Abstract
A new type of levitating droplet cluster composed of often transforming small aggregates of water droplets is described for the first time. Unlike earlier observed droplet clusters controlled by aerodynamic forces, which formed either an ordered hexagonal structure or a chain structure, the cluster under consideration has a hierarchical organization. Small groups of closely spaced or packed droplets with interactions controlled by the electrostatic force are combined into larger structures controlled by aerodynamic forces. Since charged droplets in the nucleus of the cluster do not have dynamically stable configurations, droplet aggregates keep continuously restructuring. However, droplets with lower charges in external layers of the cluster form a stable hexagonal structure.
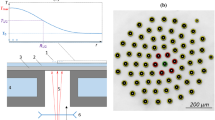
Graphical abstract
Levitating hierarchical droplet cluster build of small droplet groups.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Close-packed colloidal crystals made of rigid microparticles are a natural phenomenon that has been studied for decades. Cooperative motion, self-assembly of ordered structures, and structural phase transitions in colloidal crystals are similar to effects observed in the condensed matter (Grzybowski et al. 2017). Recently, small colloidal clusters have attracted the attention of researchers (Perry et al. 2015; Lim et al. 2019). Such clusters of several particles to several dozens of particles can form close-packed structures while levitating due to acoustic waves or due to another mechanism. Small clusters often possess properties absent in large crystals.
Unlike close-packed structures with neighboring particles touching each other, levitating particles can form configurations and possess symmetries not typical of large crystals. Perry et al. (2015) investigated 2D spherical particles of solid sulfate polystyrene suspended in a fluid that formed clusters demonstrating structural rearrangements. In certain configurations, individual particles in these clusters may have bonds between them due to aerodynamic forces. For a system of six particles, Perry et al. (2015) identified seven-bond and eight-bond configurations. Lim et al. (2019) reported transitions between sticky and ergodic configurations in similar six-particle and seven-particle systems which could form various arrangements, with different characteristic probabilities likely associated with the Zipf probability distribution (Nosonovsky and Roy 2020).
Self-assembled clusters of condensed microdroplets levitating over a locally heated water surface have been first reported by Fedorets in 2004 (Fedorets 2004). When a thin (about 1 mm thick) layer of water is heated locally to the temperatures at which water evaporates actively (60–90 °C) small water droplets (usually from 5 to 50 μm in radius) are condensed in the ascending flow of mixed air and water vapor. Droplets tend to form monolayer clusters levitating at heights comparable with their radii. Such clusters are typically arranged into an ordered hexagonal structure due to an interplay of aerodynamic forces which drag the droplets towards the center of the heated flow facilitating closed packing and aerodynamic repulsion forces between droplets facilitating a distance between them. Besides large (dozens to hundreds of droplets) hexagonally ordered droplets, small clusters (from one to dozens of droplets) have been discovered and a methodology to synthesize them has been developed. Small clusters may possess symmetries absent from the large clusters including the fourfold, fivefold, and sevenfold symmetries (Fedorets et al. 2017). It has been hypothesized that the unusual symmetries can be classified with the simply laced Dynkin diagrams (the ADE-classification) (Fedorets et al. 2020a). Small clusters demonstrate collective behavior including simultaneous horizontal and vertical oscillations (Fedorets et al. 2019a, 2021).
Besides the clusters with a hexagonal arrangement of droplets, the chain-like arrangement was found under certain conditions. The transition between the hexagonal and chain arrangements is reversible and it possesses characteristics of a phase transition (Fedorets et al. 2019b). When the thermocapillary flow is not suppressed by surfactants, a ring cluster surrounding the convective vortex flow can also form (Fedorets et al. 2020b).
Here we report a new type of cluster arrangement, hierarchical clusters (H-clusters). These are relatively large clusters consisting of small groups or aggregates of droplets with bonds between individual droplets in each group. Unlike in previously studied clusters, droplets in the hierarchical cluster may carry electrical charges of opposite signs, and electrostatic forces play a role in a droplet group arrangement, while aerodynamic forces control interactions between the droplets as usual (Fedorets et al. 2020c).
2 Results and discussion
2.1 Experimental results
The droplet clusters were formed from microdroplet clouds created by an ultrasound nebulizer, which generated water microdroplets by ultrasonic acoustic irradiation. Ultrasonic nebulization of water and other polar liquids results in electrically charged microdroplets (the Lenard or ballo-electric effect). In a dry environment, the droplets keep their charge for a significant time. In particular (Zilch et al. 2008), both positively and negatively charged water droplets (the latter constitute about 10% of all droplets) are formed by ultrasonic nebulization. The typical charge is on the order of hundreds or thousands of elementary charges with the probability density function decreasing quickly with increasing charge. Droplets with the charge of dozens of thousands of the elementary charge also exist but they are rare (Zilch et al. 2008).
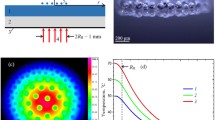
A typical lifecycle of the H-cluster is presented in Fig. 1 (also in Video 1). Similarly to earlier reported chain clusters (Fedorets et al. 2019b), the H-clusters form when droplets become large enough, so that vortex flows in between the droplets facilitate the structure formation. Such vortices are observed in some of the videos showing small particles on the water surface. While weakly charged droplets forming chains are condensed in humid air, those formed in the dry air result in H-clusters, which are the focus of the present study. For the H-clusters, complex electrostatic interactions between droplets are imposed over complex aerodynamic forces generated by the air fluid flow. Consequently, clusters are built not just of single droplets, but of small groups of droplets (typically two to four), which we call L-groups. The experiments were repeated many times, and dozens of video records of the hierarchical clusters have been obtained.
At first, many charged droplets penetrate into the cluster (Fig. 1a) causing rapid merging of droplets and coalescence of individual droplets with the water layer [which does not cause the collapse of the entire cluster (Fedorets et al. 2015)]. These processes led to the decrease in the total number of droplets in the cluster, N. Thus, in 40 s separating the snapshots in Fig. 1a and b–c, the number of droplets decreased by approximately one-third of the initial number of droplets. As the number of strongly charged droplets decreases, the intensity of droplet merging and coalescence with the substrate decreases as well, although these processes go on during the entire life cycle of the H-cluster. The cluster was not subject to external infrared irradiation, which stabilizes droplet sizes (Dombrovsky et al. 2020). Consequently, when a critical droplet diameter was reached, the known effect of the cluster collapse was triggered (Fedorets and Marchuk 2014). The coalescence of the cluster with the water layer is a chain reaction due to capillary waves on the surface of the layer, so the disappearance tends to be a very fast process on the order of one millisecond (Fig. 1c, d).
High-frequency video recordings show some details of the merging and coalescence (Fig. 2 and Video 1), which occurs at the time on the order of several microseconds. Frames (a) and (b) are consecutive (with the time distance of 1/50 = 0.02 s), as well as frames (c) and (d).
The coalescences of two droplets seen in the video (100 frames per second) are the merge of the two droplets into a new large droplet (Fig. 2, droplets 3 + 4, 8 + 9). When two droplets coalesce, their total volume is conserved. Thus, before the coalescence, the total volume of droplets 3 and 4 was 20.75 + 24.21 = 44.96 pl, while after the event it was 44.88 ± 0.73 pl. For droplets 8 and 9, the volume before and after the coalescence was 18.64 + 18.64 = 37.28 before the merger and 37.27 ± 0.73 pl after (Table 1). The margins of error (± 0.4 µm and ± 0.73 pl) were calculated from the pixel size. In both cases, the difference is attributed to the accuracy of the measurements. Besides that, the coalescence with the layer is observed (Fig. 2, droplets 5, 6, 7).
The analysis of a series of frames shows that at the initial stage of H-cluster formation, the number of single droplets decreases sharply (Table 2). The most common groups are of four or fewer droplets, which is consistent with various rank-frequency statistical distributions (such as the Zipf law).
It is observed that some of the droplets have a light spot on them, while other droplets of similar size do not have it. The difference is caused by levitation heights of the droplets. For example, the droplet 1 is above the droplet 2, with their images overlapping at about 10% of the diameters (Fig. 3). One can conclude that the difference in their levitation heights is approximately equal to their radius. Similarly, heavy droplets close to the water layer’s surface due to their increased mass have a light spot (droplets 3, 4, 5 in Fig. 3). We will denote lower levitating droplets as type “n” and higher levitating droplets as type “p”. Thus in the same cluster, different droplets can levitate at significantly different heights. This is another indication of the presence of droplets of opposite charges in the H-cluster.
It is known that droplets tend to attain a small negative charge during their condensational growth (Fedorets et al. 2020c). The surface of the evaporating water layer can charge positively. Consequently, an electrostatic interaction force between the droplet and the layer is added to the aerodynamic drag force of the ascending gas flow. As the size of the p- and n-droplets may be the same, different height indicates the different signs of the electric charge, so that there is an attraction or repulsion force component. The positive charge of the water layer surface is a result of evaporation. The equilibrium levitation height of positively charged droplets grows, while for negatively charged droplets the levitation height decreases down to the coalescence with the water layer.
The observed property of the L-groups is that droplets are continuously moving the groups are restructuring exchanging droplets and fragments of several droplets. The type of the droplet can change as well, as evidenced by rapid video recording. Thus, it is observed that a group of five droplets is divided into groups of two and three droplets within dozens of milliseconds. Droplet 2 changes its n-type into the p-type, while droplet 3 changes the p-type to n-type (Fig. 4). This indicates that the levitation height of the droplet at any instance of time depends on its closest neighbors.
Discussion of results. The H-clusters are formed with the use of the nebulizer and they emerge only in dry air. The relative humidity of the ambient air in the lab (measured with the ThermoHygrometer IVTM-7 M, eksis.ru) was at the levels not exceeding 8–12%. At higher levels of the relative humidity, the charge is lost quickly and a usual hexagonal cluster is formed. The level of humidity determines whether the electric charge relaxes from the droplets. Note that droplets cannot be treated as point charges, they clearly have a dipole moment. Due to the large fraction of initially weakly charged droplets in the aerosol and the loss of charge, only some droplets in the cluster participate in the formation of L-groups. Thus, at the final stage (Fig. 1c), only 177 droplets out of the total N = 437 (i.e., about 40%) participate in L-groups.
The structure and dynamic behavior of the central part of the H-cluster can be explained by assuming that some droplets are charged positively, while others are charged negatively. The charge results in electrostatic forces, whose magnitude is comparable with the aerodynamic forces acting upon a regular droplet cluster in the absence of the electric charge. However, the aerodynamic forces still dominate (Fedorets et al. 2019c, 2020c). Unlike in a regular cluster of uncharged droplets, which has a relatively stable hexagonal equilibrium configuration (as observed at the periphery of the H-cluster), the central part of the cluster does not possess the equilibrium configuration. On the contrary, one can see continuous rearrangement of the droplets and their groups.
Since the small L-groups of water droplets in the central part of the H-cluster are arranged approximately the same way as individual droplets in a normal hexagonal cluster, the distance between the L-groups is related only to the peculiarities of the flow of humid air between them and the electric charge of droplets in the L-groups has no effect on this.
Consider now the droplets in L-groups, separated by the distance which is much smaller than the radii of the droplets, is observed. What is the reason for this non-coalescence? Obviously, the coalescence diminishes the interfacial energy of droplets, resulting in the effective attraction force, acting between the droplets. Let us estimate the characteristic scale \({R}^{*}\) at which electrostatic effects become comparable to the interfacial ones. We compare the energy of electrostatic interaction between two charged droplets possessing electrical charge \(Q\) and radii R separated by the negligible distance to the interfacial energy of the droplets:
where k is the Coulomb’s constant. The length scale at which these energies become comparable are given by
The realistic estimation is \(Q\cong 1000e=1.6\times {10}^{-16}C,\) substitution this value and assuming \(\gamma =70\times {10}^{-3}\frac{\mathrm{J}}{{\mathrm{m}}^{2}}\) yields \({R}^{*}\cong 1.0\times {10}^{-7}\mathrm{m}=100 \mathrm{nm}.\) For the droplets larger than \({R}^{*}\) (and this is the case in our experiments) interfacial effects will dominate on the electrostatic ones, and interfacial attraction will prevail upon the electrostatic repulsion. Thus, electrostatic repulsion is definitely not responsible for the non-coalescence. It seems plausible to relate the effect of non-coalescence to the rotation of droplets or convective motion of water neat the surface of non-isothermal droplets (Fedorets et al. 2015) drawing thin air layer adjacent to the surface of droplets, as shown in Dell’Aversana et al. (1996). It was demonstrated that the presence of explicitly moving interfaces may provide a lubrication pressure which can resist coalescence for macroscopically long times. Thus, from the observation of a cluster with fluorescent bio-particles (Chlorella vulgaris Beijer single-celled algae) embedded in its droplets (Fedorets and Biocluster 2021), it is found that a typical revolution period of a droplet is on the order of 1 s.
Both the droplet rotation and water flow along the surface inside the droplet are accompanied by formation of a thin layer of a moving gas in the gap between the neighboring droplets of L-groups. In this gap, the gas velocity varies linearly between the local velocities of water at the surfaces of two water droplets. The viscous gas layer works as a kind of lubricant that promotes the continuation of the almost independent rotation of the droplet (or the water flow under their surface) (Dell’Aversana et al. 1996; Dell'Aversana and Neitzel 2004). The small value of the dynamic viscosity of a gas ensures the high quality of such “gas lubrication”. Judging by the rare coalescence of the droplets in L-groups, complete overlap of the gas gap in a group of closely spaced droplets rarely occurs. This means that maintaining a thin gas gap between the droplets is a typical situation for the L-groups, explaining the observed frequent rearrangement of droplets.
In the gaps between the L-group droplets, the gas pressure is constant (as, for example, in the viscous boundary layer of any gas flow). Therefore, the only force acting from the gas side on the droplets inside the L-group is the viscous force, which can neither bring the droplets closer to each other nor remove them from each other, because this force is directed along the droplet surface. Obviously, water droplets join into L-groups and remain in them only due to the droplet charge and electrostatic forces. Forces on the gas flow side have nothing to do with this phenomenon.
The electric charge is created due to the Lenard (or ballo-electric) effect during the dispersion of liquid. Droplets’ electrical charge is very sensitive to the ambient humidity since the charge remains only in dry air. In humid air, the electric charges are lost, in particular, those of the positive sign, as condensational growth of the droplets often creates negative charges. Note that the evaporation of microdroplets may be very fast, especially in a low-humidity environment (Shang et al. 2022).
Unlike the H-cluster, the chain cluster is controlled by pure aerodynamic origin, and it is observed when droplets are electrically neutral. The interplay of aerodynamic and electrostatic forces creates any effects in the H-cluster which are of potential interest for the 2D aerosol technology.
The H-cluster can be characterized as chaotic or unstable, because it keeps rearranging. At the same time, it demonstrates certain orderliness, although it is less ordered than the hexagonally ordered droplet cluster and the chain droplet cluster. The H-cluster can therefore be viewed as a new type of colloidal crystal, which is chaotic but still ordered.
3 Materials and methods
The experimental setup is presented in Fig. 5. The cluster (1) was formed over a locally heated spot of a horizontal layer of distilled water, (2) containing surfactant microadditives (sodium dodecyl sulfate) suppressing thermocapillary flows. A sitall substrate (400 μm thick), (3) was attached to the bottom of the cuvette, (4) the body of the cuvette contained channels connected to the cryothermostat CC805 (Huber, Germany) allowing temperature stabilization in the water layer. Local heating of the water layer can be performed with a laser beam (MRL-III-660D-1 W, CNI, China), (5) directed towards the lower side of the substrate, (6) is the camera lens.
Temperature field on the water layer surface was detected by an IR camera A655sc (FLIR, USA). Visual recording of the cluster was conducted with a stereomicroscope AXIO Zoom.V16, (Zeiss, Germany), with the attached high-speed camera PCO.EDGE 5.5C (PCO, Germany). In all experiments the thickness of the water layer was maintained at 400 ± 2 μm, and thermostatic temperature T0 = 15 ± 1 °C. The cluster was generated in an open cuvette, with the ambient air temperature in the lab 26 ± 1 °C.
3.1 Synthesis of H-clusters
There are two requirements for the H-cluster synthesis. First, the H-cluster is formed from a cloud of microdroplets generated by an ultrasonic nebulizer. Second, the ambient air in the laboratory must be very dry: the relative humidity is about ten percent or less.
The microdroplet cloud was created by an ultrasound nebulizer (Omron, Japan), which generated aerosol microdroplets (diameter about 5 μm) by ultrasonic acoustic irradiation. The aerosol was injected when the heating power level was decreased (the local temperature of the water layer at the center of the heating spot was Tmax = 60 ± 2 °C), which facilitated the penetration of small droplets to the cluster area. It was also important to keep the density of droplets at the heating spot is high. After that, the heating power was abruptly increased with temperature stabilized at 75 ± 2 °C, and an active condensational growth of the droplets as well as their coalescence occurred. As a consequence of the growth of the droplet sizes, at a certain point, various local multi-droplet structures started to form. With further increasing size of the droplets, the cluster collapses due to coalescence of the droplets.
References
Dell’Aversana P, Banavar JR, Koplik J (1996) Suppression of coalescence by shear and temperature gradients. Phys Fluids 8:15–28
Dell’Aversana P, Neitzel GP (2004) Behavior of noncoalescing and nonwetting drops in stable and marginally stable states. Exp Fluids 36(2):299–308
Dombrovsky LA, Fedorets AA, Levashov VY, Kryukov AP, Bormashenko E, Nosonovsky M (2020) Stable cluster of identical water droplets formed under the infrared irradiation: experimental study and theoretical modeling. Int J Heat Mass Transfer 161:120255
Fedorets AA (2004) Droplet cluster. JETP Lett 79:372–374
Fedorets AA IV, Marchuk OAK (2014) On the role of capillary waves in the mechanism of coalescence of a droplet cluster. JETP Lett 99:266–269
Fedorets AA, Dombrovsky LA, Smirnov AM (2015) The use of infrared self-emission measurements to retrieve surface temperature of levitating water droplets. Infrared Phys Technol 69:238–243
Fedorets AA, Frenkel M, Bormashenko E, Nosonovsky M (2017) Small levitating ordered droplet clusters: stability, symmetry, and Voronoi entropy. J Phys Chem Lett 8(22):5599–5602
Fedorets AA, Aktaev NE, Gabyshev DN, Bormashenko E, Dombrovsky LA, Nosonovsky M (2019a) Oscillatory motion of a droplet cluster. J Phys Chem C 123(38):23572–23576
Fedorets AA, Frenkel M, Legchenkova I, Shcherbakov D, Dombrovsky LA, Nosonovsky M (2019b) Self-arranged levitating droplet clusters: a reversible transition from hexagonal to chain structure. Langmuir 35(47):15330–15334
Fedorets AA, Dombrovsky LA, Bormashenko E, Nosonovsky M (2019c) On relative contribution of electrostatic and aerodynamic effects to dynamics of a levitating droplet cluster. Int J Heat Mass Transfer 133:712–717
Fedorets AA, Bormashenko E, Dombrovsky LA, Nosonovsky M (2020a) Symmetry of small clusters of levitating water droplets. Phys Chem Chem Phys 22(21):12239–12244
Fedorets AA, Shcherbakov DV, Dombrovsky LA, Bormashenko E, Nosonovsky M (2020b) Impact of surfactants on the formation and properties of droplet clusters. Langmuir 36(37):11154–11160
Fedorets AA, Dombrovsky LA, Gabyshev DN, Bormashenko E, Nosonovsky M (2020c) Effect of external electric field on dynamics of levitating water droplets. Int J Therm Sci 153:106375
Fedorets A (2021) Biocluster. Chlorella vulgaris Beijer (08.12.18) https://www.youtube.com/watch?v=Ihc4cgtLUg8. Accessed 15 Dec 2021
Fedorets AA, Gabyshev DN, Shcherbakov D, Bormashenko E, Dombrovsky LA, Nosonovsky M (2021) Vertical oscillations of droplets in small droplet clusters. Colloids Surf A 628:127271
Grzybowski BA, Fitzner K, Paczesny J, Granick S (2017) From dynamic self-assembly to networked chemical systems. Chem Soc Rev 46:5647–5678
Lim MX, Souslov A, Vitelli V, Jaeger HM (2019) Cluster formation by acoustic forces and active fluctuations in levitated granular matter. Nat Phys 15:460–464
Nosonovsky M, Roy P (2020) Scaling in colloidal and biological networks. Entropy 22(6):622
Perry RW, Holmes-Cerfon MC, Brenner MP, Manoharan VN (2015) Two-dimensional clusters of colloidal spheres: ground states, excited states, and structural rearrangements. Phys Rev Lett 114:228301
Shang X, Zhang X, Nguyen TB, Tran T (2022) Direct numerical simulation of evaporating droplets based on a sharp-interface algebraic VOF approach. Int J Heat Mass Transfer 184:122282
Zilch LW, Maze JT, Smith JW, Ewing GE, Jarrold MF (2008) Charge separation in the aerodynamic breakup of micrometer-sized water droplets. J Phys Chem A 112:13352–13363
Acknowledgements
The authors gratefully acknowledge the Ministry of Science and Higher Education of the Russian Federation (Project No. A20-120051490005-9) for the financial support of this work.
Funding
This research was supported by Russian Science Foundation (Grant 19-19-00076).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supporting information. Video 1. A typical lifecycle of the H-cluster (AVI 313728 KB)
Rights and permissions
About this article
Cite this article
Fedorets, A.A., Dombrovsky, L.A., Bormashenko, E. et al. A hierarchical levitating cluster containing transforming small aggregates of water droplets. Microfluid Nanofluid 26, 52 (2022). https://doi.org/10.1007/s10404-022-02557-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-022-02557-9