Abstract
We investigate analytically and experimentally the flow rate through a biochip in a circuit involving a peristaltic pump and reservoirs with liquid/air interfaces. Peristaltic pumps are a convenient way to achieve recirculation in microfluidic circuits. We consider different cases: reservoirs in contact with ambient air, tight reservoirs, and imperfect tightness leading to air or liquid leaks. We demonstrate that if changes in hydraulic resistance are slow enough, i.e., if cells do not proliferate too fast, the system may reach an equilibrium, with a difference in liquid height between inlet and outlet reservoir compensating the pressure drop in the biochip. We compute the flow rate through the biochip in the transient regime as well as the characteristic time. We also show that depending on the circuit dimensions, this equilibrium may never be reached. We provide guidelines to design tubings and reservoirs to avoid this situation and ensure a smooth recirculation at a desired flow rate, which is a necessary condition for dynamic cell culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Different technologies exist to pump fluids through microfluidic cell culture chambers (Byun et al. 2013). Passive pumping can be achieved (Narayanamurthy et al. 2020), thanks to gravity (Huh et al. 2007), surface tension (Jang et al. 2021), or osmosis (Xu et al. 2010). Placing the microfluidic circuit on a rotating disk allows to use centrifugal force as a driving force (Burger et al. 2012). In many microfluidic cell culture systems, however, medium is actively pumped through the culture chambers.
All types of pumps can either push the fluid through the inlet or suck it from the outlet (Teo et al. 2017). Syringe pumps are commonly encoutered in microfluidics laboratories but flows driven by syringe pumps are subjected to fluctuations (Li et al. 2014). It has been shown that the type of flow control can influence the polydispersity of emulsions generated in microsystems (Zeng et al. 2015). Pressure control has been proposed as an alternative. The difference between flow-rate control using syringe or peristaltic pumps and pressure pumps has mostly been studied in the case of immiscible (Ward et al. 2005) or miscible (Bihi et al. 2019) two-phase flows. Guidelines have been provided to adapt the design of channels to pressure flow control (Oh et al. 2012; Minetti et al. 2020). Positive pressure at the inlet can be imposed by a pressure manifold fed with compressed air (Bong et al. 2011; Mavrogiannis et al. 2016) or by fluid columns (Gnyawali et al. 2017).
For organ-on-chip applications, the fluid needs to be recirculated through the system to mimic the closed loop circulation of blood in the body (de Graaf et al. 2022). Liquid recirculation in such microfluidic circuits is a challenge (Futai et al. 2006; Garcia-Cordero et al. 2010) and requires pumps (Byun et al. 2013) and/or valves (Debski et al. 2018). In this manuscript, we investigate the flow in a microfluidic biochip installed between an inlet and an outlet reservoir, in the presence of cells. The biochip accommodates cells that proliferate over time during the experiment, and require feeding and oxygenation with a culture medium. To achieve recirculation, a peristaltic pump drives the liquid from the outlet to the inlet reservoir. We study the influence of cell growth on the overall circuit resistance and compute the difference between the target flow rate Q imposed by the pump and the flow rate through the biochip \(Q'\) under different conditions: we consider a tight circuit, a leaking seal, and a leaking connector.
2 Methods
2.1 Bioreactor design and operation
Dynamic cell culture is performed in PDMS biochips. Each chip has a volume of 40 \(\mu\)L and a surface of 2 cm\(^2\). Cells reside at the textured bottom while medium flows above them through the smooth top chamber. To operate in parallel with 12 biochips, we use an Integrated Dynamic Cell Cultures in Microsystems (IDCCM) tool box, previously developed in our team and illustrated in supplementary figure A1 (Baudoin et al. 2012). Briefly, this platform, made of polycarbonate, consists of 3 parts: a 24-well plate equipped with connectors at the bottom of each reservoir that can be plugged into the biochips, a cover with connectors, and a PDMS seal. Tightness is achieved by inserting the assembled platform in a metal frame maintained by a clamp whose strength can be adjusted.
2.2 Tightness assays
The test is conducted using the 24-well platform. Using silicon tubes and T-connectors, the outlet of the pressure controller is connected to the inlet of 12 wells as illustrated in Fig. 1a. The pressurized wells are those of the rows placed at the edges of the platform. Their bottom outlets are sealed. This way, half of the wells are pressurized, mimicking what happens when the peristaltic pump is used to circulate the culture medium in the biochips. Wells that are not connected to the pressure controller can be either sealed or left open. The platform is then immersed in water in a glass container. The inlet pressure is increased by 20 mbar steps until visible bubbles indicate a leak. The test is performed twice with two degrees of fastening force. Two clamps on each side of the device squeeze the three layer between their jaws and tighten the wells. A threaded rod is used to adjust the tightening strength. We used two positions of the threaded rod in this study. The "loose" setting corresponds to a position for which the assembly seems tight after a simple visual inspection of the device. The "tight" setting corresponds to a position beyond which most users find it difficult to fasten the clamp and seal the system. The two positions were 4 screws away from one another.
2.3 Cell culture
We use the hepatic cell line HepG2/C3A, a clone of the HepG2 line derived from human hepatocellular carcinoma (ATCC CRL-10741). Cells are cultured at \(37^\circ\)C and 5% CO\(_2\) in a humid atmosphere in 75 cm\(^2\) flasks. The culture medium is Minimal Essential Medium (MEM) with phenol red (Pan Biotech, Aidenbach, Germany) supplemented with 10% (v/v) fetal bovine serum (Gibco, Waltham, MA, USA), 2 mM L-glutamine (Gibco), 0.1 mM non-essential amino acids (Gibco), 1 mM sodium pyruvate (Gibco), and 100 U/mL penicillin/streptomycin (Pan Biotech). Cells are passaged weekly at a confluence of 80–90% and the culture medium is renewed every two days. Biochips coated with collagen (Corning, NY, USA) are seeded with the desired number of cells suspended in 80 \(\mu\)L of culture medium, kept 24 h in static conditions at \(37^\circ\)C and 5% CO\(_2\). Then, a flow rate of 10\(\mu\)L/min is imposed by a peristaltic pump (ISM949, ISMATEC) using silicone tubing of 0.089 cm internal diameter (PharMed BTP, Cole Parmer). The process is illustrated in supplementary figure A1. The number of cells in a biochip is evaluated by injecting a known volume of a trypsin–EDTA solution (0.25%, Gibco) to detach the cells from the chip surface, and measure the final cell number using a Malassez slide. These measurements are performed 24 h after cell seeding to evaluate the adhesion rate and at different time points during microfluidic culture to evaluate the proliferation of adherent cells.
2.4 Flow control
For most experiments, flow is driven by a peristaltic pump (ISM949, ISMATEC) using silicone tubing of 0.089 cm internal diameter (PharMed BTP, Cole Parmer). The flow rate varies between 10 and 25 \(\mu\)L/min.
To evaluate the time evolution of hydraulic resistance in biochips during cell culture, the flow rate is imposed, instead of a peristaltic pump, by a pressure controller (MFCS-EX, Fluigent, Le Kremlin-Bicêtre, France), a flowmeter (Flow Unit type M, Fluigent) and a feedback loop. The pressure drop (pressure difference between inlet and outlet reservoirs) required to achieve the target flow rate is measured for flow rates ranging from 0 to 30 \(\mu\)L/min. The ratio between pressure drop and flow rate yields the total circuit resistance, from which the chip resistance is obtained by subtracting the constant resistance of the tubings and fittings.
2.5 Numerical calculation
Numerical integration of differential equations is performed in Python using scipy.integrate subpackage. A fifth-order implicit Runge–Kutta method is chosen to avoid non-physical oscillations. The resolution schemes for situations involving air and liquid leaks are, respectively, described in supplementary figures A2 and A3.
3 Results
3.1 Tightness assay
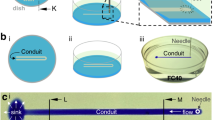
Experimental setup to assess tightness. a Configuration mimicking the perfusion of 12 biochips. Wells in the top and bottom rows are pressurized. Those in the middle rows can be closed with a plug or left open. b Configuration used to assess the tightness of a single well. All the other wells are closed. The star indicates the location at which bubbles are observed when leaks occur
The dynamic culture system consists of a lid, a silicone seal, and a 24-well plate. In cell culture experiments, biochips can be installed between two neighboring wells by plugging them to the connectors found at the bottom of each well. In a similar manner, liquid can be transferred from one well to another using a peristaltic pump and deformable tubing inserted in two of the 24 holes pierced in the lid. Lid, seal, and well plate are aligned and assembled using a metal plate holder. For tightness assays, to mimic the conditions encountered in dynamic cell culture, we pressurized the wells of the two outer rows. Wells of the two central rows were closed with a plug. We used two positions of the threaded rod, respectively, corresponding to a loose and tight clamping. For the loosest clamping, air leakage was observed at the silicone seal when the relative pressure in the wells reached 150 mbar, while for the strongest clamping, leakage at the seal only occurred for a pressure of 280 mbar, as indicated by Fig. 2a. When the central wells were left open, leaks occurred at lower relative pressure: 10 mbar for the loose clamping and 260 mbar for the tight one (Fig. 2b). In tight clamping conditions, leakage was only observed at the central non-pressurized wells (positions B3 and B4, according to Fig. 1b). In a second experiment, only one well was pressurized at a time, as illustrated in Fig. 1b. When the wells in positions A1 and A6 were pressurized, no leakage was observed (the pressure was increased up to 750 mbar). On the other hand, for the wells in positions B2–B5, leakage at the seal occurred when the pressure reached 300 mbar. Each time, the bubbles formed at the same point of the seal, indicated by a blue star in Fig. 1b and by a in Fig. 4, in the center of the edge, indicating that the sealing is less effective at this region (Fig. 2).
3.2 Cell growth and chip resistance
Cells tend to form a monolayer at the bottom of the biochip at low density and to assemble into multilayer structures when the number of seeded cells increases, as can be seen in supplementary figures A1.E and A4. This decreases the cross section available for the fluid in the biochip. The hydraulic resistance of empty biochips has been estimated at \(5.9 \pm 0.4 \cdot 10^{12}\) kg\(\cdot\)m\(^{-4}\)s\(^{-1}\) (Messelmani et al. 2022). When cells are seeded in the biochip, this resistance increases, as illustrated in Fig. 3(a), where the plain line represents the fitting equation \(R = 2.4 \cdot 10^{7} N + 5.9 \cdot 10^{12} \text{ kg }\cdot \text{ m}^{-4}\text{ s}^{-1}\). Using this correlation and taking into account the doubling time of cells, we can foresee the time evolution of the total number of cells and thus the time evolution of chip resistance for different seeding conditions. HepG2/C3A were cultured in parallel in several chips in dynamic conditions. Cells were detached and counted at different time points, which allowed us to estimated a doubling time of two days for HepG2/C3A cells cultured in dynamic conditions in the biochip. Fig. 3(b) shows that in our biochips, the predicted resistance varies from \(R_{min} = 8.3 \cdot 10^{12} \text{ kg }\cdot \text{ m}^{-4}\text{ s}^{-1}\) shortly after seeding at low density (100 000 cells in the biochip) to \(R_{max} = 10^{14} \text{ kg }\cdot \text{ m}^{-4}\text{ s}^{-1}\) after a few days of culture for cells seeded at high density (1 million per biochip). These values will be used throughout the rest of the study.
Hydrodynamic resistance of a biochip seeded with cells. a Measurements performed for biochips seeded with variable amounts of cells, using a pressure controller, a flowmeter, and a feedback loop to impose a flow rate between 0 and 30 \(\mu\)L/min. b Expected variation of biochip resistance over time, assuming a doubling time of 2 days, for three values of initial cell density (100,000, 500,000, and 1,000,000 cells)
3.3 Flow rate control in a tight circuit
In this section, we consider a biochip connecting two wells of a culture plate installed in a IDCCM tool box as sketched in Fig. 4. Initially, the wells, whose cross-section area A is considered constant, are filled with culture medium up to an height \(h_0\) inferior to the well depth \(H_w\), so there is an air–water interface in each of the inlet and outlet wells. The circulation of liquid from the outlet well back to the inlet well is achieved by the means of a peristaltic pump, which may lead to the displacement of these air–water interfaces. H and h, respectively, represent the levels in the inlet and outlet wells. If the air–water interfaces are moving, then the flow rate Q imposed by the pump in the upper part of the circuit is not the same as the flow rate \(Q'\) inside the biochip.
R represents the hydraulic resistance of the biochip, which is the ratio between the pressure drop \(\Delta P\) across the chip and the flow rate \(Q'\) through the chip. Since the fluid is at rest in the wells, the pressure at the bottom of inlet well is \(P_i + \rho g H\) (respectively, \(P_o + \rho g h\) for the outlet well) and \(\Delta P\) is the difference between the two:
where \(P_i\) and \(P_o\) are the pressures measured in the air above the liquid in the inlet and outlet wells. If \(Q'<Q\), fluid accumulates in the inlet well:
Since the bioreactors are placed in an incubator, the temperature is fixed. Considering air as a perfect gas we get \(P_i V_i = P_{atm} V_{i_0}\), with \(V_i\) the volume of air in the inlet well. This leads to \(P_i = P_{atm} \frac{H_w - h_0}{H_w - H}\), where \(H_w\) is the well depth. Similarly, \(P_o = P_{atm} \frac{H_w - h_0}{H_w - h}\).
Mass conservation yields \(H + h = 2 h_0\), which makes it possible to eliminate h from (3):
Combining (2) with (4), we get
If H reaches an equilibrium value \(H_{\infty }\), then \(\frac{\textrm{d}H}{\textrm{d}t} = 0\). We can then compute \(H_{\infty }\) by solving the third-order equation:
with
The values of a, b, c and d are computed using the numerical values of the physical parameters listed in Table 1. In Fig. 5a, pressures at equilibrium in the inlet and outlet wells are plotted against the flow rate Q imposed by the pump. For both wells, the amplitude of the relative pressure \(P_{eq}-P_{atm}\) increases with Q and with R, in a more pronounced way for the inlet well. Figure 5b shows the variations of \(H_{\infty }\) with respect to Q for two different values of R. As long as the biochip resistance is close to its initial value \(R_{min}\), \(H_{\infty }\) remains extremely close to its initial value \(h_0\). To increase the level of the free surface by 1 mm, the resistance needs to be much larger: \(R_{max} = 10^{14} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\), which is also larger than the value measured after 3 days of culture, as seen in Fig. 3.
Equilibrium in a tight circuit, for two different values of the chip resistance R. a Relative pressures \(P_{eq} - P_{atm}\) after equilibrium has been reached in the inlet (\(\diamond\)) and outlet (\(\square\)) wells. b Position \(H_{\infty }\) of the free surface in the inlet well after equilibrium has been reached. Red and black curves, respectively, indicate values obtained for \(R = R_{min} = 5\cdot 10^{12} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\) and \(R = R_{max} = 10^{14} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\)
For each condition, we compute the time \(t_{90}\) required for \(Q'\) to reach 90%Q. Figure 6 shows that Q has a limited influence on \(t_{90}\), especially at the beginning of the experiment, when R is close to \(R_{min}\). It is much more sensitive to variations in biochip resistance, with a quasi-linear dependency with R. The time to equilibrium also strongly depends on the amount of air initially present in the circuit. It takes more time to balance pressures in wells containing little liquid, while fuller circuits reach equilibrium sooner. To reach the target flow rate Q, the pressure difference \(P_i - P_o\) must match the product RQ, which, even for en empty biochip, corresponds to a liquid head of 12 cm for \(Q_{min}\) and 60 cm for \(Q_{max}\). This means that, if the wells were to be open to atmospheric pressure instead of closed, the difference \(H-h\) should be way too large for the device to be practical. This, as well as the desire to avoid possible airborne contamination, explains why inlet and outlet wells are sealed closed. When computing \(P_i(t_{90})\) we notice that the pressure variations in the inlet well remain moderate as long as the biochip is not too resistive, but it can reach the tightness limit if R increases due to cell growth.
Equilibrium in a tight circuit. Estimation of the time to equilibrium \(t_{90}\) and relative pressure in the inlet well as a function of Q for \(R = R_{min} = 5\cdot 10^{12} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\) and \(R = R_{max} = 10^{14} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\)
3.4 Influence of an air leak
As can be observed in Fig. 6, pressure in the inlet well reaches values at which leaks have been shown to occur in the system. In this paragraph, we, therefore, investigate the behavior of the free surface in the case an air leak occurs in the inlet well. We model the leak by introducing a threshold pressure \(P_m\) above which air can escape through the joint: \(P_i = \text{ min }(P_m, P_{atm} \frac{H_w - h_0}{H_w - H})\). This means that the pressure drop across the biochip is smaller than the one predicted by Eq. (3). The leak triggers a decrease of the flow rate through the chip \(Q'\) and thus tends to amplify the accumulation of fluid in the inlet well.
As long as the system is leaking, Eq. (3) is replaced by
The time evolution of the system is governed by
This time the equilibrium height is found by solving a second-order equation
with
Equilibrium height in the inlet well \(H_\infty\) in a leaking circuit: solutions for Eq. (9). a \(R = R_{min} = 5\cdot 10^{12} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\) (b) \(R = R_{max} = 10^{14} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\).
The resolution scheme is represented graphically in supplementary figure A2. In Fig. 7, Eq. (9) is solved for the extreme values of Q and R used in this study. A solution can be found but we observe that in most conditions, this solution is non-physical since the equilibrium height \(H_\infty\) is much larger than the well depth \(H_w\). We observe that \(H_\infty\) decreases with \(P_m\). If the circuit is sufficiently tight, an equilibrium might be reached. Figure 7 shows that to reach an equilibrium height \(H_\infty < H_w\), the system must be able to withstand an inlet pressure of at least 507 mbar for \(R = R_{max} = 10^{14} \text{ kg }\cdot \text{ m}^{-4}\cdot \text{ s}^{-1}\) and \(Q = 10 \ \mu\)L/min, and this value increases with both R and Q. In many cases, however, the level keeps growing in the inlet well.
Equilibrium in a leaking circuit. a Time evolution of the fluid index H in the inlet well for \(R = R_{min}\), \(h_0\) = 9 mm, \(Q = 10 \mu\)L/min and variable value of threshold pressure \(p_m\). b Variations of the minimal value of \(p_m\) compatible with an equilibrium \(p_{m_{min}}\) with initial position of the fluid index \(h_0\), for extreme values of R and Q
To determine the limit beyond which no equilibrium value is found, we proceed as sketched in Fig. 8a. We plot the time evolution of H in a tight circuit, then in a leaking circuit, starting with a large value of \(P_m\) that is progressively reduced until \(H_\infty = H_w\). Figure 8b shows how the pressure \(P_{m_{min}}\) at which \(H_\infty = H_w\) depends on the initial liquid index h in the wells.
3.5 Influence of a liquid leak
We also considered the case of a loss of liquid occurring at the inlet of the biochip due to an imperfect connector, corresponding to the situation b in Fig. 4. We modeled this liquid leak as an outward flow with a constant flow rate \(q_l\) that takes place as soon as the pressure at the bottom of the inlet well reaches a limit \(P_l\), and stops when \(P_i \le P_l\), as sketched in the diagram A3. When the circuit resistance is low (\(R = R_{min}\)), no leak is observed unless the threshold \(P_l\) is extremely low (5 mbar). For larger values of \(P_l\), more compatible with experimental evaluations, the pressure drop in the biochip is so low, even for the maximal flow rate, that the inlet pressure never reaches \(P_l\). The system keeps behaving as a tight system. On the other hand, when \(R = R_{max}\), leaks occur for relevant values of \(P_l\). The time evolution is visible in Fig. 9: the initially linear decrease of \(H_t = H + h\) indicates that the system leaks in a constant manner at short times; the leak then becomes intermittent in a second, transient phase; after which finally stops and \(H_t\) reaches a plateau. The duration of each of these steps decreases with \(q_l\), since the excess liquid is drained faster in these conditions, and with \(P_l\). When \(P_l\) is large, the final plateau is reached sooner and the cumulated liquid loss is smaller than for small values of \(P_l\). In all the studied cases, the flow rate \(Q'\) through the biochip is larger than \(90 \%\) of the requested flow rate Q, apart from a few minutes after the pump is turned on.
Effect of a leaking connector. Time evolution of the total amount of liquid in the reservoirs \(H_t\) normalized by its initial value and of the flow rate \(Q'\) through the chip normalized by the target flow rate Q, for \(R = R_{max}\), \(Q = Q_{max}\) and different values of the leaking flow rate \(q_l\)
4 Conclusion
The idea to use numerical simulations and modeling to help design microfluidic circuits and experiments is not new (Erickson 2005). As microfluidic systems grow more complex, experiments involve a vast number of physical, biological and chemical parameters. One way to deal with this increasing complexity is the use of machine learning to build microfluidics databases and experimental plans but it is time- and resource-consuming (McIntyre et al. 2022). In this work, we present a set of experimental data and simple simulations regarding the flow rate control in a microfluidic circuit involving free surfaces and living cells whose proliferation alter the circuit resistance.
By modeling the peristaltic pump-driven flow through a biochip installed between two wells containing a liquid–air interface, we demonstrate that, in most situations, an equilibrium state can be reached in which the flow rates through the pump and through the biochip are equal. We also found that the time to reach this equilibrium is shorter than the characteristic timescale of cell proliferation, which governs changes in hydraulic resistance. The system can thus be considered as quasi-static, with a slowly varying resistance that can be considered constant to compute the flow rate and the liquid head in both reservoirs. This result depends of course on the chosen cell line and the culture conditions. We chose the hepatic cell line HepG3/C3A as a test case in this study because of its tendency to form large, sometimes multilayer structures when cultured in collagen-coated biochips in dynamic conditions, causing an increase in biochip resistance. A similar behavior can be expected with other cell lines and even with induced pluripotent stem cells. The time evolution of hydraulic resistance proposed in this paper should by no means be considered universal, but it illustrates the fact that cell-induced resistance can trigger significant changes in a microfluidic setup. These changes need to be taken into account when designing long experiments to avoid risks of leaks associated with high internal pressures. One should also keep in mind that when cells proliferate in a biochip where medium circulates at constant flow rate, they are exposed to an increasing shear stress. The target flow rate should ensure sufficient nutrient supply but prevent excessive shear stress and its value could change over the course of an experiment, depending on the way the growing cells self-organize in the biochip.
We considered different leakage scenarios and demonstrated that most of them do not compromise the possibility to reach equilibrium and reach the desired flow rate. So the fact that a stable liquid head is reached in both inlet and outlet wells does not mean that there were no leaks during the experiment. Achieving tightness is crucial when manufacturing a microfluidic chip and assembling a microfluidic circuit, as leaks provoke losses of culture medium and/or increased risks of contamination. The methods described in the present paper aim at providing insight as to what is an acceptable limit of tightness, depending on the application. The objective is to help users implement meaningful quality control.
We evaluated the tightness of our device at different stages of clamping force. We found that leaks occur preferentially at the wells located at the center of the device, which suggests that these wells should be used as outlet rather than inlet wells, to avoid excessive pressure and air leaks. Else, they can be reserved to experiments in which the hydraulic resistance is expected to remain low enough so that no leaks occur. When the device is used to run different experiments in parallel, conditions associated with a high expected resistance should be tested on the most robust wells. Also, in the case of future design changes, if an additional effort should be made to enhance tightness, these wells should be addressed first.
Our results suggest that a preliminary evaluation of cell growth, 3D organization and biochip resistance over time can be useful to design long-term experiments involving recirculation and avoid leaks and contaminations, by adjusting the dimensions of biochips, volume of culture medium and degree of clamping.
References
Baudoin R, Alberto G, Paullier P, Legallais C, Leclerc E (2012) Parallelized microfluidic biochips in multi well plate applied to liver tissue engineering. Sens Actuators B Chem 173:919–926. https://doi.org/10.1016/j.snb.2012.06.050
Bihi I, Vesperini D, Kaoui B, Le Goff A (2019) Pressure-driven flow focusing of two miscible liquids. Phys Fluids 31(6):062001. https://doi.org/10.1063/1.5099897
Bong KW, Chapin SC, Pregibon DC, Baah D, Floyd-Smith TM, Doyle PS (2011) Compressed-air flow control system. Lab Chip 11(4):743–747. https://doi.org/10.1039/c0lc00303d
Burger R, Kirby D, Glynn M, Nwankire C, O’Sullivan M, Siegrist J, Kinahan D, Aguirre G, Kijanka G, Gorkin RA, Ducrée J (2012) Centrifugal microfluidics for cell analysis. Curr Opin Chem Biol 16(3–4):409–414. https://doi.org/10.1016/j.cbpa.2012.06.002
Byun CK, Abi-Samra K, Cho Y-K, Takayama S (2013) Pumps for microfluidic cell culture. Electrophoresis 35(2–3):245–257. https://doi.org/10.1002/elps.201300205
de Graaf MNS, Vivas A, van der Meer AD, Mummery CL, Orlova VV (2022) Pressure-driven perfusion system to control, multiplex and recirculate cell culture medium for organs-on-chips. Micromachines 13(8):1359. https://doi.org/10.3390/mi13081359
Debski P, Sklodowska K, Michalski J, Korczyk P, Dolata M, Jakiela S (2018) Continuous recirculation of microdroplets in a closed loop tailored for screening of bacteria cultures. Micromachines 9(9):469. https://doi.org/10.3390/mi9090469
Erickson D (2005) Towards numerical prototyping of labs-on-chip: modeling for integrated microfluidic devices. Microfluid Nanofluid 1(4):301–318. https://doi.org/10.1007/s10404-005-0041-z
Futai N, Gu W, Song JW, Takayama S (2006) Handheld recirculation system and customized media for microfluidic cell culture. Lab Chip 6(1):149–154. https://doi.org/10.1039/b510901a
Garcia-Cordero JL, Basabe-Desmonts L, Ducrée J, Ricco AJ (2010) Liquid recirculation in microfluidic channels by the interplay of capillary and centrifugal forces. Microfluid Nanofluid 9(4–5):695–703. https://doi.org/10.1007/s10404-010-0585-4
Gnyawali V, Saremi M, Kolios MC, Tsai SSH (2017) Stable microfluidic flow focusing using hydrostatics. Biomicrofluidics 11(3):034104. https://doi.org/10.1063/1.4983147
Huh D, Bahng JH, Ling Y, Wei H-H, Kripfgans OD, Fowlkes JB, Grotberg JB, Takayama S (2007) Gravity-driven microfluidic particle sorting device with hydrodynamic separation amplification. Anal Chem 79(4):1369–1376. https://doi.org/10.1021/ac061542n
Jang I, Kang H, Song S, Dandy DS, Geiss BJ, Henry CS (2021) Flow control in a laminate capillary-driven microfluidic device. Analyst 146(6):1932–1939. https://doi.org/10.1039/d0an02279a
Li Z, Mak SY, Sauret A, Shum HC (2014) Syringe-pump-induced fluctuation in all-aqueous microfluidic system implications for flow rate accuracy. Lab Chip 14:744
Mavrogiannis N, Ibo M, Fu X, Crivellari F, Gagnon Z (2016) Microfluidics made easy: a robust low-cost constant pressure flow controller for engineers and cell biologists. Biomicrofluidics 10(3):034107. https://doi.org/10.1063/1.4950753
McIntyre D, Lashkaripour A, Fordyce P, Densmore D (2022) Machine learning for microfluidic design and control. Lab Chip 22(16):2925–2937. https://doi.org/10.1039/d2lc00254j
Messelmani T, Le Goff A, Souguir Z, Maes V, Roudaut M, Vandenhaute E, Maubon N, Legallais C, Leclerc E, Jellali R (2022) Development of liver-on-chip integrating a hydroscaffold mimicking the liver’s extracellular matrix. Bioengineering 9(9):443. https://doi.org/10.3390/bioengineering9090443
Minetti F, Giorello A, Olivares ML, Berli CLA (2020) Exact solution of the hydrodynamic focusing driven by hydrostatic pressure. Microfluidics Nanofluidics. https://doi.org/10.1007/s10404-020-2322-y
Narayanamurthy V, Jeroish ZE, Bhuvaneshwari KS, Bayat P, Premkumar R, Samsuri F, Yusoff MM (2020) Advances in passively driven microfluidics and lab-on-chip devices: a comprehensive literature review and patent analysis. RSC Adv 10(20):11652–11680. https://doi.org/10.1039/d0ra00263a
Oh KW, Lee K, Ahna B, Furlani EP (2012) Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 12:515–545
Teo AJT, Li K-HH, Nguyen N-T, Guo W, Heere N, Xi H-D, Tsao C-W, Li W, Tan SH (2017) Negative pressure induced droplet generation in a microfluidic flow-focusing device. Anal Chem 89(8):4387–4391. https://doi.org/10.1021/acs.analchem.6b05053
Ward T, Faivre M, Abkarian M, Stone HA (2005) Microfluidic flow focusing: drop size and scaling in pressure versus flow-rate-driven pumping. Electrophoresis 26:3716–3724
Xu Z-R, Yang C-G, Liu C-H, Zhou Z, Fang J, Wang J-H (2010) An osmotic micro-pump integrated on a microfluidic chip for perfusion cell culture. Talanta 80(3):1088–1093. https://doi.org/10.1016/j.talanta.2009.08.031
Zeng W, Jacobi I, Li S, Stone HA (2015) Variation in polydispersity in pump- and pressure-driven micro-droplet generators. J Micromech Microeng 25(11):115015. https://doi.org/10.1088/0960-1317/25/11/115015
Acknowledgements
This research was funded by ANR MimLiverOnChip grant number ANR-19-CE19-0020-01. IZN was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (Capes) - Finance Code 001. Partial financial support was received from Fluigent. A.M. and W. C. are employed by Fluigent.
Author information
Authors and Affiliations
Contributions
ALG, RJ, and AM designed experiments. TM and IZN performed experiments. TM, IZN, and ALG analyzed results. ALG wrote the main manuscript text. EL, CL and WC edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Some of the authors are employed by Fluigent, as indicated by their affiliation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Messelmani, T., Zarpellon Nascimento, I., Leclerc, E. et al. Flow rate variations in microfluidic circuits with free surfaces. Microfluid Nanofluid 27, 79 (2023). https://doi.org/10.1007/s10404-023-02691-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-023-02691-y