Abstract
A microfluidic system for rapid nucleic acid analysis based on real-time convective PCR is developed. To perform ‘sample-in, answer-out’ nucleic acid analysis, a microfluidic chip is developed to efficiently extract nucleic acid, and meanwhile convective PCR (CPCR) is applied for rapid nucleic acid amplification. With an integrated microfluidic chip consisting of reagent pre-storage chambers, a lysis & wash chamber, an elution chamber and a waste chamber, nucleic acid extraction based on magnetic beads can be automatically performed for a large size of test sample within a limited time. Based on an easy-to-operate strategy, different pre-stored reagents can be conveniently released for consecutive reaction at different steps. To achieve efficient mixing, a portable companion device is developed to introduce properly controlled 3-D actuation to magnetic beads in nucleic acid extraction. In CPCR amplification, PCR reagent can be spontaneously and repeatedly circulated between hot and cool zones of the reactor for space-domain thermal cycling based on pseudo-isothermal heating. A handheld real-time CPCR device is developed to perform nucleic acid amplification and in-situ detection. To extend the detection throughput, multiple handheld real-time CPCR devices can be grouped together by a common control system. It is demonstrated that influenza A (H1N1) viruses with the reasonable concentration down to 1.0 TCID50/ml can be successfully detected with the microfluidic system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Molecular diagnosis is able to detect pathogen- or pathogen-related nucleic acids (NAs) with ultrasensitive sequence-specific nucleic acid amplification (Foudeh et al. 2012). With enzymatic nucleic acid amplification, the target NAs become detectable after being replicated up to more than a million-fold. Traditional molecular diagnostics relies on big and heavy instruments, for example, nucleic acid extraction instrument and conventional PCR instrument, which is normally performed with trivial manual operation in central labs. In contrast, for point-of-care molecular diagnostics, simple, convenient, easy-to-operate, and even ‘sample-in, answer-out’ nucleic acid analysis can be achieved based on other concepts, for example, microfluidics (Zhuang et al. 2020; Soni and Toley 2022). With a properly designed fluid network integrated with different functional units, for example, micro-valves, micro-channels, micro-reactors, and micro-actuators, POC nucleic acid analysis can be performed on a single microfluidic chip normally accompanied by a portable instrument (Pumford et al. 2020; Huang et al. 2021). Superior to traditional methods, point-of-care molecular diagnostics is capable of implementing immediate detection with high convenience in resource-poor settings, which is quite beneficial to efficient detection of infectious deceases, for example, COVID-19 in the global pandemic (GeneXpert 2022; Abbott. 2022).
For ‘sample-in, answer-out’ nucleic acid analysis in point-of-care molecular diagnostics, before amplification, the test sample needs to be processed to extract and purify nuclei acid templates. Traditional nucleic acid extraction is normally performed on regular large-size instrument with manual operation, which is not suitable for POC diagnostics. Therefore, different nucleic acid extraction methods based on microfluidic chips have been intensively studied for nucleic acid analysis at resource-poor settings in POC testing (Azimi et al. 2011; Mosley et al. 2016; Du et al. 2017). With microfluidic chip, nucleic acid extraction can be achieved in a rapid, simple, and convenient way at point-of-care testing (Stumpf et al. 2016; Nguyen et al. 2019).
Currently, nucleic acid amplification can be performed with PCR or other isothermal amplification methods (Qiu et al. 2010; Liu et al. 2011). Normally, PCR must be performed with a thermal cycler to provide accurate thermal cycling to implement three different reaction stages including denaturation, annealing, and extension in one amplification cycle (Sposito et al. 2016). One PCR test consists of 30–40 amplification cycles, which normally take 1–2 h (Wong et al. 2009; Houssin et al. 2016). Comparing to PCR, isothermal amplification can be performed at a constant temperature with a simplified heating strategy. However, the complexity of primer design is lower for PCR comparing to isothermal amplification, for example LAMP (Zhang et al. 2014).
As one of important divisions of PCR, microfluidic PCR enables nucleic acid amplification to be performed with high efficiency, low reagent volume, short reaction time, high throughput, and automatic operation (Chen et al. 2010; Norian et al. 2014; Ahrberg et al. 2016). Because of its characteristics with simplified heating, high sensitivity, high efficiency, and short detection time, as a division of microfluidic PCR, CPCR has drawn more and more attention on the way to applications since its invention (Krishnan et al. 2002). When the CPCR reactor is properly heated with one or two constant temperatures, a continuous circulatory flow will be developed to spontaneously and repeatedly transport the reagent through different temperature zones for amplification (Rajendran et al. 2019a, b). Comparing to conventional PCR, nucleic acid amplification with CPCR can be performed within remarkably short time with pseudo-isothermal heating (Li et al. 2016; Chou et al. 2017; Shu et al. 2017).
Previously, we have developed different CPCR reactors (Qiu et al. 2017a, 2017b, 2017c, 2019; Miao et al. 2022) for rapid nucleic acid amplification, which more focused on CPCR reactor itself. Different from our previous work, this paper demonstrated a combined platform for rapid ‘sample-in, answer-out’ virus detection by taking advantage of both convective PCR and microfluidics. More specifically, this paper reported a microfluidic nucleic acid detection system which can automatically process large-size test samples with a short turn-around time. Different from other existing microfluidic systems which can normally handle small-size test samples, for the developed microfluidic system, large-size test samples can be processed for nucleic acid extraction to provide more templates, which is critical to ensure the detection sensitivity especially for diagnosis of infectious diseases. To perform automated and simple nucleic acid extraction based on magnetic beads especially for a large size of test sample, an integrated microfluidic chip consisting of reagent pre-storage chambers, a lysis & wash chamber, an elution chamber and a waste chamber is developed. To improve the mixing efficiency between magnetic beads and liquid reagent, a portable companion device consisting of multiple linear moving stages equipped with magnets is developed for efficient nucleic acid extraction. On the other side, the nucleic acid amplification time can be reduced with convective PCR. Different from our previously developed CPCR instruments, in this manuscript, a handheld, much smaller real-time CPCR device is developed to perform amplification and in-situ detection with a capillary reactor, which is more convenient for POC test in resource-poor settings. In principle, multiple handheld real-time CPCR devices can be grouped together by a common control system to extend the detection throughput. Therefore, the handheld CPCR device can accommodate both low-throughput and relatively high-throughput applications with high flexibility. By combining microfluidics-based nucleic acid extraction with CPCR, rapid ‘sample-in, answer-out’ nucleic acid analysis to large-size test sample can be conveniently performed. Influenza A (H1N1) viruses can be successfully detected with the combined microfluidic system within 50 min, which as a proof of concept, confirms its potentiality for POC nucleic acid diagnostics.
2 Material and methods
2.1 Integrated microfluidic system for nucleic acid extraction
Traditionally, nucleic acid extraction includes a couple of trivial steps, for example, cell lysis, nucleic acid capturing, purification, and elution, which is a time-consuming process. Therefore, many efforts have been made to simplify nucleic acid extraction with automatic machines or even microfluidic systems (Mosley et al. 2016). To perform automated nucleic acid extraction, an integrated microfluidic chip is developed here. As shown in Fig. 1, the integrated microfluidic chip, which includes two major modules, e.g., a reagent storage & release module and a reaction module, consists of multiple reagent pre-storage chambers, a lysis & wash chamber, an elution chamber and a waste chamber. Different layers of the integrated microfluidic chip are made from polycarbonate (PC) with CNC machining, and then they are bonded together with acetonitrile.
As shown in Fig. 1A, B before reaction, different reagents are independently pre-stored in different chambers of the reagent storage and release module. The major reagents include lysis buffer, wash buffer A and B, elution buffer, magnetic beads, and proteinase K (Beijing Wantai Biological Pharmacy Enterprise, Ltd.). After the test sample with lysis buffer is loaded into the chamber for lysis buffer storage, a needle will be pushed down to pierce the top cover of the microfluidic chip first, and then the bottom layer of the same chamber, which will build a fluid connection between the reagent storage chamber and the lysis and wash chamber on the reaction module, e.g., reagent loading holes shown in Fig. 1B. As shown in Fig. 1C, when the vent hole is open, the lysis buffer can be released and enter the lysis and wash chamber. Similarly, other reagents, e.g., wash buffer A or B, elution buffer can be released at different steps. To simplify the procedure, magnetic beads are manually loaded into the lysis and wash chamber from outside. As shown in Fig. 1C, before the lysis or the wash buffer is discharged into the reaction chamber, the silicone tube (RUNZE FLUID Co., Ltd., Nanjing, China) is kept compressed like a closed valve to avoid the reagent enter the elution chamber. Similarly, when the elution buffer is discharged into the elution chamber, the silicone tube-based valve should be closed. Besides working as a valve, the silicone tube also provides a path for transferring of magnetic beads from the lysis and wash chamber to the elution chamber.
2.2 Portable companion device for nucleic acid extraction
To facilitate fluid control inside the microfluidic chip, especially, to introduce properly controlled 3-D actuation to magnetic beads for efficient mixing, a portable companion device (145 mm × 200 mm × 220 mm) is developed. As shown in Fig. 2A, the portable device consists of multiple liner moving stags equipped with magnets (Lalaci, Lalaci Co., Ltd., Shenzhen, China) and a heating module. A picture of the integrated microfluidic chip is shown in Fig. 2B. As shown in Fig. 2C, a resistance heater (Zhenglong Electrical Thermal Technology Co., Ltd., Yancheng, China) is adopted to heat the microfluidic chip for desired performance in nucleic acid extraction.
As shown in Fig. 2A, different liner moving stags (DINGS' Intelligent Control Technology Co., Ltd., Changzhou, China) are used for different purposes, for example, to push down needles for reagent release, to move the magnets for efficient mixing, to move the microfluidic chip for mixing or fluid control, or to compress the silicone tube for valving, et al. Because the size of the elution buffer is significantly lower than that of other reagents, magnetic beads need to be transferred from the lysis and wash chamber to the smaller elution chamber for efficient mixing.
As shown in Fig. 3A-1, A-2, after the second wash with wash buffer B, the silicone tube-based valve will be opened, which will enable wash buffer B to enter the elution chamber, and meanwhile, the silicone tube will be filled with wash buffer B, which will provide a liquid bridge for transferring of magnetic beads. After that, a magnet mounted on a linear moving stage, which is used for mixing too, is used to concentrate magnetic beads based on magnetic force first, and then transfer them from the lysis and wash chamber to the elution chamber through the liquid bridge.
As shown in Fig. 3B-1, B-2, to facilitate efficient mixing between magnetic beads and liquid reagent, a two-dimensional linear moving stage is adopted to move the integrated chip at X or Y direction, and meanwhile, two linear moving stages at both sides of the chip are adopted to respectively move two magnets up and down. Based on reasonably configured linear moving stages, properly controlled 3-D actuation to magnetic beads can be introduced to generate 3-D mixing trajectory by the companion device, which is critical to the performance of nucleic acid extraction.
2.3 Handheld real-time CPCR system
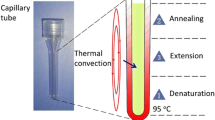
Previously, we have successfully developed CPCR systems for rapid nucleic acid amplification with capillary tube reactors within 30 min. CPCR capillary tubes (inner and outside diameter: 1.6 and 3.3 mm, inner reactor length: 19 mm), which are made from transparent polycarbonate (PC), can be made with injection molding for mass production. As shown in Fig. 4A, unlike other complicated microfluidic PCR chips, the concise capillary tube can be handled much more conveniently just like a conventional 200 μL PCR tube. As shown in Fig. 4B, when the capillary tube is heated from the bottom end with a stable temperature gradient, consistent thermal convection will be introduced because of thermosiphon, which will circulate reactants between hot and cool zones of the reaction for spatially separate melting, annealing, and extension of PCR amplification. Previously, the temperature gradient inside the capillary tube has been analyzed with mathematical modeling and in-silico simulations (Qiu et al. 2019).
As shown in Fig. 4C, to perform real-time CPCR with a capillary tube, a handheld device (60 mm × 60 mm × 60 mm) is developed to run duplicated tests at the same time. As shown in Fig. 4D, the handheld real-time CPCR device consists of two major modules, e.g., a fluorescence detection module and a heating module. In the heating module, a resistive heater is adopted to heat capillary tubes. In the fluorescence detection module, a LED (light-emitting diode, Lumileds Holding B.V) excitation source positioned close to the capillary tube is used to illuminate the reagent through a short pass optical filter (λcut-off = 495 nm, Jingyibodian Co., Ltd., Beijing, China) when the light passes through an optical fiber (Hecho Co., Ltd., Nanjing, China) until reaches the capillary tube. Meanwhile, the fluorescence signal of the reagent inside the capillary tube is collected with an optical fiber and then detected by a photodiode after it is coupled through an optical filter (to block the excitation, λcut-off = 515 nm, Jingyibodian Co., Ltd., Beijing, China). The handheld real-time CPCR device can be powered by an outside 5 V DC power supply with a working current less than 850 mA in amplification. Inside the developed portable device, it has a feedback temperature control system to ensure the accuracy of temperature control in heating. Therefore, powered by a portable mobile power supply with relatively large capacity, the handheld real-time CPCR device can run up to hundred tests, which is desired in POC test.
To increase the detection throughput, a distributed real-time CPCR system is developed. As shown in Fig. 5, a distributed and configurable real-time CPCR system is constructed by connecting multiple handheld CPCR devices to a common main controller (Mycontrol Co., Ltd., Hangzhou, China).
As shown in Fig. 5, a common controller is adopted to control multiple handheld real-time CPCR devices through Bluetooth. Different commands can be sent from the common controller to control each device independently, and meanwhile, data and state information from each device can be sent back to the common main controller for further processing and display. Custom application software based on JAVA was developed to control multiple devices via Bluetooth. The distributed system can be configured with different number of reaction units for different applications. In principle, each handheld device is allowed to independently run new tests at any time, which is beneficial to POC immediate test. Comparing to conventional PCR instrument, with the distributed real-time CPCR system, efficient nucleic acid amplification can be achieved in a more flexible way.
The CPCR reaction mix is comprised of 3 μL mixture of primers (Beijing Wantai Biological Pharmacy Enterprise, Ltd.) and a Taqman probe which is labeled with FAM at 5’end and Eclipse at the 3’end, 4 mM dNTP and 4 μL of Fast Buffer I (Mg ion buffer) (all Takara Bio Inc., Shiga, Japan), 0.4 U of AMV reverse transcriptase (Promega, USA), 1 U of SpeedSTAR HS DNA polymerase (Takara), 10 μL of the purified nucleic acid templates as per above protocol, and molecular-biology grade water to a total reaction volume of 40 μL. Instead of using normal DNA-intercalating fluorescent dye, such as SYTO-9 Green™, a Taqman probe is used for specific fluorescence labeling, which ensures the accuracy and specification of pathogen detection. The primer sequence used with influenza H1N1 template is expected to yield a 105-bp amplicon.
3 Results and discussion
3.1 Evaluation of nucleic acid extraction with the developed microfluidic chip
The performance of nucleic acid extraction with the developed microfluidic chip was systematically evaluated. HBV viruses were used as the test samples. To simplify the procedure, 200 μL of HBV test sample is manually pre-mixed with 400 μL of lysis buffer (Beijing Wantai Biological Pharmacy Enterprise, Ltd.) inside a 1.5 ml tube for 3 min, and then it is loaded into the reagent storage chamber. Meanwhile, 500 μL wash buffer A or B (Beijing Wantai Biological Pharmacy Enterprise, Ltd.), and 50 μL elution buffer (Beijing Wantai Biological Pharmacy Enterprise, Ltd.) are loaded into their corresponding reagent storage chambers, respectively. To reduce the negative effect from the proteins bonded by magnetic beads, 10 μL proteinase K is added into wash buffer A before it is loaded into the microfluidic chip. 20 μL magnetic beads (Beijing Wantai Biological Pharmacy Enterprise, Ltd.) is loaded into the reaction chamber directly. Once all the required reagents are loaded into the microfluidic chip, nucleic acid extraction can be automatically performed with the companion device. After the second wash with wash buffer B, the reaction chamber is heated up to 45 ℃ for 3 min to remove the residual alcohol. Furthermore, when magnetic beads are mixed with elution buffer, the elution chamber is heated up to 56 ℃ for 3 min for efficient elution. In parallel, nucleic acid extraction with a standard instrument (Yuandian, SLA-32, Taiwan Nanotechnology Corp.) based on the benchtop protocol was performed for comparison. Finally, 10 μL purified viral DNA templates are amplified in a conventional real-time PCR instrument. As it is well known that, the Ct value (threshold value) from each test can be used to evaluate the performance of nucleic acid extraction.
To determine the appropriate mixing mode for efficient nucleic acid extraction, we carried out a sequence of experiments in which we varied the motion of linear moving stages. In details, three different mixing modes (mode I, II, III) with different actuation to magnetic beads were evaluated and compared. In mode I, the microfluidic chip is moved back and forth at X direction. In mode II, beside the actuation from mode I, two magnets beside the chip are moved up and down at Z direction. In mode III, beside the actuation from mode II, one more motion at Y direction is included, which finally generate 3-D actuation to magnetic beads. 200 μL of HBV test sample with a medium concentration (15 IU/ml) was used as the test sample. To ensure mixing performance, the mixing cycles for nucleic acid binding with magnetic beads and elution were 160 and 180, respectively. Totally, it takes almost 20 min for nucleic acid extraction to be completed with the developed microfluidic chip.
As shown in Fig. 6A, different from mode I or mode II respectively with 1-D or 2-D actuation to magnetic beads, the performance of nucleic acid extraction of mode III with 3-D actuation to magnetic beads is comparable to that with the standard instrument (Yuandian). In Fig. 6A, each error bar represents the variation range of the threshold cycle of conventional PCR with multiple repeated tests for each operation mode. In the experiments, 200 μL test sample is mixed with 400 μL lysis buffer for nucleic acid extraction. When the reagent size is high, for example, up to 600 μL, it is difficult to dynamically and uniformly distribute magnetic beads in such a big reactor space with just 1 or 2-D actuation based on outside magnetic field. Therefore, for mode I and II, magnetic beads cannot be thoroughly mixed with reagent at each step, which will deteriorate the performance of nucleic acid extraction. In contrast, for mode III, properly controlled 3-D actuation to magnetic beads can be achieved based on programmable motion of 3-D linear moving stages, which will enable thorough mixing between magnetic beads and reagent even in a big size reactor. The purity of the extracted DNA samples was measured with a spectrophotometer (NanoDrop Lite Plus, ThermoFisher), and their OD260/OD280 values were between 1.72 and 1.76.
Beside variation of motion dimensionality of actuation to magnetic beads, the performance of nucleic acid extraction with different mixing cycles were evaluated and compared. As shown in Fig. 6B, two typical operation states with different mixing cycles are compared between each other. In Fig. 6B, each error bar represents the variation range of the threshold cycle of conventional PCR with multiple repeated tests for each operation state. In the experiments, 200 μL test sample is mixed with 400 μL lysis buffer for nucleic acid extraction. In state I, two mixing cycles for nucleic acid binding with magnetic beads and elution are 80 and 100, respectively. In state II, the two similar mixing cycles are 160 and 180, respectively. Because multiple linear moving stages are driven and controlled by a microcontroller, their operation cycles can be conveniently programmed. It is found that, when the number of the mixing cycle increases, the performance of nucleic acid extraction will be improved accordingly. The proper mixing cycles (160: nucleic acid binding with magnetic beads, 180: elution) are chosen when the performance of the microfluidic chip is quite close to that with the standard instrument (Yuandian). Therefore, the performance of nucleic acid extraction with the developed microfluidic system can be optimized with both the mode of actuation to magnetic beads and the mixing cycle, which can be conveniently configured by the system microcontroller.
3.2 Detection of influenza virus with the microfluidic system
First, the system performance was evaluated and optimized to achieve consistent and stable performance. To evaluate the performance of the heating module, a thin thermistor (diameter: 0.76 mm) was inserted into the capillary tube filled with 40 μL water sealed by mineral oil from top, and then it was connected to a temperature reader (USB-TEMP, Measurement Computing Corporation, USA) to monitor the reaction temperature. Figure 7A depicts the thermal time-response of the capillary tube when it is heated from the bottom side. It takes less than 5 min for the inner temperature of the capillary tube to reach 95 ℃.
To find out the proper LED working current for optimal fluorescence detection, signal ratios between positive and negative tests with different driving currents were measured and analyzed. As shown in Fig. 7B, when the LED working current increases, the signal ratio increase accordingly. Finally, 100 mA is chosen as the LED working current since its signal ratio is quite close to that with 120 mA. By increasing the LED working current up to 100 mA, the signal ratio can reach close to 4, which is helpful to achieve satisfied dynamic range of fluorescence signal detection in qualitative diagnosis.
The performance of the developed microfluidic system is demonstrated with the detection to H1N1 influenza virus. The viral samples (200 μL) were tenfold serially diluted from influenza A (H1N1) virus culture stock (100–1.0 TCID50/ml). Currently, for H1N1 clinical diagnostics applications, a limit of detection of 1.0 TCID50/ml or better (101.29 TCID50/ml) is feasible with commercial kits (Influenza A virus real-time PCR kit, Guangzho Hauyin Medicine Science, Ltd). Purified viral RNA was amplified in a single-tube CPCR reaction combining reverse transcription and cDNA amplification. For each test, a real-time fluorescence curve is achieved when the detected fluorescent intensity is indicated as a function of the amplification time.
After nucleic acid extraction with the developed microfluidic chip, the PCR reaction mix with templates is loaded into the capillary tube, and then the PCR mix is covered with mineral oil on top. The capillary tube is put into the handheld real-time CPCR device for amplification. Because of the uncontrollable amplification cycle of thermal cycling in CPCR, qualitative test instead of quantitative test is provided by the developed system. As shown in Fig. 8, all the positive samples have been successfully detected. Comparing to negative test, all the real-time curves for positive samples rise within 30 min, which is significant for qualitative test. The experiments were repeated multiple times with similar results.
Real-time CPCR with H1N1 influenza virus RNA template. In details: A original fluorescent images and end-point ones of amplicons with diluted H1N1 virus samples (1.0, 10, 100TCID50/ml), B analyzed real-time fluorescence curves with diluted H1N1 virus samples (1.0, 10, 100TCID50/ml) and a negative control (NC), the dotted line, short dashed line, long dashed line, and solid line correspond, respectively, to negative control (NC), and samples with concentrations of 1.0, 10, 100 TCID50/ml
As shown in Fig. 8A, for each test sample, 1.0, 10, or 100TCID50/ml, the left fluorescent image is the original one before amplification, and the right fluorescent image is the end-point one after amplification. For each test sample, it can be found that its fluorescence signal intensity is significantly increased after amplification. As shown in Fig. 8B, H1N1 viruses even with the concentration down to 1.0 TCID50/ml can be successfully detected. Comparing to conventional PCR, with CPCR, amplification time has been significantly reduced to 30 min. Comparing to traditional methods relying on large and heavy devices, with the developed portable microfluidic system, nucleic acid diagnosis can be achieved within a short time, e.g., 50 min, which is highly desired in POC testing. In principle, the total detection time can be further decreased by optimizing the protocol of nucleic acid extraction on the developed microfluidic chip.
4 Conclusions and outlook
To perform rapid nucleic acid analysis from sample to answer, a microfluidic chip for automated nucleic acid extraction and a handheld real-time CPCR system for rapid amplification are developed in a pair. With the developed system, rapid, simple, easy-to-operate and low-cost nucleic acid analysis at POC testing can be achieved. The integrated microfluidic chip is developed to automate nucleic acid extraction, which potentially enable nucleic acid analysis to be implemented out of center labs. As a rapid amplification method, CPCR can complete nucleic acid amplification within 30 min with a much-simplified heating strategy in pseudo-isothermal manner.
A concise, low-cost, and integrated microfluidic chip, which consists of a reagent storage and release module and a reaction module, is developed to perform nucleic acid extraction based on magnetic beads for a large-size test sample. To ensure the performance of nucleic acid extraction, different mixing modes as well as different mixing cycles are systematically evaluated and compared. Finally, an optimal mixing mode with proper mixing cycles are adopted to achieve comparable performance to that with the traditional method. To improve the detection throughput, a distributed real-time CPCR system consisting of multiple handheld CPCR devices is developed. Each handheld real-time CPCR device, which is independently controlled by a main controller, can start new tests at any time when test samples come in, for example, in emergency rooms or doctor's offices. As a proof of concept, experimental results with the detection to H1N1 influenza virus demonstrate that the handheld real-time CPCR system combined with microfluidic chip-based nucleic acid extraction can complete nucleic acid test from sample to answer within 50 min, which is beneficial to POC testing. In the next step, to construct a fully integrated microfluidic system, more efforts can be made to not only integrate the sample-processing microfluidic chip with the CPCR capillary tube reactor, but also optimize the strategy for long-term reagent storage.
References
Abbott (2022) Abbott ID NOW COVID-19. https://www.globalpointofcare.abbott/en/product-details/id-now-covid-19-ous.html. Accessed on 01 Aug 2022
Ahrberg CD, Manz A, Neužil P (2016) Palm-sized device for point-of-care Ebola detection. Anal Chem 88:4803–4807
Azimi SM, Nixon G, Ahern J, Balachandran W (2011) A magnetic bead-based DNA extraction and purification microfluidic device. Microfluid Nanofluidics 11:157–165
Chen D, Mauk M, Qiu X et al (2010) An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids. Biomed Microdevices 12:705–719
Chou W-P, Lee C, Hsu Z-J et al (2017) Development of capillary loop convective polymerase chain reaction platform with real-time fluorescence detection. Inventions 2:3
Du K, Cai H, Park M et al (2017) Multiplexed efficient on-chip sample preparation and sensitive amplification-free detection of Ebola virus. Biosens Bioelectron 91:489–496
Foudeh AM, Didar TF, Veres T, Tabrizian M (2012) Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip 12:3249–3266
GenxXpert (2022) Xpert® Xpress SARS-CoV-2 Instructions for Use. https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SA RSCoV2%20Assay%20ENGLISH%20Package%20Insert%203023787%20Rev.%20B.pdf. Accessed on 01 Aug 2022
Houssin T, Cramer J, Grojsman R et al (2016) Ultrafast, sensitive and large-volume on-chip real-time PCR for the molecular diagnosis of bacterial and viral infections. Lab Chip 16:1401–1411
Huang E, Wang Y, Yang N et al (2021) A fully automated microfluidic PCR-array system for rapid detection of multiple respiratory tract infection pathogens. Anal Bioanal Chem 413:1787–1798
Krishnan M, Ugaz VM, Burns MA (2002) PCR in a Rayleigh-Benard convection cell. Science 298:793–793
Li Z, Yang Z, Zhang D et al (2016) The development of a portable buoyancy-driven PCR system and its evaluation by capillary electrophoresis. Sens Actuator B-Chem 230:779–784
Liu C, Mauk MG, Hart R et al (2011) A self-heating cartridge for molecular diagnostics. Lab Chip 11:2686–2692
Miao G, Jiang X, Yang D et al (2022) A hand-held, real-time, ai-assisted capillary convection PCR system for point-of-care diagnosis of African swine fever virus. Sens Actuators B Chem 358:131476
Mosley O, Melling L, Tarn MD et al (2016) Sample introduction interface for on-chip nucleic acid-based analysis of Helicobacter pylori from stool samples. Lab Chip 16:2108–2115
Norian H, Field RM, Kymissis I, Shepard KL (2014) An integrated CMOS quantitative-polymerase-chain-reaction lab-on-chip for point-of-care diagnostics. Lab Chip 14:4076–4084
Pumford EA, Lu J, Spaczai I et al (2020) Developments in integrating nucleic acid isothermal amplification and detection systems for point-of-care diagnostics. Biosens Bioelectron 170:112674
Qiu X, Mauk MG, Chen D et al (2010) A large volume, portable, real-time PCR reactor. Lab Chip 10:3170–3177
Qiu X, Ge S, Gao P et al (2017a) A low-cost and fast real-time PCR system based on capillary convection. Slas Technol Transl Life Sci Innov 22:13–17
Qiu X, Zhang S, Mei L et al (2017b) Characterization and analysis of real-time capillary convective PCR toward commercialization. Biomicrofluidics 11:024103
Qiu X, Zhang S, Xiang F et al (2017c) Instrument-free point-of-care molecular diagnosis of H1N1 based on microfluidic convective PCR. Sens Actuators B Chem 243:738–744
Qiu X, Shu J-I, Baysal O et al (2019) Real-time capillary convective PCR based on horizontal thermal convection. Microfluid Nanofluidics 23:1–8
Rajendran VK, Bakthavathsalam P, Bergquist PL, Sunna A (2019a) Smartphone detection of antibiotic resistance using convective PCR and a lateral flow assay. Sens Actuators B Chem 298:126849
Rajendran VK, Bakthavathsalam P, Bergquist PL, Sunna A (2019b) A portable nucleic acid detection system using natural convection combined with a smartphone. Biosens Bioelectron 134:68–75
Shu B, Zhang C, Xing D (2017) A sample-to-answer, real-time convective polymerase chain reaction system for point-of-care diagnostics. Biosens Bioelectron 97:360–368
Soni S, Toley BJ (2022) Paper-based nucleic acid sample preparation for point-of-care diagnostics. Sens Actuators B Chem 355:131272
Sposito A, Hoang V, DeVoe D (2016) Rapid real-time PCR and high resolution melt analysis in a self-filling thermoplastic chip. Lab Chip 16:3524–3531
Stumpf F, Schwemmer F, Hutzenlaub T et al (2016) LabDisk with complete reagent prestorage for sample-to-answer nucleic acid based detection of respiratory pathogens verified with influenza A H3N2 virus. Lab Chip 16:199–207
Van Nguyen H, Nguyen VD, Nguyen HQ et al (2019) Nucleic acid diagnostics on the total integrated lab-on-a-disc for point-of-care testing. Biosens Bioelectron 141:111466
Wong K, Lyddon R, Dracheva S (2009) TaqMan-based, real-time quantitative polymerase chain reaction method for RNA editing analysis. Anal Biochem 390:173–180
Zhang Y, Zhang L, Sun J et al (2014) Point-of-care multiplexed assays of nucleic acids using microcapillary-based loop-mediated isothermal amplification. Anal Chem 86:7057–7062
Zhuang J, Yin J, Lv S et al (2020) Advanced “lab-on-a-chip” to detect viruses–current challenges and future perspectives. Biosens Bioelectron 163:112291
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 81871505, 61971026), the Fundamental Research Fund for the Central Universities (XK1802-4), the National Science and Technology Major Project (2018ZX10732101-001-009), and the research fund to the top scientific and technological innovation team from Beijing University of Chemical Technology (No. buctylkjcx06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, D., Jiang, X., Zou, T. et al. A microfluidic system for rapid nucleic acid analysis based on real-time convective PCR at point-of-care testing. Microfluid Nanofluid 26, 69 (2022). https://doi.org/10.1007/s10404-022-02577-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-022-02577-5