Abstract
Topical application of drugs for treatment of intraocular diseases is often limited by inadequate transport and induced toxicity in corneal tissues. To improve the drug delivery, a mathematical model was developed to numerically simulate the transport process of ethacrynic acid (ECA), a potential drug for glaucoma treatment, in the anterior segment of a typical human eye. The model considered diffusion of ECA in all tissues and the aqueous humor (AH) as well as convection of ECA in the AH. The simulation results showed that ECA concentration in the eye depended on the rate of AH production, the half-life of ECA in the precorneal tear film, and the transport parameters in the model. In addition, the main pathway for ECA clearance from the eye was the trabecular meshwork (TM) and the rate of clearance was approximately proportional to the AH production rate. The model predicted that the most effective approach to improving topical drug delivery was to prolong its half-life in the precorneal tear film. These simulation results and model prediction, which could be verified experimentally, might be useful for improving delivery of ECA and other therapeutic agents to the TM as well as other tissues in the anterior segment of the eye.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Topical application of ophthalmic drugs is currently the preferred choice for treatment of diseases in the anterior segment of the eye.8 Drug administration through this route, however, is often limited by the corneal epithelium, a physiological barrier to drug transport, and rapid drug turnover in the precorneal tear film. In this case, frequent dosing or high drug dose is required to overcome the poor drug availability within ocular tissues. For drugs with a relatively narrow therapeutic window, the requirement may result in severe toxicity in corneal tissues.
This study was focused on topical drug delivery for treatment of primary open-angle glaucoma (POAG), which is one of the common eye diseases affecting approximately 1–2% of the population over the age of 40.6,39 One of the main risk factors for POAG is the elevated intraocular pressure (IOP), which often causes retinal ganglion cell death, optic nerve damage, and visual field loss. Lowering IOP is so far the only effective therapeutic approach to the control of POAG progression.15 Although it has been shown for more than four decades that the locus of this disease resides in the outflow pathway in the trabecular meshwork (TM),11 there is no specific treatment targeted directly to the TM.6,23
Ethacrynic acid (ECA) is a phenoxyacetic acid with sulfhydryl reactivity that has been used clinically as a diuretic agent. Recently, it has also been considered as a potential TM drug.24,36 ECA acts to alter the shape and, at higher concentrations, the adhesion to substrate of cultured human TM cells.10 The shape change is reversible if the ECA concentration is <0.4 mM and the incubation time is 1 h.7 The changes in cell shape and adhesion to substrate are mediated though remodeling of both actin filaments and microtubules.10,25 In addition, ECA can inhibit the activity of Na+/K+/Cl− co-transporter, which in turn leads to a reduction in TM cell volume.26 All these changes in TM cells can cause an increase in aqueous humor (AH) outflow, which is considered to be the mechanism of ECA-mediated reduction in IOP.7,30
Different routes and methods have been considered for ECA administration. They include (i) direct infusion of ECA into the anterior chamber of living monkey’s eyes,31 enucleated human,21 calf eyes,6 and the eyes of patients with advanced open-angle glaucoma24; (ii) implantation of a polymeric film into the sclera to locally release ECA33; and (iii) topical application of ECA solutions onto the corneas of rabbits and monkeys.32 In the study of topical application of ECA, corneal edema was observed in some animals at the precorneal concentration of 100 mM, which was required for lowering IOP in these animals.32 To eliminate the adverse effects of ECA in corneal tissues while maintaining the same therapeutic efficacy, ointment with reduced concentration of ECA has been developed.36 The ointment with its high viscosity can increase the residence time of ECA in the precorneal surface and thus the time available for drug transport across the cornea. The ECA loaded ointment has been used to treat laser-induced glaucoma in cynomolgus monkeys.36 However, the long-term use of the ointment is toxic since superficial corneal erosion has been observed at the ECA concentration of 49.5 mM during a 30-day treatment.36 Therefore, the induced corneal toxicity remains to be a limiting factor for the clinical use of ECA as a POAG drug.32,33
To further reduce the corneal toxicity, several strategies can be explored. One is to modify the drug to make it less toxic and easy to diffuse across the cornea. Another is to increase drug half-life on the corneal surface, which may allow a significant reduction in drug dose. The latter strategy can be achieved through (i) applying drugs as a night-time (i.e. during sleep) medication that will reduce drug clearance in the precorneal tear film and (ii) conjugating drugs with mucoadhesive polymers, such as Pluronic® block copolymers, which form gels in situ upon exposure to physiological environments in the precorneal region to prolong the drug half-life.40 As a result, the concentration of the free drug on the corneal surface can be significantly reduced while the drug concentration in the TM is similar to those after drug administration via traditional eye drops or ointment formulations.
To evaluate advantages/disadvantages of different strategies mentioned above for POAG treatment in the clinic, it is necessary to determine the ECA availability in target tissues (e.g., the TM). To date, a direct and non-invasive measurement of spatial and temporal distributions of drugs in the eye is practically impossible.16 Thus, other approaches need to be considered. One of them is to develop mathematical models and use them to numerically simulate drug transport in the eye. A number of such models have been developed in previous studies for predicting the average concentration of drugs in the AH and thus the ocular bioavailability of drugs after topical instillation,17,43 but they cannot be used to determine the local concentration of drugs in the TM since they are based on the compartmental analysis of drug delivery. In this study, a new mathematical model of drug transport was developed, based on the anatomy of a human eye. It was used to simulate the spatial and temporal distributions of ECA in the AH and the tissues around the anterior chamber. The simulations predicted the distribution of ECA concentration in the anterior segment of the eye and the ECA concentration in the TM, which was important for determination of the therapeutic efficacy of ECA in POAG treatment.
Materials and Methods
Description of Mathematical Model
A mathematical model was developed to simulate transport of ECA in the anterior chamber and the surrounding tissues after ECA were applied topically on the surface of the cornea. It also considered the AH flow in the anterior and posterior chambers, which affected convection of ECA in the eye. To simplify the analysis, the model neglected the natural convection induced in the eye by the temperature gradients across the anterior chamber,20 and the blinking of eye lid. These assumptions were likely to be valid for ECA transport when the eye lids were completely closed during sleep. Practically, it is also more convenient for patients to have the formulated drugs on the corneal surface for a few hours during the sleep if the active drug or the materials for drug formulation are not 100% transparent. To further simplify the model, it was assumed that the gravity had minimal effects on the AH flow.
Model Geometry
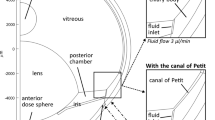
The geometry of the model was obtained first from data in the literature,5,14 and then modified slightly based on an optical coherence tomography (OCT) image of a human eye.4 The final geometry is shown in Fig. 1, and the dimensions of different portions are listed in Table 1. One geometrical parameter that could not be precisely determined was the minimum distance between iris and lens. Data in the literature suggest that it is on the order of 10 μm.13 In the OCT image, the iris appeared to be in direct contact with the lens since the dimension of the smallest gap was below the spatial resolution of OCT. In a preliminary study, we observed that the gap size had only minor effects on the flow field in the middle of the anterior chamber of the eye although it could have a significant impact on the local velocity of AH within the gap. When the minimum distance between iris and lens was varied between 5 and 10 μm, the simulated ECA concentration at the TM was not changed significantly. In fact, the two curves were on top of each other when the concentration vs. time curves were plotted. Therefore, the minimum distance was assumed to be 10 μm in this study. The model also assumed that tissue structures in the eye were axisymmetric. Thus, the transport of fluid and solutes was also axisymmetric in this study.
Governing Equations
ECA is relatively a hydrophobic molecule. Our previous and current studies suggested that it could diffuse rapidly through both intracellular and interstitial spaces in the sclera and the cornea.23 Thus, interstitial convection of ECA was neglected in the model. The transport of ECA in tissues around the anterior chamber, i.e., cornea, sclera, iris, and ciliary body (CB), involved only passive diffusion, which was governed by the Fick’s second law of diffusion:
where C is the ECA concentration, D is the diffusion coefficient of ECA in tissues, and R d is the rate of ECA clearance in tissues. We assumed that there was no ECA degradation/inactivation in tissues. Thus, the potential mechanism of drug clearance could only be the drug absorption into blood and lymph microvessels. Under normal physiological conditions, there are no blood and lymph vessels in the cornea. Sclera tissues contain large vessels,38 which have minimal effects on drug absorption due to the low vascular permeability and the small surface area compared to microvascular networks. Cells in the sclera receive nutrient supplies mainly from microvessels in episcleral and choroidal tissues.38 Therefore, drug absorption does not happen in the cornea and can be neglected in the sclera. The iris and ciliary body contain rich microvascular networks, which may significantly affect drug transport in these tissues. In this study, drug absorption in the iris was lumped together and considered by assuming that the concentration boundary condition at the posterior surface of the iris to be zero (see also the discussion below). In the ciliary body, the drug absorption was assumed to be zero since the drug concentration was negligible, compared to those in other tissues as observed in our preliminary study and demonstrated by the simulation results shown later.
Within the anterior chamber, ECA transport included convection and diffusion in the AH, a transparent filtrate solution produced actively by the CB that provides nutrition for avascular tissues (i.e., the lens and cornea) around the anterior chamber.14,28 After being secreted from the CB, the AH passes through the gap between the lens and the iris to enter the anterior chamber. The AH exits the anterior chamber primarily through the TM, which is connected to the Schlemm’s canal. The AH will eventually enter the systemic circulation through aqueous veins. The drainage structure is termed as the conventional TM outflow pathway. Under normal physiological conditions, it constitutes approximately 90% of total aqueous outflow in human eyes.30 The rest AH (~10%) flows through interstitial spaces of iris, adjacent sclera, and ciliary muscles to enter episcleral tissues via the uveoscleral outflow pathway. The latter pathway is less pressure-sensitive and has a different outflow mechanism compared to the former. The model developed in this study assumed that the outflow via the uveoscleral pathway had minimal effects on the IOP and thus was neglected in the transport analysis. Additionally, the model assumed that the AH was an incompressible Newtonian fluid. Thus, the AH flow in the anterior chamber of the eye was governed by the continuity equation and the Navier–Stokes equation for incompressible fluid at the steady state:
The governing equation for ECA transport in the AH was
where D 0 is the diffusion coefficient of ECA in the AH, \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {\upsilon } \) is the fluid velocity, ρ is the mass density of the AH, μ is the viscosity of the AH, t is the time, and P is the fluid pressure. In the derivation of Eq. (4), we had assumed that there was no ECA degradation in the AH.
Boundary Conditions
All boundary conditions are shown in Fig. 2. The justifications of these conditions are as follows.
Boundary Conditions for AH Flow
It was assumed that there was only one inlet, i.e., the CB, and one outlet, i.e., the TM, for AH flow in the eye. On other boundary surfaces, the AH velocity was assumed to be zero, i.e., the no-slip and no-penetration condition. The AH velocity profile at the inlet surface was assumed to be parabolic
where \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {\upsilon }_{\text{CB}} \) is the AH velocity, Q aq is the AH production rate, A CB is the surface area of the inlet, and S is a dimensionless coordinate that varied from 0 to 1 in the z direction on the inlet surface. At the outlet surface, the viscous stresses were assumed to be zero
where \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {n} \) is the outward normal vector of the outlet surface.
Concentration Boundary Conditions
It is well known that only a small fraction of topically applied drugs can eventually reach intraocular tissues, mainly in the anterior chamber, and the rest is cleared with the tear. Therefore, we assumed that the pre-corneal ECA concentration was independent of the drug diffusion across the cornea. Three different cases of topical administration of ECA were simulated in the study. They corresponded to three different boundary conditions of the ECA concentration on the pre-corneal surface (C cor). In the first case, C cor was assumed to be a constant (C 0), which was an ideal situation with the half-life of ECA being infinite. This scenario will not happen exactly in patients but can exist approximately in short-term, drug delivery experiments by using controlled drug release technologies. This scenario was used in this study to predict the maximum amount of ECA that would be delivered to the TM for a given dose of ECA. If this amount of ECA was less than the minimum requirement for effective treatment of POAG, it would be in vain to improve topical delivery of ECA via any other approaches. The second case was the traditional eye drop-mediated drug delivery. To date, it has been limited by the rapid drug clearance due to the tear turnover.9 In previous studies, the drug clearance was observed to follow the first-order kinetics with the half-life of 1–3 min.29,40 The half-life of ECA was chosen to be 177.7 s in this study, based on the data in the literature.29 Thus,
where C 0 is the initial value of C cor. The third case was the sustained release of ECA from a Pluronic® copolymer film, consisting of 21% F127 and 10% F68. The drug release from the gel in an in vivo study also follows the first-order kinetics with a half-life of 30.3 ± 17.4 min.40 Therefore, the half-time of ECA was chosen to be 1818 s in this study and
In Eqs. (7) and (8), the coefficient in the exponential function was calculated as ln(2) divided by the corresponding half-life. Diffusion of ECA from the AH into the TM was negligible because it was slower than convection. At the steady state of convective transport, the concentration gradient is zero along streamlines of fluid, which was approximately perpendicular to the surface of the TM. Thus, we assumed
on the inner surface of the TM. ECA in the anterior chamber might diffuse into the lens and the vitreous body but the rates of diffusion were likely to be slow and thus were neglected in our model. The topically applied ECA could also diffuse into the subconjunctival space via conjunctiva and then diffuse into the sclera and the TM. However, data in the literature have shown that the half-life of small molecules in peri-ocular regions or more specifically in the subconjunctival space is on the order of 10 min after local injection, and the short half-life is presumably caused by considerable drug clearance via both blood and lymph microvessels.1,18,41,42 These data indicate that after topical application, the majority of ECA molecules are absorbed by the microvessels if they diffuse into the subconjunctival space via conjunctiva. Therefore, we neglected the diffusion of ECA from the subconjunctival space into the sclera. On the other hand, ECA in the sclera could also diffuse into the subconjunctival space before it could reach the TM, thereby reducing ECA delivery to the TM. The amount of reduction should be minimal, compared to the total amount of ECA delivered to the TM via the AH and the sclera. Thus, Eq. (9) was also applied to the outer surface of the sclera. Furthermore, the iris is a highly vascularized tissue, in which small molecules are absorbed rapidly by local microvessels and then removed from the tissue through blood microcirculation, which is similar to that in the subconjunctival space. Therefore, the concentration of ECA at the posterior side of the iris was assumed to be zero.
Initial Conditions
The initial drug concentration was set to zero in all regions involved in the simulation, i.e.
In addition, the initial concentration of ECA on the outer surface of the cornea, C 0, was chosen to be 75 μM, at which we observed in a preliminary study that 98% of primary porcine corneal epithelial cells could survive after they were treated with ECA for 6 h.22
Model Constants
The constants in the model were either estimated or obtained from the literature. The baseline values of these constants are shown in Table 2. The diffusion coefficients of ECA in the cornea and the sclera were determined experimentally in our previous studies.22,23 The experiments were performed at 4 °C in order to minimize tissue degradation. The diffusion coefficients of ECA in the iris and CB were unavailable. However, the structures of iris were similar to those of cornea, both contain multiple layers of epithelium (5–7 layers in the cornea and 2 layers in the iris). Thus, it was assumed that the diffusion coefficient in the iris was the same as that in the cornea. The diffusion coefficient in the CB was assumed to be the same as that in the sclera. The surface areas of TM and CB, facing the anterior chamber, were 18 mm2 14 and 32.6 mm2 (calculated from model geometry), respectively. The mass density ρ and the viscosity μ of AH at 37 °C were assumed to be 1000 kg m−3 and 7.5 × 10−4 kg m−1 s−1, respectively.14 In addition, it was estimated that the diffusion coefficient of ECA in the AH was 7.0 × 10−6 cm2/s based on our previous data.22,23 The IOP was assumed to be 2.1 kPa (16 mmHg), a normal level in patients. The rate of AH production was varied from 0.3 to 2.4 μL/min.
Numerical Methods and Simulation Procedures
Equations (1) through (4) were coupled together and thus solved simultaneously by using a finite element method. The simulations were performed with commercially available software, FEMLAB 3.1 (COMSOL, Inc., Burlington, MA, USA). The procedures included generating Lagrange-quadratic meshwork in all regions and solving the equations numerically. During the simulation, the adaptive time points were adjusted automatically by the time-dependent solver. The convergence of the simulation was tested by refining the meshes. It was observed that there were insignificant changes in simulation results when the number of meshes varied between 13,752 and 38,368. Thus, the final, refined meshwork consisted of 38,368 elements with 186,893 degrees of freedom. The relative tolerance and absolute tolerance were set at 0.01 and 0.001, respectively. The simulation was performed until the transport time reached 6 h.
Results
The steady-state flow field of the AH was simulated and the results are shown in Fig. 3, in which each vector indicates both direction and magnitude of the local velocity of AH. For the AH production rate (Q aq) of 2.4 μL/min, the maximum velocity of the AH was 0.2 mm/s, which occurred at the minimum distance between the iris and lens. The maximum velocity was two to three orders of magnitude higher than the average velocity in the anterior chamber. The pressure difference between the anterior and posterior chambers was on the order of 1 Pa, which was much smaller than the IOP (2100 Pa).
The spatial distributions of ECA concentration at 1 and 6 h are shown in Figs. 4a and 4b, respectively, for Q aq = 2.4 μL/min. The concentration gradient from the cornea to the anterior chamber could be clearly observed, indicating that the main barrier for topical drug delivery was the cornea. Local concentration of ECA in the TM (C TM) depended on Q aq and the concentration of ECA in the precorneal region (C cor). When C cor was maintained at 75 μM and Q aq was 2.4 μL/min, the corresponding values of C TM at 1 and 6 h were 14.0 and 24.1 μM, respectively. As illustrated in the figure, most ECA molecules applied topically accumulated in the cornea, and virtually no ECA could penetrate into the posterior chamber through the iris–lens junction due to the AH flow in the opposite direction. Only a small fraction of ECA could eventually reach the CB through diffusion in the sclera over a 6-h period (Fig. 4b). When Q aq was reduced to 0.3 μL/min and other model constants were unchanged, the distribution of ECA concentration in the anterior chamber is shown in Fig. 5 and the average concentration increased slightly compared with that when Q aq was 2.4 μL/min, suggesting that the average ECA concentration in the anterior chamber was not very sensitive to changes in the AH production rate in the range between 0.3 and 2.4 μL/min.
In addition to Q aq, ECA concentration in the eye depended on the half-life (t 1/2) of ECA in the precorneal region. For the simulation data shown in Figs. 4 and 5, t 1/2 was infinite, which was an ideal situation for maximum drug delivery into the eye. In experiments, t 1/2 is always finite and can be controlled by using different methods of drug administration. Two methods were considered and compared in this study. One was the direct application of a drop of ECA solution on the cornea surface and another was to slowly release ECA on the cornea surface using a Pluronic® block copolymer. The initial concentrations of ECA in both the eye drop and the polymeric gel were 75 μM. The simulated C TM, which had been normalized by 75 μM, are shown in Fig. 6. The dotted line shown in Fig. 6 indicates the minimum effective C TM, which was chosen to be 10 μM because Liang et al. have shown that TM outflow facility in excised human eyes is increased by 28% after the eyes are perfused with ECA solution at 10 μM for 1 h.21
Time-dependent changes in ECA concentration at the TM. It depended on the concentration of ECA in the precorneal region (C cor) and the rate of AH production (Q aq). (●) C cor = 75 μM and Q aq = 2.4 μL/min; (○) C cor = 75 μM and Q aq = 0.3 μL/min. (▲ and Δ) ECA was released from the Pluronic® copolymer film with C 0 = 75 μM and Q aq = 2.4 μL/min (▲) or 0.3 μL/min (Δ). (♦ and ◊) ECA was administered as an eye drop with C 0 = 75 μM and Q aq = 2.4 μL/min (♦) or 0.3 μL/min (◊). The dotted line indicates the minimum effective concentration of ECA at the TM, which was 10 μM in this study. The diffusion coefficients used were determined at 4 °C. All ECA concentrations were normalized by 75 μM
When t 1/2 was infinite, C TM increased with time in a biphasic manner. The increase was rapid initially and then became gradual, presumably due to the reduction in the concentration gradient across the cornea. C TM reached the minimal effective concentration (10 μM) at 40 and 50 min for Q aq to be 2.4 and 0.3 μL/min, respectively. When ECA was administered via an eye drop, C TM was a bell-shaped curve with the peak value at 1.4 μM for Q aq = 2.4 μL/min. The peak occurred at approximately 20 min after the eye drop application. Most ECA molecules applied were cleared from the external surface of the cornea before they had a chance to diffuse into the cornea. When ECA was delivered via the Pluronic® block copolymer, the levels of C TM were significantly higher than those after the eye drop application for both values of Q aq. More specifically, the peak values of C TM were 7.5 and 6.0 μM for Q aq being 2.4 and 0.3 μL/min, respectively. They were reached at approximately 1 h after the drug loaded polymer was applied. It was also observed that C TM decreased slightly with reducing the AH production rate (see Fig. 6).
The diffusion coefficient of ECA used in this study was measured at 4 °C instead of 37 °C in order to minimize tissue degeneration during the measurement. On the other hand, it is well known that the diffusion coefficient increases with increasing the temperature. According to the Stokes–Einstein equation, the diffusion coefficient is proportional to the ratio of absolute temperature and fluid viscosity. When the temperature is increased from 4 to 37 °C, the viscosity of water is decreased by ~56%.3 Thus, the diffusion coefficient is increased by a factor of 2.5. The temperature-dependence of the diffusion coefficient suggested that the ECA transport was underestimated in the simulated results shown in Figs. 4–6. To determine the extent of underestimation, we increased all diffusion coefficients of ECA shown in Table 2 by a factor of 2.5, and repeated the simulations. The results for Q aq at 2.4 μL/min are shown in Fig. 7, which indicated that the concentration of ECA in the TM was insensitive to changes in the diffusion coefficient. Therefore, the predicted concentrations of ECA shown in Figs. 4–6 were also approximately valid at 37 °C. In addition, the data suggested that the dominant mode of ECA transport in the anterior chamber was convection, except in the region near the surfaces of the surrounding tissues where the AH velocity was close to zero and diffusion was the dominant mode of transport.
Dependence of ECA concentration at the TM on time and diffusion coefficients. The diffusion coefficients at 4 °C (i.e., D 4C) were measured experimentally whereas the diffusion coefficients at 37 °C (i.e., D 37C) were calculated, which were equal to 2.5 times those at 4 °C. (●) C cor = 75 μM and D = D 4C; (○) C cor = 75 μM and D = D 37C. (▲ and Δ) ECA was released from the Pluronic® copolymer film with C 0 = 75 μM and D = D 4C (▲) or D 37C (Δ). (♦ and ◊) ECA was administered as an eye drop with C 0 = 75 μM and D = D 4C (♦) or D 37C (◊). The dotted line indicates the minimum effective concentration of ECA in the TM, which was 10 μM. All ECA concentrations were normalized by 75 μM and the AH production rate was maintained at 2.4 μL/min
Discussion
The objective of this study was to develop a mathematical model to evaluate different strategies for improving ECA delivery into the TM via topical application. The local concentration of ECA in the TM (C TM) depended on several key factors, including the AH production rate (Q aq), the diffusion coefficient of ECA, and the half-life of ECA (t 1/2) in the precorneal region. The simulated data indicated that the most important factor for determining C TM was t 1/2. The diffusion coefficient of ECA and Q aq could affect the transport of ECA in the eye but had only minor effects on C TM.
In the clinic, an ocular drug applied topically is often administered at a concentration that is at least 100 times higher than the minimal threshold concentration of the drug in target tissues in order to achieve effective treatment. For instance, the minimal threshold concentration of ECA in the TM is on the order of 10 μM,21 whereas the concentration of ECA in eye drops has to be between 16.5 and 100 mM in order to reduce the IOP.31,36 It has been estimated that less than 5% of a topically applied drug will eventually reach its target in the anterior chamber. The poor ocular bioavailability is caused by physiological barriers to drug transport, chemical inactivation of the drug, and rapid clearance of the drug in the precorneal region. If the target tissue is located in the posterior region of the eye (i.e., retina), it will be more difficult to reach it by administering drugs topically since the drug penetration into the posterior chamber and the vitreous is minimal.8 Our simulation results were in a good agreement with these observations. The maximum concentration of ECA in the TM after topical application of eye drops was <2% of that in the pre-corneal region (see Figs. 6 and 7) and virtually no ECA could penetrate into the posterior chamber through the iris–lens junction (see Figs. 4 and 5), due to the AH flow from posterior to anterior chambers. Only a small fraction of ECA could eventually reach the CB by diffusion through the sclera. Therefore, other strategies have to be developed to deliver drugs to the posterior region of the eye.2,19
In order to improve ECA delivery into the TM after it is applied topically, it is critical to reduce the drug clearance from the precorneal region or increase the drug half-life in this region as suggested by the simulation data. One approach to achieving it is to apply the drug at the night time since the clearance rate during sleep is lower than that during the day. As a result, it is possible that the eye drop applied at the night time is more effective than the sustained release of ECA from the Pluronic® copolymer film applied during the day because of the potential increase in the half-life of ECA in the precorneal region. Another approach is to increase the viscosity of drug solution or load the drug into polymeric gels. However, it is difficult to topically apply drugs formulated in highly viscous solution or gels by patients at home. Thus, an ideal eye drop product should be convenient to use and have a long residence time on the cornea. One formulation strategy for developing such products is based on environment-sensitive polymers,12,27,34,35,37,40 such as the temperature-sensitive Pluronic® block copolymer with 21% F127 and 10% F68. This copolymer is a liquid at low temperature and undergoes a rapid phase transition at 26.5 °C.40 Thus, Pluronic® block copolymer prepared at a low temperature (<20 °C) is in a liquid form with low viscosity and it will undergo phase transition to become a gel film after topical administration onto the cornea. The t 1/2 of drug in this formation (i.e., ~30 min) is much longer than that in traditional eye drops (<3 min), thereby leading to >5-fold increase in the peak value of C TM. It is expected that t 1/2 and thus C TM can be further increased through innovative design of drug delivery systems. The increase in t 1/2 may also allow reducing the dose of ECA, and thus the corneal toxicity of the drug, without compromising its therapeutic efficacy.
Comparison of the simulation results shown in Figs. 4 and 5 revealed that the ECA concentration was increased in the anterior chamber but decreased in the TM when Q aq was decreased from 2.4 to 0.3 μL/min. Qualitatively, the effects of Q aq on C TM were independent of the half-life of ECA in the precorneal region since C TM in all cases was decreased when Q aq was reduced although the amount of decrease was small (see Fig. 6). These observations could be explained by considering the coupling between ECA delivery into the anterior chamber through diffusion across the cornea and ECA clearance from the anterior chamber through both convection into the TM and diffusion into the surrounding tissues of the anterior chamber. When Q aq was decreased by a factor of 8, it would cause a significant reduction in the AH velocity in the anterior chamber and an 8-fold reduction in the rate of AH flow into the TM, which in turn would decrease the rate of ECA clearance through the TM and allow ECA to accumulate in the anterior chamber. The accumulation would (i) impair ECA diffusion across the cornea, due to the reduction in driving force, and (ii) enhance ECA diffusion into surrounding tissues of the anterior chamber and thus ECA clearance through tissues other than the TM.
In summary, an axisymmetric model was developed to simulate transport of ECA within the anterior chamber and its surrounding tissues in a human eye. The simulation results suggested that the most efficient approach to improving topical drug delivery to the TM was to prolong the t 1/2 of ECA in the precorneal region, which could be a design criterion for future controlled release devices. The model presented in this paper might also be useful for optimizing delivery of other therapeutic agents in the anterior segment of the eye.
Abbreviations
- ECA:
-
Ethacrynic acid
- AH:
-
Aqueous humor
- TM:
-
Trabecular meshwork
- CB:
-
Ciliary body
- POAG:
-
Primary open-angle glaucoma
- IOP:
-
Intraocular pressure
References
Amrite, A. C., H. F. Edelhauser, and U. B. Kompella. Modeling of corneal and retinal pharmacokinetics after periocular drug administration. Invest. Ophthalmol. Vis. Sci. 49:320–332, 2008.
Aukunuru, J. V., G. Sunkara, S. P. Ayalasomayajula, J. DeRuiter, R. C. Clark, and U. B. Kompella. A biodegradable injectable implant sustains systemic and ocular delivery of an aldose reductase inhibitor and ameliorates biochemical changes in a galactose-fed rat model for diabetic complications. Pharm. Res. 19:278–285, 2002.
Bird, R. B., W. E. Stewart, and E. N. Lightfoot. Transport Phenomena. New York: John Wiley and Sons, Inc., 2002.
Chalita, M. R., Y. Li, S. Smith, C. Patil, V. Westphal, A. M. Rollins, J. A. Izatt, and D. Huang. High-speed optical coherence tomography of laser iridotomy. Am. J. Ophthalmol. 140:1133–1136, 2005.
Dobler, B., and R. Bendl. Precise modelling of the eye for proton therapy of intra-ocular tumours. Phys. Med. Biol. 47:593–613, 2002.
Epstein, D. L., L. B. Hooshmand, and M. P. Epstein. Thiol adducts of ethacrynic acid increase outflow facility in enucleated calf eyes. Curr. Eye Res. 11:253–258, 1992.
Erickson-Lamy, K., A. Schroeder, and D. L. Epstein. Ethacrynic acid induces reversible shape and cytoskeletal changes in cultured cells. Invest. Ophthalmol. Vis. Sci. 33:2631–2640, 1992.
Geroski, D. H., and H. F. Edelhauser. Drug delivery for posterior segment eye disease. Invest. Ophthalmol. Vis. Sci. 41:961–964, 2000.
Geroski, D. H., and H. F. Edelhauser. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 52:37–48, 2001.
Gills, J. P., B. C. Roberts, and D. L. Epstein. Microtubule disruption leads to cellular contraction in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 39:653–658, 1998.
Grant, W. Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthalmol. 69:783–801, 1963.
He, C., S. W. Kim, and D. S. Lee. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control Release 127:189–207, 2008.
Heys, J. J., and V. H. Barocas. A boussinesq model of natural convection in the human eye and the formation of Krukenberg’s spindle. Ann. Biomed. Eng. 30:392–401, 2002.
Heys, J. J., V. H. Barocas, and M. J. Taravella. Modeling passive mechanical interaction between aqueous humor and iris. J. Biomech. Eng. 123:540–547, 2001.
Kass, M. A., D. K. Heuer, E. J. Higginbotham, C. A. Johnson, J. L. Keltner, J. P. Miller, R. K. Parrish, 2nd, M. R. Wilsonm, and M. O. Gordon. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120:701–713, 2002; discussion 829–830.
Keister, J. C., E. R. Cooper, P. J. Missel, J. C. Lang, and D. F. Hager. Limits on optimizing ocular drug delivery. J. Pharm. Sci. 80:50–53, 1991.
Keister, J. C., P. S. Heidmann, and P. J. Missel. Transient analysis of ocular drug delivery: zero-volume effect. J. Pharm. Sci. 86:1040–1045, 1997.
Kim, S. H., K. G. Csaky, N. S. Wang, and R. J. Lutz. Drug elimination kinetics following subconjunctival injection using dynamic contrast-enhanced magnetic resonance imaging. Pharm. Res. 25:512–520, 2008.
Kompella, U. B., N. Bandi, and S. P. Ayalasomayajula. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest. Ophthalmol. Vis. Sci. 44:1192–1201, 2003.
Kumar, S., S. Acharya, R. Beuerman, and A. Palkama. Numerical solution of ocular fluid dynamics in a rabbit eye: parametric effects. Ann. Biomed. Eng. 34:530–544, 2006.
Liang, L. L., D. L. Epstein, A. W. de Kater, A. Shahsafaei, and K. A. Erickson-Lamy. Ethacrynic acid increases facility of outflow in the human eye in vitro. Arch. Ophthalmol. 110:106–109, 1992.
Lin, C. W. Drug delivery to the eye for treatment of primary open-angle glaucoma. Ph.D. Thesis in Department of Biomedical Engineering, Duke University, Durham, NC, 2006.
Lin, C. W., Y. Wang, P. Challa, D. L. Epstein, and F. Yuan. Transscleral diffusion of ethacrynic acid and sodium fluorescein. Mol. Vis. 13:243–251, 2007.
Melamed, S., R. Kotas-Neumann, A. Barak, and D. L. Epstein. The effect of intracamerally injected ethacrynic acid on intraocular pressure in patients with glaucoma. Am. J. Ophthalmol. 113:508–512, 1992.
O’Brien, E. T., M. Kinch, T. W. Harding, and D. L. Epstein. A mechanism for trabecular meshwork cell retraction: ethacrynic acid initiates the dephosphorylation of focal adhesion proteins. Exp. Eye Res. 65:471–483, 1997.
O’Donnell, M. E., J. D. Brandt, and F. R. Curry. Na-K-Cl cotransport regulates intracellular volume and monolayer permeability of trabecular meshwork cells. Am. J. Physiol. 268:C1067–C1074, 1995.
Qiu, Y., and K. Park. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 53:321–339, 2001.
Sabah, J. R., H. Davidson, E. N. McConkey, and L. Takemoto. In vivo passage of albumin from the aqueous humor into the lens. Mol. Vis. 10:254–259, 2004.
Scuderi, A. C., A. De Lazzari, F. Miano, and P. Zola. Residence time of netilmicin in tears. Cornea 21:48–50, 2002.
Shimazaki, A., M. Ichikawa, P. V. Rao, T. Kirihara, K. Konomi, D. L. Epstein, and H. Hara. Effects of the new ethacrynic acid derivative SA9000 on intraocular pressure in cats and monkeys. Biol. Pharm. Bull. 27:1019–1024, 2004.
Tingey, D. P., R. R. Ozment, A. Schroeder, and D. L. Epstein. The effect of intracameral ethacrynic acid on the intraocular pressure of living monkeys. Am. J. Ophthalmol. 113:706–711, 1992.
Tingey, D. P., A. Schroeder, M. P. Epstein, and D. L. Epstein. Effects of topical ethacrynic acid adducts on intraocular pressure in rabbits and monkeys. Arch. Ophthalmol. 110:699–702, 1992.
Wang, Y., P. Challa, D. L. Epstein, and F. Yuan. Controlled release of ethacrynic acid from poly(lactide-co-glycolide) films for glaucoma treatment. Biomaterials 25:4279–4285, 2004.
Wang, Y., J. K. Hu, A. Krol, Y. P. Li, C. Y. Li, and F. Yuan. Systemic dissemination of viral vectors during intratumoral injection. Mol. Cancer Ther. 2:1233–1242, 2003.
Wang, Y., S. Liu, C. Y. Li, and F. Yuan. A novel method for viral gene delivery in solid tumors. Cancer Res. 65:7541–7545, 2005.
Wang, R. F., S. M. Podos, J. B. Serle, P. Y. Lee, A. H. Neufeld, and R. Deschenes. Effects of topical ethacrynic acid ointment vs timolol on intraocular pressure in glaucomatous monkey eyes. Arch. Ophthalmol. 112:390–394, 1994.
Wang, Y., and F. Yuan. Delivery of viral vectors to tumor cells: extracellular transport, systemic distribution, and strategies for improvement. Ann. Biomed. Eng. 34:114–127, 2006.
Watson, P. G., and R. D. Young. Scleral structure, organisation and disease. A review. Exp. Eye Res. 78:609–623, 2004.
Wax, M. B., C. B. Camras, R. G. Fiscella, C. Girkin, K. Singh, and R. N. Weinreb. Emerging perspectives in glaucoma: optimizing 24-hour control of intraocular pressure. Am. J. Ophthalmol. 133:S1–S10, 2002.
Wei, G., H. Xu, P. T. Ding, S. M. Li, and J. M. Zheng. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J. Control Release 83:65–74, 2002.
Weijtens, O., E. J. Feron, R. C. Schoemaker, A. F. Cohen, E. G. Lentjes, F. P. Romijn, and J. C. van Meurs. High concentration of dexamethasone in aqueous and vitreous after subconjunctival injection. Am. J. Ophthalmol. 128:192–197, 1999.
Yu, D. Y., W. H. Morgan, X. Sun, E. N. Su, S. J. Cringle, P. K. Yu, P. House, W. Guo, and X. Yu. The critical role of the conjunctiva in glaucoma filtration surgery. Prog. Retin. Eye Res. 28:303–328, 2009.
Zhang, W., M. R. Prausnitz, and A. Edwards. Model of transient drug diffusion across cornea. J. Control Release 99:241–258, 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Gerald Saidel oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Lin, CW., Yuan, F. Numerical Simulations of Ethacrynic Acid Transport from Precorneal Region to Trabecular Meshwork. Ann Biomed Eng 38, 935–944 (2010). https://doi.org/10.1007/s10439-010-9947-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9947-z