setup” section. A fine mesh with over 500,000 tetrahedral volume elements resolved the pulsating velocity field over the entire domain. The continuity and Navier–Stokes equations were used to calculate the CSF flow and pressure distribution within the system as given in Eqs. (1) and (2). The inlet velocity is implemented using the oscillatory inlet flow profile given in Eq. (3).
where \( \rho \) is the density, \( \nu \) is the kinematic viscosity, \( \vec{F} \) is the external force term, and \( \vec{u} \) and \( p \) describe the periodic velocity and pressure fields, respectively. Using experimental measurements and curve-fitting methods, a Fourier series matching the periodic waveform was defined as shown in Fig. 2, which was then made use of to impose the pulsatile velocity profile at one end of the spinal canal. A Dirichlet pressure boundary condition was specified at the distal end to ensure no net bulk fluid or tracer loss. All boundary conditions used in the simulation are listed in Table 1. The drug dispersion in the bulk CSF fluid takes place through a convection–diffusion process according the Eq. (4) as,
where C is the concentration, and D is the drug diffusivity. The transport equations for the bulk and the drug are fully coupled via the convective velocity field, \( \vec{u}(t) \), occurring in all transport equations. Drug injection rate, concentration, and duration parameters were identical to those in experiments. The solution of the fundamental conservation laws of mass, momentum, and species transport lead to the desired predictions of species concentration, C, as well as velocity and pressure fields, \( \vec{u} \) and p, respectively. A finite volume-based CFD scheme was used to solve the conservation equations describing convection and diffusion transport processes.
Results
We will first report the experimental measurements of the species dispersion under pulsatile and stagnant flow conditions using florescent dye and nuclear medicine methods. The florescence dye experiments are employed to visualize the dispersion patterns and to prepare the infusion apparatus for the quantitative analysis with SPECT. SPECT images (10-s time frame and 128 × 128 matrix size) of the radionuclide distribution were acquired using a dual-headed camera equipped with high-resolution collimators. The validity of the computational model is demonstrated by comparing against the SPECT experimental data. The convergence criteria for the computational simulations are maintained below 10−6 for all the dependent variables.
Experimental Measurements of the Accelerated Dispersion Associated with Pulsatile Flow
Figure 4 shows the advancement of the net dye front with time for pulsatile and stagnant flow cases. The data indicate a clear difference between the pulsatile case and non-pulsatile control. The spinal column experiment results show that after 5 min, pulsatile motion induces an almost twofold increase in dye front displacement compared to that in stagnant fluid. The SPECT experimental results show a large difference in the speed of the dispersion front between the pulsatile and non-pulsatile experiment: at 7 min, pulsatile flow shows more than a 500% increase in the displacement of the dye front compared to the stagnant case as shown in Fig. 5.
Effect of Oscillation Frequency and Stroke Volume on the Drug Dispersion
We conducted dye experiments to investigate the effect of pulse frequency and stoke volume on the rate of dispersion as shown in Fig. 6. The human pulse range can vary as low as 30 bpm during a rest to almost as high as 180 bpm during an exercise,33 but for the delivery of IT drugs, the range is much narrower. The speeds of dye dispersion at 0.5, 1, and 2-Hz oscillatory frequencies are shown in Fig. 6a for a stroke volume of 1.5 mL/s. The MRI measurements of the CSF flow in the spinal canal suggest that the CSF stroke volume could vary in the range of 0.1–2.5 mL/s in humans.10 Figure 6b shows the effect of different pulsatile stroke volumes (0.5, 1.0, and 1.5 mL/s) on the speed of dye dispersion while keeping the pulse frequency at 1 Hz. The dye dispersion results shows that the both frequency and the stroke-volume have a significant effect the dispersion speed.
Comparison of SPECT Experiments with Rigorous CFD
The Technetium distribution patterns obtained by SPECT and the corresponding 3D simulations are shown in Fig. 7. After bolus injection, the tracer spreads symmetrically from the infusion point mainly because of convection caused by oscillatory flow. Dispersion due to molecular diffusion has much smaller contribution. The distribution patterns in SPECT experiments and simulations show good agreement for all time frames.
Figure 8 summarizes the experimentally observed species dispersion patterns of Technetium-99m at different counts in time compared to the computer simulations for pulsatile and stagnant conditions. Periodic flow was induced right at the end of the 30-s species infusion. In a second device, the fluid was kept stagnant as a control. The distribution of Technetium shows 35-cm displacement after 4 min. In the stagnant case, the front advances only 7 cm after 4 min. The accelerated speed with oscillations corresponds to 500% increase in displacement compared to the stagnant case. The 3D computational simulations show a similar trend as observed in the SPECT experiment. Initially, both computational and experimental dispersion fronts are in very good agreement. After 2 min of the infusion, the computational simulations tend to predict slightly higher species dispersion than observed with SPECT as shown in Fig. 8. This difference may be caused by the decay in the resolution of the SPECT imaging method at lower concentrations, when the transport front approaches the detection threshold of the gamma camera. Specialized calibration techniques as described elsewhere36 could be an option to acquire more precise measurements. Within the present experimental uncertainty, it can be concluded that the current SPECT data and computational predictions are in reasonable agreement.
The normalized species concentrations profiles obtained for Technetium in the nuclear medicine experiment and 3D simulations are shown in Fig. 9 for pulsatile and stagnant cases at the end of 3 min. The local Technetium concentration peaks could be due the variations in the counts over the 10-s exposure time of the gamma camera. In both cases, simulations closely predict the experimental concentration profiles as shown in Fig. 9. The rapid dispersion of the species along the spinal canal model due to pulsatile flow field is shown in Fig. 10 at different time intervals: after 4 min, tracer dispersion in pulsatile flow is five times faster than in the stagnant case.
Patient-Specific CNS Model and Dispersion of an Intrathecally Administered Therapeutic Agent
In a preliminary effort to demonstrate the potential of computational methods for therapy design, a patient-specific model of the human CNS was created from MR images using image reconstruction techniques.22 – 24 , 43 The dispersion of the anti-spasticity drug, baclofen,31 administered intrathecally via a bolus injection was computed using rigorous computational fluid mechanics methods introduced in “Methodology” section. The simulations predict the outcome of a treatment with a 100-μg bolus injection of baclofen into the second lumbar vertebrae of the spinal canal of a virtual patient.
The computational mesh of CSF spaces inside the human CNS was constructed via image reconstruction from patient specific MRI.43 The oscillatory boundary conditions of Table 2 reproduced realistic CSF pulsations leading to a periodic velocity waveform with 0.9 cm3/s volumetric CSF flow amplitude in the cervical spine region. A steady CSF production of 0.4 mL/min was incorporated via inflow from the lateral and third ventricles. CSF reabsorption was modeled via outflow zone at the arachnoid granulation of the superior sagittal sinus. More details on how to model the CSF flow in the human CNS are described elsewhere.43
Intrathecal Drug Delivery in a Patient-Specific CNS
The simulated pulsatile velocity profile at the lateral C4 level shown in Fig. 11 is in good agreement with the measured CSF velocities.16 Note that the CSF pulsatile waveform has a higher maximum systolic velocity than the diastolic minimum. In addition, the systolic half-wave is shorter than the backflow during diastole. Figure 12a depicts drug distribution inside the CNS of the patient after bolus IT infusion at different time intervals. The drug concentration along the midline of the dorsal side is shown in Fig. 12b. The drug moves upward along the spinal canal with a similar speed as observed in our experiments. After 5 min of infusion, approximately 30% of the initial baclofen concentration was found in the subarachnoid space at the T10 vertebral level. The rapid dispersion of the drug was observed within the first several minutes of the infusion. The drug dispersion front reached at the upper thoracic level after 15 min as shown in Fig. 12b. After 30 min, approximately 10% of the initial drug concentration was found at the upper cervical region of the spinal canal.
Ventral velocity profile at the C4 level in the spinal canal compared to the measured velocities using MR imaging method16
Simulated IT drug infusion into the lumbar region. (a) Drug distribution inside the human CNS after IT infusion in the 2nd lumbar vertebrae. (b) The drug concentration along the spinal canal (midline of the dorsal side) at different time intervals after the bolus infusion. The drug transport is accelerated by a pulsatile CSF flow field. The drug is already found in the cervical region 30 min after IT infusion
Discussion
Effect of Pulsatile Flow on the Accelerated Drug Dispersion
The experimental results and computational predictions showed that the effect of the pulsatile flow increases the speed of the drug transport by a factor of five compared to transport in stagnant fluid. Also, the Technetium-99m distribution patterns along the spinal canal obtained with SPECT show similar profiles to that of the computer simulations. The local peaks observed in the experimentally measured distribution profiles using Technetium-99m could be due to the exposure time used in the gamma camera to record the radionucleotide counts. The computational predictions are in good agreement with the SPECT experiment. This finding confirms the suitability of the laminar incompressible flow assumption in the computational model. The increased speed of drug transport can be attributed to the combined effect of pulsations and diffusion. Even though the current study does not render all the unknown parameters relevant in IT transport including open issues concerning the spinal epidural space, spinal nerves, microstructures, and binding reactions, the experimental and simulation data presented here confirm pulsations are a significant factor in enhancing the speed of the drug dispersion in the spinal canal. It shows that CSF pulsations are the key elements in predicting the fate of drug administered intrathecally.
Computational Fluid Mechanics to Study Species Transport in Pulsating Flow
To better understand the mechanisms of enhanced transport in oscillatory flow, computational simulations based on the finite volume method were performed. Direct numerical simulation of species transport in oscillatory flow requires fine spatiotemporal discretization. Owing to the demanding computational requirements, several authors have proposed specialized alternative techniques. Lattice Boltzmann simulations were proposed to quantify the effect of obstacles in the spinal canal, such as nerves and trabeculae on CSF dynamics and species dispersion.41 , 42 The steady streaming method was used to model solute dispersion in oscillatory flow.11 , 20 In our study, parameters for mesh discretization and time-step integration were selected carefully, so that the numerical simulation was reproducible and mesh independent. The numerical solutions executed with 500,000 mesh cells and time steps of 0.05 s agreed reasonably well with data of experiments. We concluded that careful spatiotemporal discretization was suitable in this case for performing rigorous 3D simulations of oscillatory flow with 1 cm3 stroke volume at the 1 Hz frequency. Yet, simulation time requirement using such discretization schemes should be expected to be demanding: for example, 32 processors on Teragrid required about 4 h to complete simulation for 1 min in real time.
Patient Specific Drug Delivery
The prediction of drug dispersion profiles in a patient specific model shows the upward motion of the intrathecally infused drug along the spinal canal. These distribution patterns show the potential of using patient-specific computational modeling in rational IT therapy design. The accurate prediction of dynamic drug dispersion in patient specific CNS can provide answers to important questions in IT therapy design, such as the amount of dose, infusion site and duration, effective therapeutic drug concentration levels at specific regions, and efficacy of the infusion.
Conclusions
Experimental studies were conducted and interpreted theoretically with computational studies to quantify the effect of the pulsatile flow on the acceleration of drug transport in the spinal canal. An experimental model of the spinal canal model was built with realistic dimensions and pulsatile flow field. In parallel, a 3D computational model of the spinal canal was generated. A SPECT nuclear medicine technique using Technetium-99m was carried out to quantify the spatiotemporal distribution patterns. Also, florescent dye experiments were conducted to qualitatively demonstrate the increase in apparent drug dispersion due to pulsating flow field. Experimental results show that pulsatile flow can increases the speed of species dispersion by a factor of five. Computational predictions with incompressible viscous laminar flow yield good agreement with the experimental observations. The computations also provide a theoretical explanation for increased drug dispersion due to enhanced micromixing between oscillating laminar sheets covering the drug fate than by pure diffusion in stagnant fluid. In addition, a 2D computational model representing the CSF space inside the human CNS was constructed via image reconstruction from patient-specific MRI data to investigate the transport of a therapeutic agent administered intrathecally via a bolus injection. Our results show the potential of using computational predictions to enhance the efficacy of IT drug therapies.
References
Abbott, N. J., and I. A. Romero. Transporting therapeutics across the blood–brain barrier. Mol. Med. Today 2:106–113, 1996.
Airaksinen, M. S., and M. Saarma. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3:383–394, 2002.
Alam, M. I., S. Beg, A. Samad, S. Baboota, K. Kohli, J. Ali, A. Ahuja, and M. Akbar. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 40:385–403, 2010.
Aris, R. On the dispersion of a solute in pulsating flow through a tube. Proc. R. Soc. Lond. A 259:370–376, 1960.
Bandyopadhyay, S., and B. Mazumder. Unsteady convective diffusion in a pulsatile flow through a channel. Acta Mech. 134:1–16, 1999.
Bhadelia, R. A., A. R. Bogdan, R. F. Kaplan, and S. M. Wolpert. Cerebrospinal fluid pulsation amplitude and its quantitative relationship to cerebral blood flow pulsations: a phase-contrast MR flow imaging study. Neuroradiology 39:258–264, 1997.
Chatwin, P. C. On the longitudinal dispersion of passive contaminant in oscillatory flows in tubes. J. Fluid Mech. 71:513–527, 1975.
Elad, D., D. Halpern, and J. B. Grotberg. Gas dispersion in volume-cycled tube flow. I. Theory. J. Appl. Physiol. 72:312–320, 1992.
Fettes, P. D. W., J.-R. Jansson, and J. A. W. Wildsmith. Failed spinal anaesthesia: mechanisms, management, and prevention. Br. J. Anaesth. 102:739–748, 2009.
Freund, M., M. Adwan, H. Kooijman, S. Heiland, M. Thomsen, S. Hahnel, K. Jensen, H. J. Gerner, and K. Sartor. Measurement of CSF flow in the spinal canal using MRI with an optimized MRI protocol: experimental and clinical studies. Rofo 173:306–314, 2001.
Gaver, D. P., and J. B. Grotberg. An experimental investigation of oscillating flow in a tapered channel. J. Fluid Mech. 172:47–61, 1986.
Ghafoor, V. L., M. Epshteyn, G. H. Carlson, D. M. Terhaar, O. Charry, and P. K. Phelps. Intrathecal drug therapy for long-term pain management. Am. J. Health Syst. Pharm. 64:2447–2461, 2007.
Gordon Harris, H., Jr., and S. L. Goren. Axial diffusion in a cylinder with pulsed flow. Chem. Eng. Sci. 22:1571–1576, 1967.
Hassenbusch, S. J., and R. K. Portenoy. Current practices in intraspinal therapy—a survey of clinical trends and decision making. J. Pain Symptom Manage. 20:S4–S11, 2000.
Hazra, S., A. Gupta, and P. Niyogi. On the dispersion of a solute in oscillating flow through a channel. Heat Mass Transfer 31:249–256, 1996.
Henry-Feugeas, M. C., I. Idy-Peretti, B. Blanchet, D. Hassine, G. Zannoli, and E. Schouman-Claeys. Temporal and spatial assessment of normal cerebrospinal fluid dynamics with MR imaging. Magn. Reson. Imaging 11:1107–1118, 1993.
Holley, E. R., D. R. F. Harleman, and H. B. Fischer. Dispersion in homogeneous estuary flow. J. Hydraul. Div. 96:1691–1709, 1970.
Ko, H. Y., J. H. Park, Y. B. Shin, and S. Y. Baek. Gross quantitative measurements of spinal cord segments in human. Spinal Cord. 42:35–40, 2004.
Kroin, J. S., A. Ali, M. York, and R. D. Penn. The distribution of medication along the spinal canal after chronic intrathecal administration. Neurosurgery 33:226–230, 1993; discussion 30.
Kuttler, A., T. Dimke, S. Kern, G. Helmlinger, D. Stanski, and L. Finelli. Understanding pharmacokinetics using realistic computational models of fluid dynamics: biosimulation of drug distribution within the CSF space for intrathecal drugs. J. Pharmacokinet. Pharmacodyn. 37:629–644, 2010.
Lamer, T. J. Treatment of cancer-related pain—when orally-administered medications fail. Mayo Clin. Proc. 69:473–480, 1994.
Linninger, A. A., M. R. Somayaji, L. Zhang, M. Smitha Hariharan, and R. D. Penn. Rigorous mathematical modeling techniques for optimal delivery of macromolecules to the brain. IEEE Trans. Biomed. Eng. 55:2303–2313, 2008.
Linninger, A. A., C. Tsakiris, D. C. Zhu, M. Xenos, P. Roycewicz, Z. Danziger, and R. Penn. Pulsatile cerebrospinal fluid dynamics in the human brain. IEEE Trans. Biomed. Eng. 52:557–565, 2005.
Linninger, A. A., M. Xenos, B. Sweetman, S. Ponkshe, X. Guo, and R. Penn. A mathematical model of blood, cerebrospinal fluid and brain dynamics. J. Math. Biol. 59:729–759, 2009.
Linninger, A. A., M. Xenos, D. C. Zhu, M. R. Somayaji, S. Kondapalli, and R. D. Penn. Cerebrospinal fluid flow in the normal and hydrocephalic human brain. IEEE Trans. Biomed. Eng. 54:291–302, 2007.
Misra, A., S. Ganesh, A. Shahiwala, and S. P. Shah. Drug delivery to the central nervous system: a review. J. Pharm. Pharm. Sci. 6:252–273, 2003.
Mukherjee, A., and B. S. Mazumder. Dispersion of contaminant in oscillatory flows. Acta Mech. 74:107–122, 1988.
Onofrio, B. M., and T. L. Yaksh. Long-term pain relief produced by intrathecal morphine infusion in 53 patients. J. Neurosurg. 72:200–209, 1990.
Penn, R. D. Intrathecal baclofen for spasticity of spinal origin: seven years of experience. J. Neurosurg. 77:236–240, 1992.
Penn, R. D. Intrathecal medication delivery. Neurosurg. Clin. N. Am. 14:381–387, 2003.
Penn, R. D., S. M. Savoy, D. Corcos, M. Latash, G. Gottlieb, B. Parke, and J. S. Kroin. Intrathecal baclofen for severe spinal spasticity. N. Engl. J. Med. 320:1517–1521, 1989.
Piechnik, S. K., P. E. Summers, P. Jezzard, and J. V. Byrne. Magnetic resonance measurement of blood and CSF flow rates with phase contrast—normal values, repeatability and CO2 reactivity. In: Acta Neurochirurgica Supplements, edited by H. J. Steiger. Vienna: Springer, 2009, pp. 263–270.
Rodeheffer, R. J., G. Gerstenblith, L. C. Becker, J. L. Fleg, M. L. Weisfeldt, and E. G. Lakatta. Exercise cardiac-output is maintained with advancing age in healthy-human subjects—cardiac dilatation and increased stroke volume compensate for a diminished heart-rate. Circulation 69:203–213, 1984.
Ros, L., J. Mota, A. Guedea, and D. Bidgood. Quantitative measurements of the spinal cord and canal by MR imaging and myelography. Eur. Radiol. 8:966–970, 1998.
Sawatsky, N. G., and D. W. Oscarson. Diffusion of technetium in dense bentonite. Water Air Soil Pollut. 57–58:449–456, 1991.
Sindhwani, N., O. Ivanchenko, E. Lueshen, K. Prem, and A. A. Linninger. Methods for determining agent concentration profiles in agarose gel during convection-enhanced delivery. IEEE Trans. Biomed. Eng. 58:626–632, 2011.
Smith, R. Contaminant dispersion in oscillatory flows. J. Fluid Mech. 114:379–398, 1982.
Somayaji, M. R., M. Xenos, L. Zhang, M. Mekarski, and A. A. Linninger. Systematic design of drug delivery therapies. Comput. Chem. Eng. 32:89–98, 2008.
Stearns, L., R. Boortz-Marx, S. Du Pen, G. Friehs, M. Gordon, M. Halyard, L. Herbst, and J. Kiser. Intrathecal drug delivery for the management of cancer pain: a multidisciplinary consensus of best clinical practices. J. Support Oncol. 3:399–408, 2005.
Stienstra, R., and N. M. Greene. Factors affecting the subarachnoid spread of local anesthetic solutions. Reg. Anesth. Pain Med. 16:1–6, 1991.
Stockman, H. W. Effect of anatomical fine structure on the flow of cerebrospinal fluid in the spinal subarachnoid space. J. Biomech. Eng. 128:106–114, 2006.
Stockman, H. W. Effect of anatomical fine structure on the dispersion of solutes in the spinal subarachnoid space. J. Biomech. Eng. 129:666–675, 2007.
Sweetman, B., and A. A. Linninger. Cerebrospinal fluid flow dynamics in the central nervous system. Ann. Biomed. Eng. 39:12, 2010.
Sykova, E., and C. Nicholson. Diffusion in brain extracellular space. Physiol. Rev. 88:1277–1340, 2008.
Watson, E. J. Diffusion in oscillatory pipe flow. J. Fluid Mech. 133:233–244, 1983.
Yasuda, H. Longitudinal dispersion of matter due to the shear effect of steady and oscillatory currents. J. Fluid Mech. 148:383–403, 1984.
Acknowledgments
The authors would like to thank Dr. Amjad Ali at the Nuclear Medicine Department, Rush University medical center for facilitating the SPECT nuclear medicine experiments. We also appreciate the useful discussions on baclofen with Dr. R. Penn. Timothy J. Harris Jr. is gratefully indebted to NSF REU program (NSF EEC 0754590, PI A.A. Linninger) for having supported his undergraduate research during the summer of 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Peter E. McHugh oversaw the review of this article.
An erratum to this article can be found at http://dx.doi.org/10.1007/s10439-011-0376-4
Rights and permissions
About this article
Cite this article
Hettiarachchi, H.D.M., Hsu, Y., Harris, T.J. et al. The Effect of Pulsatile Flow on Intrathecal Drug Delivery in the Spinal Canal. Ann Biomed Eng 39, 2592–2602 (2011). https://doi.org/10.1007/s10439-011-0346-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0346-x