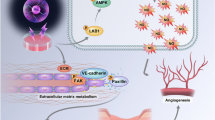
Abstract
Non-thermal dielectric barrier discharge plasma is being developed for a wide range of medical applications, including wound healing, blood coagulation, and malignant cell apoptosis. However, the effect of non-thermal plasma on the vasculature is unclear. Blood vessels are affected during plasma treatment of many tissues and may be an important potential target for clinical plasma therapy. Porcine aortic endothelial cells were treated in vitro with a custom non-thermal plasma device. Low dose plasma (up to 30 s or 4 J cm−2) was relatively non-toxic to endothelial cells while treatment at longer exposures (60 s and higher or 8 J cm−2) led to cell death. Endothelial cells treated with plasma for 30 s demonstrated twice as much proliferation as untreated cells five days after plasma treatment. Endothelial cell release of fibroblast growth factor-2 (FGF2) peaked 3 h after plasma treatment. The plasma proliferative effect was abrogated by an FGF2 neutralizing antibody, and FGF2 release was blocked by reactive oxygen species scavengers. These data suggest that low dose non-thermal plasma enhances endothelial cell proliferation due to reactive oxygen species mediated FGF2 release. Plasma may be a novel therapy for dose-dependent promotion or inhibition of endothelial cell mediated angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-thermal dielectric barrier discharge (DBD) plasma has recently emerged as a novel tool in medicine. DBD occurs at atmospheric pressure in air or other gases when high voltage of sinusoidal waveform or short duration pulses is applied between two electrodes, with at least one electrode being insulated.10,35 The insulator prevents current build-up between the electrodes, creating electrically safe plasma without substantial gas heating. This approach allows direct treatment of biological systems without the thermal damage observed in conventional thermal plasma.38 Non-thermal plasma can kill bacteria or induce apoptosis in malignant cells.2,15,17,27 It can be applied in sub-lethal doses for gene transfection,7,8 cell detachment,26,27 wound healing,2,21,34 and blood coagulation.2,25 In recent studies of plasma blood coagulation2,25 and bacteria deactivation,2,15 plasma did not demonstrate measurable toxicity in the surrounding living tissue.2,7
Non-thermal plasmas can be used in medicine for either direct or indirect treatment.15 Plasma is composed of charged particles (electrons, ions), electronically excited atoms and molecules, radicals, and ultraviolet photons. Both direct and indirect plasma expose cells or the tissue surface to short and long lived neutral atoms and molecules, including ozone (O3), NO, OH radicals, and singlet oxygen (O2 1Δg). However, direct plasma allows a significant charged particle flux, including electrons and positive and negative ions like superoxide radicals O2 −, to reach the surface. Non-thermal plasma temperature and composition can be changed to control plasma products.
Non-thermal plasma interaction with the vasculature must be understood prior to treating vascularized tissue. We hypothesize that plasma can grow or regress blood vessels in a dose-dependent manner. Endothelial cells control many aspects of the vasculature from vascular tone to coagulation to inflammation. Endothelial cells also play a guiding role in angiogenesis.13 Endothelial cells produce and secrete angiogenic growth factors such as fibroblast growth factor-2 (FGF2), which in conjunction with many other signals induces cells to invade the surrounding tissue, proliferate, and develop into new blood vessels.32 Angiogenesis can be both helpful and harmful. In wound healing, angiogenesis is required at the wound site for healing, whereas in cancer, angiogenesis blockade may prevent tumor growth.12
Using an in vitro model, we investigated the effect of non-thermal DBD plasma on endothelial cells. Endothelial cell proliferation and death following plasma were measured. FGF2 release from endothelial cells and its effect on cell proliferation were quantified. Finally, mechanisms of non-thermal plasma effects were explored. We now show that while high dose non-thermal plasma induces endothelial cell death, lower doses induce endothelial cell proliferation. This proliferative effect is likely related to FGF2 release due to plasma-produced reactive oxygen species.
Methods
Endothelial Cell Culture
Porcine aortic endothelial cells (PAEC) were isolated from porcine aortae by the collagenase dispersion method and used between passages 4 and 9.40 PAEC were maintained in low glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech) with 5% fetal bovine serum (Hyclone), 1% L-glutamine, and 1% penicillin-streptomycin (Invitrogen). Medium was changed every two days. For plasma treatment, cells were washed with phosphate buffered saline, detached with 0.1% trypsin (Invitrogen), and seeded near confluence (4 × 105 cells per well) on 18 mm uncoated glass cover slips (VWR) in 12-well plates (Corning). PAEC adhere well to bare glass, with close to 100% seeding efficiency. Cells were incubated for 24 h prior to plasma treatment in 1.5 mL supplemented medium at 37 °C and 5% CO2.
Porcine tumor necrosis factor-α (TNF-α) was from R&D Systems. Recombinant human FGF2 was from Peprotech, and neutralizing FGF2 antibody was from Upstate Biotechnology. N-Acetyl-L-cysteine (NAC, Sigma), an intracellular reactive oxygen species (ROS) scavenger and sodium pyruvate (Sigma), an extracellular ROS scavenger, were used to block plasma-produced ROS.
Endothelial Cell Plasma Treatment
Non-thermal DBD plasma was produced using the device in Fig. 1 (Fig. 1a shows the device schematic and Fig. 1b shows the actual device).2,17 Plasma was generated by applying alternating polarity pulsed (500 Hz to 1.5 kHz) voltage of ~20 kV magnitude (peak-to-peak) between the insulated high voltage electrode and the sample using a variable voltage and frequency power supply (Quinta). 1 mm thick clear quartz was the insulating dielectric barrier covering the 1 in. diameter copper electrode. The discharge gap between quartz and the sample was fixed at 2 mm. The pulse waveform was 20 kV, 1.65 μs width, with rise time 5 V ns−1 (Fig. 1c). Discharge power density was 0.13 W cm−2 (500 Hz) and 0.31 W cm−2 (1.5 kHz) using electrical characterization and a custom calorimetric system.1 The plasma treatment dose in J cm−2 was calculated by multiplying the plasma discharge power density by the plasma treatment duration. The non-thermal DBD plasma has a g-factor (number of ROS generated per electron volt or eV) of between 0.3 and 0.5.14 For a plasma dose of 3.9 J cm−2, 7.32 × 1016–1.22 × 1017 ROS are generated. The values for specific plasma parameters are provided in Fig. 1d.
PAEC on glass cover slips were exposed to plasma for 5–120 s. Each cover slip was removed from the 12-well plate and placed on a microscope slide, which was positioned on the plasma device grounded base. 50 μL serum free medium was added to the sample to prevent drying. Following plasma, the cover slip was immediately placed in a new 12-well plate, 1.5 mL supplemented medium was added, and the samples were returned to the incubator.
Three approaches were used for plasma-treatment of cells: direct, indirect and separated. In direct treatment, the sample was one of the electrodes creating the plasma (Figs. 1a and 1b). Plasma discharge occurred between the quartz and the sample, which exposed the sample directly to neutral reactive species and charged particles. For indirect treatment, a grounded mesh was placed between the high voltage electrode and the sample to eliminate charged particles. In separated plasma treatment, medium was plasma treated separately from cells and then immediately applied to cells. In this case, cells were not in direct contact with any plasma component.
Non-Thermal Plasma Induced Cell Death
Non-thermal plasma endothelial cell cytotoxicity was measured via cell counts and Live/Dead assay. For cell counts, PAEC were plasma treated as described. Three and twenty-four hours following plasma treatment, attached (live) cells were trypsinized and counted using a Coulter counter (Beckman Coulter). These time points were selected to examine immediate and medium-term plasma toxicity effects. Since no change was detected between 3 and 24 h, no longer time points were investigated. For the Live/Dead assay, 3 and 24 h post treatment cells were labeled with 1 μM ethidium homodimer and 0.25 μM calcein (Invitrogen), incubated at room temperature for 45 min, and imaged by fluorescent microscopy (Olympus, USA) with a digital high performance CCD camera (Diagnostic Instruments). Live cells convert cell-permeant calcein to a FITC fluorescent form via intracellular esterases, whereas cell impermeant ethidium homodimer binds nucleic acids in membrane damaged dead cells to enhance TRITC fluorescence. Dead cells were manually counted in five distinct sample areas.
Endothelial cell apoptosis was measured via annexin V-propidium iodide labeling. Annexin V binds phosphatidylserine translocated from the inner to the outer cell membrane. Cells in early apoptosis are identified as annexin V-positive and propidium iodide-negative. PAEC were prepared by combining floating and trypsin-released attached cells. Samples were centrifuged to pellet cells, washed thoroughly, resuspended in annexin binding buffer, and labeled with annexin V-fluorescein and propidium iodide as per manufacturer instructions (BD Pharmingen). Samples were analyzed immediately by flow cytometry (BD FACScanto).
Endothelial Cell Membrane Damage and FGF2 Release
Endothelial cell membrane damage following non-thermal plasma was quantified through lactase dehydrogenase (LDH) release. PAEC were plasma treated as described, however DMEM without sodium pyruvate was used since sodium pyruvate interferes with the LDH assay. TNF-α (10 ng mL−1) was the positive control. 0.5 mL conditioned medium was collected 2, 4, 6, 8, 12, and 24 h after plasma, and LDH was quantified using the Cytotox-ONE Homogeneous Membrane Integrity Assay (Promega) as per manufacturer instructions. FGF2 release from plasma treated cells was measured in collected medium 0.5 to 24 h after plasma treatment. FGF2 levels were quantified via FGF ELISA (R&D Systems).
Non-Thermal Plasma Induced Cell Proliferation
Endothelial cell proliferation was measured through cell counts and BrdU incorporation on treated cells or using conditioned medium. For cell counts, 10,000 PAEC were seeded on coverslips and plasma treated as described. Cell number was quantified on days 1, 3, 5, and 7 by counting trypsin-detached cells using a Coulter counter, with medium changes on days 2, 4, and 6. For directly treated cells, fold proliferation was determined by comparing cell number on day five to day one. For conditioned medium, confluent PAEC were plasma treated as described and incubated for 3 h in 1 mL serum-free DMEM. Conditioned medium was collected and centrifuged to remove dead cells. 0.5 mL conditioned medium, along with 1 mL supplemented medium, was added to subconfluent PAEC (10,000 cells per well) on days 2, 4, and 6 and cell proliferation was assessed. Conditioned medium from untreated cells and serum-free medium were controls. FGF2 effects were blocked by pre-incubating conditioned medium with FGF2 neutralizing antibody (1 μg mL−1) for 30 min prior to adding it to cells.
DNA synthesis induced by plasma-treated cell conditioned medium was determined by BrdU incorporation. Thymidine analogue 5-bromo-2-deoxyuridine (BrdU) is incorporated instead of thymidine into newly synthesized DNA. 10,000 cells per well were seeded in a 96-well plate in supplemented medium. Conditioned medium was collected from plasma-treated cells as described and added to each well in a 1:2 ratio with supplemented medium. After 18 h, 20 μL BrdU labeling solution (Chemicon) was added to each well for 3 h. Cells were fixed and incubated with anti-BrdU conjugated with peroxidase. The optical density (450/570), which was directly proportional to DNA synthesis level, was determined using a microplate reader (TECAN).
Statistical Analysis
Statistical analyses were performed with Prism software (Graphpad). Data were normally distributed and expressed as the mean ± SD. Comparisons between two groups were analyzed by Student’s t test, and comparisons between more than two groups were analyzed by ANOVA. A value of p ≤ 0.05 was considered statistically significant and is indicated with a pound sign (#). p ≤ 0.01 is indicated with an asterisk (*).
Results
Endothelial Cell Proliferation in Response to Non-Thermal Plasma
Endothelial cell proliferation was enhanced by low dose non-thermal plasma treatment. Five days after treatment, cells treated with plasma showed greater viable cell number than control up to 30 s of plasma. PAEC exposed to 30 s of plasma demonstrated twice as many viable cells as untreated controls (Fig. 2a). However, plasma beyond 30 s decreased cell number. A similar increase in cell number with 30 s of plasma treatment was observed for cells grown on collagen coated coverslips (data not shown). To determine if increased cell number was related to a cell-secreted soluble factor, PAEC were incubated in conditioned medium from untreated or plasma treated cells (3.9 J cm−2, 30 s) (Fig. 2b). Serum-free media, which does not contain soluble growth factors, was the negative control. Plasma dose was selected based on maximal observed effect. Viable cell number was twice as high in cells incubated with plasma-treated cell conditioned medium on days 3, 5, and 7 compared to cells incubated with untreated cell conditioned medium.
Plasma induces endothelial cell proliferation by direct treatment and through conditioned medium from treated cells. (a) PAEC were plasma treated on day 0, and counted on days 1 and 5, with medium changes on days 1 and 3. Data are presented as fold change, since plasma leads to some cell death on day 1. * p < 0.01 compared to untreated cells. (b) Conditioned medium was collected after 3 h from untreated or plasma-treated cells and applied to untreated PAEC. Serum-free media, which does not contain soluble growth factors, was the negative control. Cell number was counted with a Coulter counter. * p < 0.01 compared to day 1; # p < 0.01 comparing untreated cells with 30 s plasma
Endothelial Cell Death in Response to Non-Thermal Plasma
Decreased viable cell number was observed at high non-thermal plasma levels; therefore we investigated endothelial cell death in response to plasma. Plasma was relatively non-toxic to PAEC up to 60 s. While the number of live, attached cells decreased as plasma exposure increased, more than 75% of cells remained viable up to 60 s plasma (Fig. 3a). There was no significant difference in cell viability 3 and 24 h following plasma exposure, suggesting no long term plasma toxicity effects. A Live/Dead assay was used to confirm cell count results. Endothelial cells treated with plasma for short exposure times (up to 30 s) showed few dead cells (Fig. 3b, quantified in Fig. 3c), confirming that plasma is relatively non-toxic at short exposures. Dead cell number increased with increasing plasma exposure time (p < 0.01 by ANOVA). While dead cell number increased slightly with 60 s plasma, at 120 s a significant number of dead cells and few live cells were evident. This extensive cytotoxicity may be related to sample drying. Therefore, 120 s plasma was not used for subsequent assays.
Low dose plasma is relatively non-toxic to cells, but high dose induces apoptotic cell death. (a) Attached cells, confirmed as alive by Trypan blue, were counted 3 and 24 h after plasma (p < 0.01 by ANOVA). (b) Endothelial cell death was measured by Live/Dead assay. Live cells appear green, whereas dead cells appear red. Scale bar is 200 μm. (c) Quantified Live/Dead images (n = 5). (d) Apoptosis was measured by Annexin V-propidium iodide 24 h after plasma
To determine the endothelial cell death mechanism induced by plasma, PAEC were analyzed 24 h post-plasma for apoptosis (Fig. 3d). Apoptosis increased with plasma treatment (p < 0.01 by ANOVA). At 30 and 60 s plasma, 20% of cells were apoptotic compared to 10% of untreated cells. At 120 s, nearly 60% of cells were apoptotic. These data confirm that shorter plasma exposures are non-toxic, and apoptosis is one mechanism of plasma-induced cell death.
Endothelial Cell FGF2 Release Post Plasma
We next considered whether FGF2 was released from endothelial cells following plasma, and whether released FGF2 contributed to enhanced cell proliferation. FGF2 has no signal sequence for secretion, and therefore is primarily thought to be released during sub-lethal cell membrane damage. Cell-released FGF2 increased up to 3 h after plasma treatment (3.9 J cm−2, 30 s) and then rapidly decreased up to 24 h after plasma (Fig. 4a). In contrast, FGF2 levels for cells treated with 10 ng mL−1 TNF-α as a positive control rose more slowly but continued to rise up to 24 h. Endothelial cell membrane damage was assessed by LDH release following plasma. Medium LDH increased significantly by 4 h after plasma and continued to rise throughout the first 24 h (Fig. 4b, p < 0.01 by ANOVA), comparable to TNF-α positive control.
Endothelial cells release FGF2 after plasma, and cell-released FGF2 enhances proliferation. (a) FGF2 was quantified in cell medium after plasma by ELISA. Inset shows medium FGF2 up to 3 h after treatment. (b) LDH release was measured in cell medium post-plasma. (c) FGF2 effects were blocked by incubating plasma-treated cell conditioned medium with FGF2 neutralizing antibody. (d) FGF2 blockade reduced DNA synthesis in response to plasma-treated cell conditioned medium, measured by BrdU incorporation. Serum free media, which does not contain FGF2, was the negative control
The role of released FGF2 in plasma-enhanced endothelial cell proliferation was investigated by treating conditioned medium from plasma-treated cells with an FGF2 neutralizing antibody to block FGF2 effects. Serum free media, which does not contain FGF2, was the negative control. The FGF2 neutralizing antibody significantly suppressed proliferation in PAEC exposed to plasma-treated cell conditioned medium (Fig. 4c). Viable cell number for samples with FGF2 blocked was similar to untreated cell conditioned medium. These data were confirmed with a BrdU assay (Fig. 4d). BrdU incorporation was enhanced for cells incubated in plasma-treated cell conditioned medium, but the FGF2 neutralizing antibody abrogated the effect. These data suggest that plasma leads to FGF2 release, which contributes to enhanced endothelial cell proliferation following plasma.
Mechanism of Release of FGF2 from Endothelial Cells Following Plasma Exposure
Plasma produces neutral short and long lived reactive species and charged particles like ions and electrons, yet which plasma component led to endothelial cell FGF2 release was unknown. To better understand FGF2 release mechanisms, PAEC were exposed to plasma directly, indirectly, or in a separated configuration. There was no statistically significant difference in FGF2 release between direct and indirect treatment (Fig. 5a). Both direct and indirect cell plasma treatment induced significantly greater endothelial cell FGF2 release as compared to separated treatment, however separated treatment still produced significantly more FGF2 release than untreated control.
Endothelial cell FGF2 release is linked to neutral ROS (a) PAEC were plasma treated directly, with a grounded mesh to remove charged particles (indirect), or medium was plasma treated and then applied to cells (separated). Medium FGF2 was measured by ELISA 3 h after plasma. # p < 0.05 compared to direct (b) PAEC were pretreated with 4 mM (NAC, intracellular) or 10 mM sodium pyruvate (SP, extracellular), or both ROS scavengers (NSP). Samples were directly plasma treated, and cells pretreated with ROS scavengers were compared to cells directly treated with plasma alone (D). Medium FGF2 was quantified by FGF ELISA 3 h after plasma
Non-thermal plasma produces large amounts of ROS. These ROS may interact with endothelial cells, leading to FGF2 release. To determine the role of ROS in plasma-induced cell FGF2 release, endothelial cells were pre-incubated in 4 mM NAC (intracellular ROS) and then plasma-treated in supplemented medium with or without 10 mM sodium pyruvate (extracellular ROS). Both NAC and sodium pyruvate significantly suppressed FGF2 release from plasma treated cells (Fig. 5b), suggesting that intracellular and extracellular ROS may contribute to plasma effects.
Discussion
Non-thermal plasma interacts with the vasculature during tissue treatment, and plasma may be able to induce dose-dependent blood vessel growth and regression. We now show that high dose plasma induces endothelial cell death, whereas low dose enhances endothelial cell proliferation. The plasma proliferative effect is likely related to sub-lethal cell membrane damage by ROS, which leads to FGF2 release. FGF2, together with vascular endothelial growth factor and other critical signals, induces endothelial cell proliferation, migration, and tube formation.32 Angiogenesis is a complicated process, and endothelial cell proliferation plays only a small initial role.13 However, our data suggest that low-dose plasma could promote angiogenesis to accelerate wound healing, whereas high-dose plasma could inhibit angiogenesis to prevent cancer growth. In cases where plasma cannot be used directly, fluid from plasma treated cells could also be used.
While FGF2 alone will likely not complete the angiogenic process, additional subsequent plasma treatments could be tuned to induce other angiogenic signals. For example, ROS play an important role in vascular endothelial growth factor signaling, and thermal plasmas that produce nitric oxide could also promote angiogenesis. Thus repeated plasma treatments of different doses or with different plasma types could be timed to maximize the angiogenic response. During these repeated treatments, plasma effects on the surrounding tissue must also be considered. In our previous work, high plasma doses did not induce gross or histological skin damage in an animal model, and malignant epithelial cells were more sensitive to plasma-induced apoptosis.16,17 These data suggest that plasma may induce angiogenesis without harming the surrounding tissue, whereas plasma inhibition of angiogenesis may synergistically kill malignant cells. However, since each of these studies were performed under slightly different plasma and cell or tissue conditions, additional studies should be performed to directly compare plasma sensitivity of various cell types.
Non-thermal plasma can induce endothelial cell death via the apoptotic pathway. In our previous work on ROS and endothelial cells, we showed that low ROS levels induce sub-lethal cell membrane damage, higher ROS levels induce apoptosis, and extremely high ROS levels induce non-specific cell death which is likely necrosis.30 Our data suggest that plasma dose can similarly be used to modulate the cell death mechanism, which is an important consideration both in vivo and in vitro. Apoptosis is programmed cell death initiated by physiological or pathological signals. Apoptotic cells are broken up into apoptotic bodies, which are engulfed by neighboring cells, leading to clean cell death without significant inflammatory response.11,29 On the contrary, necrosis is cell death accompanied by swelling, blebbing and increased membrane permeability leading to cytosolic content spillage. This typically leads to inflammation in surrounding tissue.11,29 By controlling plasma dose, we may be able to kill endothelial cells without significant necrosis and subsequent inflammation.
Plasma induces endothelial cell FGF2 release. FGF2 is thought to be released only at cell injury or death, since it has no signal sequence for secretion.32 Since plasma effects occur shortly after treatment, plasma may induce sub-lethal endothelial cell membrane damage, rendering the cells leaky to intracellular contents like FGF2. Other stimuli which induce cell membrane damage lead to FGF2 release. Biochemical changes, such as high glucose, enhance FGF2 release also through ROS.30 Transient plasma membrane disruption by mechanical forces leads to rapid cytosolic FGF2 release. This FGF2 release initiates growth required for tissue integrity maintenance and/or repair after injury.31 Mechanical strain also stimulates a proliferative response in coronary vascular smooth muscle cells via FGF2 release, and strain can even enhance endothelial cell FGF2 mRNA expression.28,36 In vivo, FGF2 released into the coronary circulation after vascular injury promotes human vascular smooth muscle cell proliferation.4
Similar to mechanical damage, cell membrane injury from ionizing radiation induces FGF2 expression and release in endothelial and epithelial cells in vitro 5,23,24 and in vivo.41 FGF2 enhances endothelial, epithelial, and hematopoetic cell survival after ionizing radiation,18,19,22,23 and FGF2 release is critical to radiation damage repair.23 Pulsed electromagnetic fields can stimulate endothelial cell growth, angiogenesis, and wound healing through endogenous FGF2 release.3,37,42 Non-thermal plasma differs from irradiation and electromagnetic fields in that the latter are penetrating and injure surrounding tissue, or they need an expensive setup to be generated safely. Plasma provides a novel and safer means to induce FGF2 release and angiogenesis since it provides precise control of treatment area and depth. Non-thermal plasma devices are also small and relatively simple to produce.
When endothelial cells are exposed to plasma, the conditioned medium FGF2 level peaks three hours after treatment and then declines. In contrast, cells treated with TNF-α show a gradual increase in medium FGF2. Thus plasma FGF2 release kinetics are essentially different from TNF-α. One possible reason is that TNF-α remains in the medium continuously, whereas plasma treatment occurs over a short, finite time. An alternative is that while both plasma and TNF-α likely release FGF2 related to ROS, TNF-α takes longer to produce ROS. While TNF-α activates a variety of biochemical signaling pathways in endothelial cells, the most likely path for TNF-α FGF2 release is cell membrane damage, since FGF2 has no known signal sequence for secretion. TNF-α must bind the membrane-bound TNF receptor, which activates intracellular signaling cascades leading to ROS.33,39 Thus the indirect and extended FGF2 release from TNF-α cell damage differs greatly from the finite and direct nature of plasma-induced rapid FGF2 release. The released FGF2 may then be bound by remaining viable cells, which explains the drop in medium FGF2 level after 3 h and enhanced endothelial cell proliferation. This brief and defined FGF2 release may be critical to angiogenesis, since timing and local biochemical environment play important roles in FGF2 signaling.6
Both FGF2 and LDH are released from cells due to cell membrane damage, yet in our experiments, FGF2 and LDH release from plasma treated cells followed different trends. Whereas FGF2 release peaked 3 h post-plasma and then declined, LDH release became significant only 4 h after plasma treatment but then increased up to 24 h. This difference may be related to the relative LDH (134 kDa) and FGF2 (18 kDa) sizes. FGF2 may be released after early sub-lethal plasma membrane damage, whereas LDH release occurs gradually after more extensive cell membrane damage. Additionally the gradual post-plasma LDH release may indicate that non-thermal plasma does not lead to immediate irreversible membrane integrity loss normally associated with severe trauma or cell death. Viable cells release low LDH amounts without hampering cell function.9 LDH also has no extracellular function, whereas FGF2 binds to cell membrane receptors, thus cell-released LDH is not metabolized by other cells whereas FGF2 is. This correlates with FGF2 decrease up to 24 h after plasma. Non-thermal plasma may lead to sub-lethal membrane damage which is gradually repaired as living cells uptake released FGF2 and remain viable.
Plasma-induced FGF2 release is likely related to neutral ROS. Non-thermal plasmas produce long lived (O3, NO, HO2, H2O2) and short lived (OH, O, electronically excited O) neutral particles and charged particles (ions and electrons). Both charged and neutral particles can lead to ROS in treated fluid. When endothelial cells were treated directly or indirectly (excluding charged particles), endothelial cell FGF2 release was not significantly different. However, FGF2 release decreased in separated treatment, in which medium was treated prior to applying it to cells. The time required to collect separately treated medium and apply it to untreated cells eliminated short lived neutral species and direct contact between plasma and cells. Direct plasma effects could include local heating by plasma streamers or UV radiation. Since separated treatment decreased FGF2 release by 50%, and FGF2 release remained significantly greater than in control cells, we believe that both short and long lived neutral species play a major role in plasma-induced FGF2 release. While a wide variety of plasma-produced ROS could affect cells, both atomic and singlet oxygen are short lived and therefore highly likely to recombine before reaching the sample surface during treatment. The plasma-produced ROS most likely to contribute to endothelial cell FGF2 release are OH radicals, hydrogen peroxide, and HO2.
Non-thermal plasma produces a large ROS concentration in extracellular medium during treatment. However, it is unclear if these ROS go inside cells. Both intracellular and extracellular ROS scavengers decreased FGF2 release following plasma. Combined scavengers reduced FGF2 release more than either scavenger alone. ROS produced by plasma extracellularly may move across the cell membrane through lipid peroxidation, opening transient cell membrane pores, or signaling pathways which modify ROS inside cells. Active species produced by plasma may also modify the cell medium, which in turn interacts with cells. Since many of active species have a short life span, they may immediately interact with medium components including amino acids and proteins, leading to production of long lived reactive organic hydroperoxides.20 These hydroperoxides may then induce lipid peroxidation and cell membrane damage, or they may bind to cell membrane receptors and activate intracellular signaling pathways leading to FGF2 expression and release.
We believe that non-thermal plasma could be used in vitro and in vivo to stimulate angiogenesis. Potential plasma applications include vascularizing tissue engineering structures, enhancing transplanted tissue incorporation, and accelerating wound healing. Our two-dimensional treatment model—an endothelial cell monolayer on a glass substrate covered with a thin medium film (~100 μm)—is likely more severe than what would be experienced by cells either in vivo or in three-dimensional in vitro models. Both sample geometry and the amount of liquid covering the sample are crucial to plasma treatment efficacy. We previously showed that increasing media depth over malignant epithelial cells decreased plasma-induced cell death.17 ROS are highly reactive and may be inactivated prior to reaching cells if the distance between the plasma and the cells is too great. In these situations, a higher plasma dose could be used to maintain plasma effects. In the future, we will examine plasma penetration depth variation with environmental conditions by treating endothelial cells within three-dimensional collagen gels. We observed similar plasma-induced proliferation results for cells seeded on uncoated and collagen-coated substrates in two dimensions, suggesting that plasma effects will be similar in a more tissue-like environment.
References
Ayan, H., G. Fridman, D. Staack, A. F. Gutsol, V. N. Vasilets, A. A. Fridman, and G. Friedman. Heating effect of dielectric barrier discharges for direct medical treatment. IEEE Trans. Plasma Sci. 37(1):113–120, 2009.
Balasubramanian, M., A. Sebastian, M. Peddinghaus, G. Fridman, A. Fridman, A. Gutsol, G. Friedman, and B. Ari. Dielectric barrier discharge plasma in coagulation and sterilization. Blood 108(11):89b-89b, 2006.
Callaghan, M. J., E. I. Chang, N. Seiser, S. Aarabi, S. Ghali, E. R. Kinnucan, B. J. Simon, and G. C. Gurtner. Pulsed electromagnetic fields accelerate normal and diabetic wound healing by increasing endogenous FGF-2 release. Plast. Reconstr. Surg. 121(1):130–141, 2008.
Caplice, N. M., C. N. Aroney, J. H. N. Bett, J. Cameron, J. H. Campbell, N. Hoffmann, P. T. McEniery, and M. J. West. Growth factors released into the coronary circulation after vascular injury promote proliferation of human vascular smooth muscle cells in culture. J. Am. Coll. Cardiol. 29(7):1536–1541, 1997.
Chang, P. Y., K. A. Bjornstad, E. Chang, M. McNamara, M. H. Barcellos-Hoff, S. P. Lin, G. Aragon, J. R. Polansky, G. M. Lui, and E. A. Blakely. Particle irradiation induces FGF2 expression in normal human lens cells. Radiat. Res. 154(5):477–484, 2000.
Clyne, A. M., H. Zhu, and E. R. Edelman. Elevated fibroblast growth factor-2 increases tumor necrosis factor-alpha, induced endothelial cell death in high glucose. J. Cell. Physiol. 217(1):86–92, 2008.
Coulombe, S. Live cell permeabilization using the APGD-t. 1st International Conference on Plasma Medicine (ICPM), Corpus Christi, TX, 2007.
Coulombe, S., V. Leveille, S. Yonson, and R. L. Leask. Miniature atmospheric pressure glow discharge torch (APGD-t) for local biomedical applications. Pure Appl. Chem. 78(6):1147–1156, 2006.
Danpure, C. J. Lactate-dehydrogenase and cell injury. Cell Biochem. Funct. 2(3):144–148, 1994.
Eliasson, B., W. Egli, and U. Kogelschatz. Modeling of dielectric barrier discharge chemistry. Pure Appl. Chem. 66(8):U1766–U1778, 1994.
Fiers, W., R. Beyaert, W. Declercq, and P. Vandenabeele. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene 18(54):7719–7730, 1999.
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1(1):27–31, 1995.
Folkman, J. Angiogenesis. Annu. Rev. Med. 57:1–18, 2006.
Fridman, A. Plasma Biology and Plasma Medicine. New York: Cambridge University Press, 2008.
Fridman, G., A. D. Brooks, M. Balasubramanian, A. Fridman, A. Gutsol, V. N. Vasilets, H. Ayan, and G. Friedman. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Processes Polym. 4(4):370–375, 2007.
Fridman, G., M. Peddinghaus, H. Ayan, A. Fridman, M. Balasubramanian, A. Gutsol, A. Brooks, and G. Friedman. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma. Chem. Plasma Process. 26(4):425–442, 2006.
Fridman, G., A. Shereshevsky, M. M. Jost, A. D. Brooks, A. Fridman, A. Gutsol, V. Vasilets, and G. Friedman. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma. Chem. Plasma Process. 27(2):163–176, 2007.
Fuks, Z., R. S. Persaud, A. Alfieri, M. Mcloughlin, D. Ehleiter, J. L. Schwartz, A. P. Seddon, C. Cordoncardo, and A. Haimovitzfriedman. Basic fibroblast growth-factor protects endothelial-cells against radiation-induced programmed cell-death in-vitro, and in-vivo. Cancer Res. 54(10):2582–2590, 1994.
Gallicchio, V. S., N. K. Hughes, B. C. Hulette, R. Dellapuca, and L. Noblitt. Basic fibroblast growth-factor (B-Fgf) induces early-stage (Cfu-S) and late-stage hematopoietic progenitor-cell colony formation (Cfu-Gm, Cfu-Meg, and Bfu-E) by synergizing with Gm-Csf, Meg-Csf, and erythropoietin, and is a radioprotective agent in vitro. Int. J. Cell Cloning 9(3):220–232, 1991.
Gebicki, S., and J. M. Gebicki. Formation of peroxides in amino-acids and proteins exposed to oxygen free-radicals. Biochem. J. 289:743–749, 1993.
Gostev, V., and D. Dobrynin. Medical microplasmatron. 3rd International Workshop on Microplasmas (IWM-2006), Greifswald, Germany, 2006.
Haimovitzfriedman, A., N. Balaban, M. Mcloughlin, D. Ehleiter, J. Michaeli, I. Vlodavsky, and Z. Fuks. Protein-kinase-C mediates basic fibroblast growth-factor protection of endothelial-cells against radiation-induced apoptosis. Cancer Res. 54(10):2591–2597, 1994.
Haimovitzfriedman, A., I. Vlodavsky, A. Chaudhuri, L. Witte, and Z. Fuks. Autocrine effects of fibroblast growth-factor in repair of radiation-damage in endothelial-cells. Cancer Res. 51(10):2552–2558, 1991.
Houchen, C. W., R. J. George, M. A. Sturmoski, and S. M. Cohn. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am. J. Physiol. Gastrointest. Liver Physiol. 276(1):G249–G258, 1999.
Kalghatgi, S. U., G. Fridman, M. Cooper, G. Nagaraj, M. Peddinghaus, M. Balasubramanian, V. N. Vasilets, A. F. Gutsol, A. Fridman, and G. Friedman. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 35(5):1559–1566, 2007.
Kieft, I. E., D. Darios, A. J. M. Roks, and E. Stoffels. Plasma treatment of mammalian vascular cells: a quantitative description. IEEE Trans. Plasma Sci. 33(2):771–775, 2005.
Kieft, I. E., M. Kurdi, and E. Stoffels. Reattachment and apoptosis after plasma-needle treatment of cultured cells. IEEE Trans. Plasma Sci. 34(4):1331–1336, 2006.
Ku, P. T., and P. A. Damore. Regulation of basic fibroblast growth-factor (Bfgf) gene and protein expression following its release from sublethally injured endothelial-cells. J. Cell. Biochem. 58(3):328–343, 1995.
Majno, G., and I. Joris. Apoptosis, oncosis, and necrosis—an overview of cell-death. Am. J. Pathol. 146(1):3–15, 1995.
Morss, A. S., and E. R. Edelman. Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J. Biol. Chem. 282(19):14635–14644, 2007.
Muthukrishnan, L., E. Warder, and P. L. Mcneil. Basic fibroblast growth-factor is efficiently released from a cytolsolic storage site through plasma-membrane disruptions of endothelial-cells. J. Cell. Physiol. 148(1):1–16, 1991.
Nugent, M. A., and R. V. Iozzo. Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 32(2):115–120, 2000.
Rath, P. C., and B. B. Aggarwal. TNF-induced signaling in apoptosis. J. Clin. Immunol. 19(6):350–364, 1999.
Shekhter, A. B., V. A. Serezhenkov, T. G. Rudenko, A. V. Pekshev, and A. F. Vanin. Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric Oxide Biol. Chem. 12(4):210–219, 2005.
Siemens, C. W. On the electrical tests employed during the construction of the Malta and Alexandria Telegraph, and on insulating and protecting submarine cables. J. Franklin Inst. 74(3):166–170, 1862.
Sudhir, K., K. Hashimura, A. Bobik, R. J. Dilley, G. L. Jennings, and P. J. Little. Mechanical strain stimulates a mitogenic response in coronary vascular smooth muscle cells via release of basic fibroblast growth factor. Am. J. Hypertens. 14(11):1128–1134, 2001.
Tepper, O. M., M. J. Callaghan, E. I. Chang, R. D. Galiano, K. A. Bhatt, S. Baharestani, J. Gan, B. Simon, R. A. Hopper, J. P. Levine, et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 18(9):1231, 2004.
Vargo, J. J. Clinical applications of the argon plasma coagulator. Gastrointest. Endosc. 59(1):81–88, 2004.
Wajant, H., K. Pfizenmaier, and P. Scheurich. Tumor necrosis factor signaling. Cell Death Differ. 10(1):45–65, 2003.
Wong, M. K. K., and A. I. Gotlieb. In vitro reendothelialization of a single-cell wound—role of microfilament bundles in rapid lamellipodia-mediated wound closure. Lab. Invest. 51(1):75–81, 1984.
Yamada, H., E. Yamada, N. Kwak, A. Ando, A. Suzuki, N. Esumi, D. J. Zack, and P. A. Campochiaro. Cell injury unmasks a latent proangiogenic phenotype in mice with increased expression of FGF2 in the retina. J. Cell. Physiol. 185(1):135–142, 2000.
Yenpatton, G. P. A., W. F. Patton, D. M. Beer, and B. S. Jacobson. Endothelial-cell response to pulsed electromagnetic-fields—stimulation of growth-rate and angiogenesis in vitro. J. Cell. Physiol. 134(1):37–46, 1988.
Acknowledgment
We would like to thank Gregory Fridman for building the plasma device.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Julia E. Babensee oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Kalghatgi, S., Friedman, G., Fridman, A. et al. Endothelial Cell Proliferation is Enhanced by Low Dose Non-Thermal Plasma Through Fibroblast Growth Factor-2 Release. Ann Biomed Eng 38, 748–757 (2010). https://doi.org/10.1007/s10439-009-9868-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9868-x