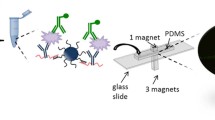
Abstract
The enhanced capture of the antigen (Ag) by the surface-immobilized antibodies (Ab) in heterogeneous microfluidic immunosensors lowers (improves) the detection limit thus facilitating the early disease detection. The efficient capture of the antigen is subjected to the interaction of the transport parameters, reaction parameters and the microfluidic system geometry. In the present work, the role of active mixing in facilitating the transport in flow-based heterogeneous immunosensor was studied, both numerically and experimentally. The experiments were performed with the capture of prostate-specific antigen (PSA) by anti-PSA and the results were validated using numerical simulations. The average Sherwood number (Sh) for the experiments and simulations was comparable. First, the effect of the nature of the flow on the capture efficiency was tested, which showed the enhancement in the antigen capture using active mixing. Further, studies of individual active mixing parameter (average velocity, frequency and amplitude) on analyte capture were conducted. The increase in average velocity (uavg) increases the antigen capture but the corresponding pressure drop also increases, and the figure of merit (FOM) attains a maximum at Re ~ 0.6. Similar behavior was observed with the increase of the frequency. The continuous increase in the frequency reduces the reaction time scale and decreases the analyte capture. The amplitude has the least effect on the antigen capture. The flow profiles were also seen and the temporal variation was compared at different flow conditions with micro-PIV. We also proposed two correlations utilizing the parameters—the average surface concentration (Cs,avg), the Reynolds number (Re) and the Strouhal number (St). The correlations indicate that the dependency coefficient for St is higher than Re, and signify the impact of vortex propagation (i.e., active mixing) on antigen capture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microfluidic immunosensors have attracted great interest for point-of-care applications. The beneficial features of microfluidic devices include a high surface area–volume ratio, reduced reagent consumption, short analysis times, and high efficiency. In a flow-based microfluidic immunosensor, antibodies (Ab) are immobilized on the channel walls via intermediate linkers, and antigens (Ag) are transported from the bulk solution to the surface of the microchannel to react with and be captured by the Ab. An important performance parameter is the “capture efficiency”, which is defined as the ratio of the number of antigens captured to the maximum available antibodies on the surface of the immunosensor (Rath and Panda 2012, 2015; Verma and Panda 2020). High capture efficiency will reduce (and, thus, improve) the limit of detection and, thus, facilitate early detection of disease. It can be improved either by increasing the Ab density on the surface of the immunosensor or by facilitating Ag transport. A significant body of research exists that uses physical (Rath and Panda 2012) and chemical (Rath and Panda 2015, 2016; Chepyala and Panda 2013, 2014; Vijayendran and Leckband 2001; Squires et al. 2008; Hu et al. 2007; Nelson et al. 2007; Reverberi and Reverberi 2007; Lebedev et al. 2006; Ibii et al. 2010) methods to increase the antibody density by modifying the surface of an immunosensor. Both active and passive mixers have been designed to facilitate the Ag transport to the channel walls.
For immunosensing (Wang et al. 1998; Fujii et al. 2003; Renaudin et al. 2010; Hart et al. 2010; Sarvazyan 2010; Kardous et al. 2011; Yu et al. 2015; Chen et al. 2016) and non-immunosensing (Bange et al. 2005; Hessel et al. 2005; Nguyen and Wu 2005; Lee et al. 2011; Mansur et al. 2008; Asgharzadehahmadi et al. 2016) applications, active mixing can be achieved using different forms of external energy inputs, such as acoustic-induced vibrations, electrokinetic instabilities, periodic variation of pumping capacity, small impellers, and integrated micro-valves or micro-pumps. Several researchers (Abbott et al. 2012, 2014) have demonstrated active mixing processes that utilize constant flow and moving geometry to increase mass transfer (i.e., improving the detection limit) and have the ability to be scaled up (oscillatory flow and oscillatory baffled reactors). Another approach is to utilize the pulse mode for the inlet velocity. The parameters of active mixing include velocity, frequency, and amplitude of the mixing pulse. Fujii et al. (2003) achieved better mixing performance using a pulsed flow and demonstrated the effect of frequency (switching frequency of pump) on immunosensing. To analyze the unsteady and oscillating behavior of fluid flow, the dimensionless number, Strouhal number (St) (which measures the effective vortex propagation and mixing intensity) is used. St (St = \( (L/V) /(1/f), \) Chen and Cho (2008)) is defined as the ratio of the characteristic flow time (L/V) to the pulsing time period (1/f), where L is the characteristic length, V is the average flow velocity, and f is the frequency of oscillation. However, the effect of pulsing in a microfluidic immunosensor, which includes the individual effects of all the governing parameters for immunosensing surface reactions, along with the correlation between the average surface concentration and process parameters has not been reported till date. Therefore, in the present study, we obtain the optimum process parameters and a figure of merit for a given system. A knowledge of flow patterns and mixing behavior is important when selecting an appropriate reactor design.
In this work, the effect of active mixing by means of pulse flow was analysed, both numerically and experimentally, along with the effect of the governing parameters (frequency, amplitude and velocity) on the capture efficiency for flow-based heterogeneous immunosensors. The experiments were performed to provide empirical validation of the simulation results (similar geometrical and process parameters could not be taken due to fabrication and computational limitations). A square wave was utilized for the simulation, and the effects of the governing parameters were studied using a convection–diffusion–reaction model. From this, dependence of the governing parameters on the transport and reaction parameters was obtained. Variation in any of the parameters affects the flow profile and Ag transport. Few experiments were conducted for detecting prostate-specific antigen (PSA) molecules to verify whether active mixing improved Ag transport and enhanced the extent of the reaction. Cyclic voltammetry (CV) peaks were used to quantify the presence of biomolecules in the experimental system. For flow visualization at different flow conditions, the positions of the streamlines were captured with the help of particle image velocimetry (micro-PIV).
2 Simulations and experiments
2.1 Simulation technique
The procedure for simulating the transport of the antigen (Ag) and capture by the antibody (Ab) is governed by the equations mentioned in the next Sect. 2.1.1. The computational method employed here made use of the finite element method (FEM) using COMSOL multiphysics software. The required modules were the “Navier–Stokes equations”, “transport of dilute species” and “surface reactions”. We have utilized piecewise function to define velocity (pulse form) for different cases. The PARDISO solver was used to solve all the modules. The straight microchannel of dimensions 3 mm × 800 µm × 50 µm was used for simulation study. We could not go beyond a channel length of 3 mm due to computational limitations. The studied geometry consisted of 173,238 number of elements. The computation times for the fluid flow and the transport of the dilute species were 20 s (total time) in intervals of 0.1 s; while for the surface reaction, it was 20 s in intervals of 0.01 s. The validity of the outflow boundary conditions was checked.
2.1.1 Governing equations
The transport of Ag to the Ab has been described by (Rath and Panda 2012):
where C is the analyte concentration at time t, D is the diffusivity of the antigens in the solution flowing in the micro-channels, u, v, and w are the velocity components in the x, y and z directions, respectively, B is the body force, ∇.P is the pressure gradient, θt is the surface density of antigen–antibody bound complex at time t, θmax is the maximum surface density of the immobilized antibodies, and Kon and Koff are the forward and backward rate constants, respectively, h is the channel height, w is the channel width. The schematic of the computational domain is shown in Fig. 1a with the schematic information provided below.
The microchannel dimensions (shown in Fig. 1a) are 3 mm × 800 µm × 50 µm; u, v, and w are the velocity components in the x, y and z directions, respectively (shown in the schematic); inlet plane: ABCD, outlet plane: EFGH, channel reactive surface or plane: BCGF.
The initial and boundary conditions for the above equations are given by (Rath and Panda 2012):
The velocity equation is given (From MathWorld) by:
where A0 and A are constant and x0 varies with the parameters.
The dimensionless numbers,
are used in this study, where Dh = hydraulic diameter of the channel, uavg = average inlet flow velocity, μ = viscosity, A = center to peak amplitude of the oscillation, and f = frequency of the oscillation.
To check the applicability of the outflow boundary conditions, we calculated the entrance length (Lh) for the geometry (hydraulic diameter, D = 94 µm) and flow (Re = 1.2) at constant flow (no pulse) to be about 7 µm (which far less than the channel length of 3 mm). The St considered here is small (i.e., of the order 10−3) in this study and the generated perturbations are also smaller/lower. Thus, we believe that the use of the outflow boundary conditions is reasonable.
The values of the input parameters were taken from the range defined in the literature (Rath and Panda 2012, 2015; Verma and Panda 2020) and are mentioned in Table 1:
2.2 Chemicals
All chemicals and experimental steps are nearly the same as mentioned in our earlier work (Verma and Panda 2020). Briefly, Mercapto propionic acid (MPA) and EDC-NHS were obtained from Sigma Aldrich (Germany) and Merck (Germany) respectively; isopropanol (IPA) was obtained from Loba Chemie (India); potassium ferricyanide and potassium ferrocyanide were obtained from Rankem (India). The components required for the PBS solution (KCl, NaCl, Na2HPO4, and KH2PO4) were purchased from Qualigens (India); the pH of PBS for all the experiments was maintained at 7.4. PDMS and glass slides were obtained from SYLGARD 184 (Dow Corning, USA), and Bioplus (India), respectively; SU-8 2050 epoxy for moulds fabrication was purchased from MicroChem Corporation (USA); prostate-specific antigen (PSA) was obtained from Fitzgerald Industries International (USA), anti-PSA antibodies were purchased from Genetech Laboratory (India), and silicon wafers ((100), surface roughness < 2 nm) were purchased from Wafer World (USA).
2.3 Channel fabrication
Glass-PDMS (Srivastava et al. 2011; Zhang et al. 2015; Chen et al. 2019; Meng-Di et al. 2019) straight microchannels of dimensions 10 mm × 800 µm × 50 µm were used in this study. The glass-PDMS microchannel fabrication protocol is the same as mentioned in our earlier work (Verma and Panda 2020). Briefly, all the fabrication steps were processed in a controlled clean room environment. Glass slides were cleaned using piranha solution and followed by the deposition of chromium (10 nm) and gold (90 nm) by means of thermal deposition. Masks used for metal deposition as well as epoxy moulds were fabricated using a laser system (Nd:YAG Laser, Laservall, Italy). PDMS flow channels were made (Di et al. 2019; Art et al. 2018; Kadilak et al. 2014; Fujii 2002) using the SU-8 epoxy. For epoxy moulds fabrication, epoxy was spin coated on silicon for consecutive steps at 500 and 2500 RPM for 10 and 45 s, respectively. The spun epoxy was prebaked at 95 °C for 7 min, exposed for 8 min to a 240 mJ/cm2 UV lamp with a copper mask, followed by baking of 7 min at 95 °C. The baked epoxy was developed in 7 min using the SU-8 developer and followed by rinsing with a fresh developer (for 10 s) and IPA (for 15 s). Further, the PDMS mixture was prepared (base and cross linker) in 10:1 ratio (by weight), degassed, poured over the epoxy moulds, and was heated for 1 h at 70–72 °C, in vacuum. This was cooled down up to room temperature and the microchannel was separated out from the mould. The inlet/outlet ports were drilled on the PDMS microchannel before being bonded to the glass slide (on which the gold was deposited). The oxygen plasma method was used to bond channels. In the above-described way, the microchannels were fabricated. We used the similar glass-PDMS microchannel for the micro-PIV study, without a gold layer to observe the flow profile directly as the gold layer blocked optical assessment of the flow profile.
2.4 Channel functionalization
All the process steps for stack immobilization and functionalization, utilizing processes described in the literature (Verma and Panda 2020; Bhuvana et al. 2013) are shown in (Fig. 1(b)(i)–1(b)(v)). Briefly, the non-functionalized microchannel was washed with ultrapure water and then dried with nitrogen followed by incubating the channel surface (gold surface) in a 1-mM MPA solution (PBS solution) for 2 h and washing with PBS. The terminal carboxylic acid (–COOH) groups were activated, followed by immobilization of 0.2 M NHS/0.05 M EDC (1:1 mixture) for 4 h at room temperature. The channel was incubated overnight in an antibody solution (100 μg/mL). Finally, after rinsing with PBS, the antigen (1 μg/mL) in PBS was flown through the microchannel in constant and in pulse mode using a syringe pump (PHD Ultra, Harvard Apparatus). Due to a leakage problem at higher flow rates, we limited our flow rates for the experiments up to 90 μL/min. We did not notice (using an optical microscope) any change in the microchannel geometry (height and width of the microchannel) for the flow rates used here.
2.5 Determination of capture efficiency
The solution of potassium ferrocyanide (K4[Fe(CN)6]) and potassium ferricyanide (K3[Fe(CN)6], 1 mM (1:1)) in 10 mM (pH = 7.4) PBS solution (Imran et al. 2019; Bhuvana et al. 2013; Verma and Panda 2020) was used as the electrolyte. Platinum was used as the working and counter electrode and Ag/AgCl as a reference electrode (Zhang et al. 2019). The number of active sites (Kalita et al. 2012; Klink et al. 2011; Singh et al. 2011; Solanki et al. 2008; Chauhan et al. 2016) with respect to peak current was calculated utilizing the CV plot and the Brown–Anson model (Brown and Anson 1977) (the detailed information with sample calculation has given in the Supplementary Information). A larger decrease in the value of the peak current represents a lower number of active sites and this indicates a higher extent of reaction.
2.6 Flow characteristics using micro-PIV
To understand the pulse flow profile, the flow profiles inside the channel were characterized by micro-PIV (TSI, USA) at flow rates 30–90 μL/min (average 60 μL/min) and at different planes (bottom, an intermediate plane, and the mid plane). The results from the micro-PIV help in comparing the temporal variation in the streamlines (at 0.6 vector length-scale) at different flow conditions and help elucidate the role and significance of active mixing in generation of secondary flows. But due to the computational limitations, the dimensions of the microchannels used for experimental and simulations were different and, hence, a one-to-one comparison of the flow lines was not possible. This is the limitation for our study.
3 Results and discussion
3.1 Enhanced captured efficiency with pulsed flow
The effect of the nature of flow on the capture efficiency was studied experimentally using PSA/anti-PSA and also verified numerically. In Fig. 2, the experimental results of the percentage reduction of the peak current at Re = 2.35 for the constant and the pulse flow are shown on the left-hand side, and the simulation results of the average surface concentration at Re = 0.48, for the constant and the pulsed flow are shown on the right-hand side. In experiments, the magnitude of the reduction of peak current gives the extent of the reaction or the captured Ag; while in simulations, the amount of Ag captured is characterized in moles per unit area. We consider different values for Re (i.e., 0.48 and 2.35, that is, different velocities for experiments and simulations) because the length of the microchannel is different for the experiments and the simulations. Due to computational limitations, we could not go beyond 3 mm and experimentally such small lengths (3 mm) do not provide sufficient signal to measure; hence, we chose longer (10-mm) microchannels. The average Sherwood number (Shavg) for the experiments and simulations were compared for the specific case—for experiments (Shavg = 23.15 at Re = 2.35, Sc = 11,764.7) and for simulations (Shavg = 21.82 at Re = 1.2, Sc = 27,000) (Subramanian; Wu et al. 2001). The values are comparable (i.e., within 6%). With the pulse flow conditions both in experiments (amplitude = 12.50 mm/s, f = 0.05 Hz, an average velocity = 60 (30–90) µL/min) and in simulations (amplitude = 2.50 mm/s, f = 0.25 Hz, an average velocity = 12 (6–18) µL/min), we observed an enhancement in the capture efficiency. For PSA/anti-PSA, using the previously described (Sect. 2.4) immunoassay protocol, the peak current obtained from the experiments after anti-PSA immobilization was 5.600 ± 0.208 μA. After PSA immobilization, the peak currents were 2.402 ± 0.079 μA and 1.831 ± 0.024 μA, for the constant and pulse flow, respectively. The reduction in the peak currents was 57.10 ± 2.84% and 67.29 ± 2.65% for the constant and pulse flow, respectively. In the case of pulse flow, a higher reduction in the peak current indicates a greater extent of reaction, when compared to constant flow. And in the case of the simulations, the average surface concentration (Cs,avg) was higher (29% more) for the pulse flow.
A dynamic PIV technique was used to characterize the flow in the pulse mode for which we had validated the no-slip condition for constant flow in our previous work (Verma and Panda 2020). Figure 3a illustrates the velocity as a function of time in the microchannel; while, Fig. 3b, c show the flow lines for the constant and pulse flow, respectively. The plane (for flow visualization) is 5 µm above the bottom surface of the channel. For constant flow, the flow lines are unidirectional; whereas for pulse flow, the flow lines are multi-directional (random circulation/distribution of streamlines). The presence of random and asymmetrical flow lines in the pulse flow improves the transport near the surface leading to better mixing than constant flow. Hart et al. (2010) had also discussed the enhancement of the heterogeneous immunoassays (using AC electroosmosis) near the electrode surface.
The pulse flow perturbs the bulk fluid and improves transport of the antigen molecule towards the microchannel surface. The presence of random movements and swirling motion of eddies improves the mixing locally, reduces the boundary layer thickness and enhances the Ag transport to the surface (Fig. 3c shows the flow lines near the bottom surface). At Re ~ 1 used in this study, the inertial forces cannot be neglected. In the pulsed flow here, the fluid packets respond to the change in the velocity obeying the principle of mass conservation. This response is depicted in the randomness or the temporal variation of the flow lines. We have also calculated the Womersley number (W0) which represents the ratio of transient inertial force to the viscous force (\( Wo = L\sqrt {\omega /v} \)) (Kumar et al. 2011). The calculated value of W0 of about 0.06 which shows the viscous force dominates the flow (as W0< 2). This result is in agreement with Fujii et al. (2003), who demonstrated a better mixing performance using pulsed flow, and Yu et al. (2015), who obtained a higher signal intensity with the electrothermal effect at all concentrations of myeloperoxidase (MPO) used in their work. Thus, the results obtained here validate our observation that active mixing enhances the capture efficiency. The effects of different parameters on the capture efficiency are discussed in the subsequent sections.
3.2 Effect of velocity on capture efficiency
In pulsed flow, velocity, frequency, and amplitude affect the transport of Ag to the surface immobilized Ab. The effect of velocity is studied first, keeping amplitude (2.5 mm/s) and frequency (0.25 Hz) constant. Here, the flow is in the laminar regime with Re in the range 0.48–1.2, and the St in the range is 0.0005–0.0013.
The simulated values of the Cs,avg are plotted on the z-axis as a function Re (a measure of velocity), and St in Fig. 4a. Re0, Re and St, are the dimensionless numbers (Eqs. 13, 14, 15) useful in analysing the effect of pulse flow and its parameters. Reo, a function of amplitude and frequency, is the measure of the mixing intensity. The amplitude and frequency are constant here; thus, the degree of mixing (Reo) is constant. St is the ratio of the characteristic flow time to the characteristic pulsing time. In this case, the Re increases with the increase in the average velocity. The increased velocity reduces the residence time and thus St decreases which results in a decline in the propagation of the effective eddies (which keeps the fluid perturbed) and reduces the mixing extent. To see the combined effect of Re (increasing average velocity) and St (eddies propagation), i.e., increasing inertial forces and reducing eddies propagation (St) on average surface concentration (Cs,avg), we introduced a correlation among Cs,avg, Re and St (Fig. 4a) which is as follows:
The data points were fitted using a curve fitting tool (poly line) and the R2 value was 0.99. The above correlation illustrates that the coefficient of St is higher than that of Re, indicating that the average surface concentration (Cs,avg) is dependent more on St than Re. This indicates that eddies propagation has a more capability to affect the capture efficiency of Ag than Re and elucidates the impact of active mixing on capture efficiency. Glasgow et al. (2004) also mentioned that the higher St lead to better mixing, while using the pulse flow for the mixing two fluids in the microchannel.
To understand better the 3D plot shown in Fig. 4a, we have divided it into two 2D plots: i) Cs,avg as a function Re, Fig. 4b and ii) Cs,avg as a function of St, Fig. 4c. In the range of Re studied (suitable for microfluidic immunosensors, Re ~ 1), it is seen that the capture efficiency increases monotonically with velocity. A similar behavior was reported with a constant flow rate in our previous work (Verma and Panda 2020) and this was attributed to the reduction in the mass transfer boundary layer thickness. The lowering of the resistance to the mass transport can be seen as lowering of the characteristic time of transport (Rath and Panda 2015). However, at high values of Re, where the characteristic time of flow, i.e., (the residence time) will be low, lesser capture of the antigen is expected. Similar results were also seen by Alonso et al. (2014) and Cherkasov et al. (2020). Alonso et al. (2014) obtained a maxima in fluorescence signal (response) in E. coli detection and Cherkasov et al. (2020) obtained a maxima in the semi-hydrogenation (% conversion) of 2-methyl-3-butyn-2-ol (MBY) for the catalyst loading as a function of inlet velocity. Although, an increased velocity (within a range) facilitates a higher capture, it is associated with a higher pressure drop. Hence, a “figure of merit (FOM)”, defined here as the ratio of the Cs,avg to the pressure drop at a given flow rate, is calculated for the different conditions and plotted on the secondary y-axis in Fig. 4b. A maxima in the FOM was seen at Re = 0.6 (Mandal et al. 2010, 2011; Singh et al. 2014 obtained either increasing or decreasing behavior in FOM for their improved coiled flow heat transfer system.). This indicates that higher Re can provide better analyte capture (the desired performance) but with a higher corresponding pressure drop. Figure 4c shows, the Cs,avg as a function of St (at constant frequency and amplitude). At constant frequency and amplitude, the St decreases with the increase in the average velocity, i.e., Re. And we knew from above Fig. 4a that higher Re provide higher analyte capture. Thus, the increase in the Cs,avg with the decrease in St again confirms the above-mentioned conclusion.
3.3 Effect of frequency
Frequency is an important parameter in the study of active mixing as it affects the characteristic time of the mass transport. Antigen capture depends on the interplay between transport, reaction, and geometrical parameters (Rath and Panda 2015). In this study, the geometry (straight microchannel) is the same for all the cases; hence, transport and reaction are the deciding factors for the extent of capture. Figure 5a shows Cs,avg at two frequencies when Re = 0.71 (simulation), and Fig. 5b shows the reduction in the peak current at Re = 2.35 for two frequencies (experimental validation) for PSA/anti-PSA. We have chosen different frequency ranges due to experimental and computational limitations (Similar to Sect. 3.2, we are validating here the frequency effect empirically). We obtained a higher Cs,avg (~ 24%) at f = 0.167 than at f = 0.20 in the simulations. In the experiments, the flow velocity was within a range of 30–90 μl/min (average 60 μl/min). The frequencies (f) were 0.05 Hz, and 0.10 Hz and the peak reduction was higher (67.29 ± 2.65% at f = 0.05 and 61.40 ± 3.45% at f = 0.10) for the lower frequency. An increase in the frequency improves the transport up to a certain scale, but beyond that, it will not provide sufficient time to react and therefore, the capture decreases. This above result clarifies the fact, numerically as well as experimentally, that a higher frequency decreases the extent of the reaction and leads to reduced capture. Glasgow et al. (2004) had also observed a higher mixing (utilizing pulse flow) at lower frequencies due to greater contact time between the fluids. Similar effect of frequency was also observed by Fujii et al. (2003), Hart et al. (2010) and Chen et al. (2016), who obtained a relatively lower signal at higher frequencies. At a constant average velocity and amplitude, Re0 and St (among Re, Re0 and St) are a function of frequency only. The St increases with the increase in the frequency. However, at very high values of St, the fluid flow lines motion is so rapid, such that the species (Ag) does not have the sufficient time to reach the surface and bind with the Ab. Consequently, the performance is poorer than that achieved at lower values of St, and this finally leads to a reduced value of the Cs,avg.
a Average surface concentration (Cs,avg) at different frequencies at Re = 0.71 (simulation), b reduction in the peak current at Re = 2.35 at different frequencies (experimental validation) for PSA/anti PSA capture for frequency effect, c average surface concentration (Cs,avg) as a function of frequency, d figure of merit (FOM) of frequency, e Reo and St as a function of frequency at Re = 0.71
To get more insight on the effect of frequency on the capture of Ag, the average velocity and the amplitude were kept at 7.53 mm/s and 2.51 mm/s, respectively. The frequency was varied over the range 0.045–1.0 Hz. Figure 5c presents Cs,avg as a function of frequency at two values of Kd:10−6 and 10−7. From Fig. 5c, it is observed that, initially for short span of frequency (0.0454–0.0625 Hz), the average surface concentration (Cs,avg) increases with the increase in frequency, and remains nearly constant for 0.0625–0.1667-Hz frequency range and decrease afterwards. A similar behavior was observed for both values of Kd. To explain this observation, two time scales were considered: one is the characteristic time of reaction and the other is the residence time scale, which are linked with the reaction and transport phenomena, respectively (Rath 2016). At lower frequencies, the reactive time ≥ residence time; while at higher frequencies, the fluid motion is rapid due to which the reactive time < residence time which results in the lesser analyte capture. This result agrees with Chen and Cho (2008), who obtained the maxima in the extent of mixing as a function of St. A reason for the maxima being flat could be that we have considered many points in a small range (0.0454–0.0625 Hz), and the total frequency range is also small (0.0454–1); while other researchers have considered wider range of frequency. However, the pressure drop increases with frequency (for all values); hence, the FOM will also vary with frequency, which shown in Fig. 5d. The FOM also attains maxima as a function of frequency and this pattern is the same for the both values of Kd. Such a behavior of frequency is not only limited to micro-range or to immunosensing applications; similar results were also obtained by Masngut and Harvey (2012) in butanol production in batch oscillatory baffled bioreactor at a higher Re0 as a function of time. Figure 5e illustrates Reo and St as a function of frequency. Reo represents the mixing intensity, and it increases (linearly) with the frequency. At a higher mixing intensity, the eddies propagation is also more and enhances the transport. This explains the increasing behavior of Reo and St with increased frequency.
In this section, we discuss the effect of frequency at a fixed plane with time. For pulse flow (in active mixing), the movement of the flow lines is random and time dependent. Figure 6a, b shows the flow profiles at different frequencies, and times and these pictures were selected where significant changes were seen. The direction of the flow lines at different times can be distinguished visually at various frequencies. We find that the direction of the flow lines are different at different times and turbulence is more at higher frequencies leading to improved transport but provides less time for reaction; hence, due to insufficient reaction time, the extent of capture decreases.
Until now, we have seen the effect of velocity at a constant frequency and the effect of frequency at constant velocity on the average surface concentration (Cs,avg). For a more detailed understanding, we extend our analysis and split the components of St. For a combined effect of frequency and velocity (Re), we varied the frequency (with range of 0.0667–0.50 Hz) and velocity (Re = 0.52–0.90) and conducted a numerical analysis. Figure 7 shows St as a function of frequency (0.0667–0.50) and velocity (Re = 0.52–0.90), and we obtain correlation (R2 = 0.964) as follows:
Figure 7 demonstrates a lower Re (i.e., velocity) and higher frequency for a higher value of St and validates that St is directly proportional to frequency (f) and inversely proportional to velocity (v). The correlation (Eq. 17) also indicates that frequency has a greater impact on St and effective eddy propagation because its coefficient is (about three times) higher than that of the velocity, thus, clarifying the impact of frequency on the St. In our earlier correlation also (Eq. 16), we found that frequency carries higher significance than velocity. Consequently, a high frequency with low velocity is considered to be a favorable combination for higher capture of Ag.
3.4 Effect of amplitude
The third key factor that affects the mass transfer is the amplitude of the pulse. To observe the effect of amplitude, we fixed the frequency at 0.25 Hz and average velocity at 7.06 mm/s and varied the amplitude from 0.5 to 2.5 mm/s. Figure 8a shows Cs,avg for different amplitudes at Re = 0.71 (simulation) and Fig. 8b shows the reduction in the peak current at Re = 2.35 for different amplitudes (experimental validation) for PSA/anti PSA capture. The response improves with the enhancement in the amplitude, but the impact is not as much as it is for velocity and frequency. In numerical studies, by increasing the amplitude from 2.01 mm/s to 2.51 mm/s (~ 25%), the enhancement obtained in Cs,avg was just 4.04%. The experimental analysis also showed a similar behavior. In the experiments, the two distinct amplitudes were considered, and flow rates were ranged from 40 to 80 µl/min (amplitude = 8.33 mm/s) to 30–90 µl/min (amplitude = 12.50 mm/s), keeping the average flow rate was same (60 µl/min). We obtained a slight enhancement in the peak reduction (~ 2.1%) with higher amplitude. These results are in agreement with the Ni et al. (1994, 1995), who also obtained an enhanced mass transfer at higher amplitudes as a function of frequency.
a Average surface concentration (Cs,avg) at different amplitudes at Re = 0.71 (simulation), b reduction in the peak current at Re = 2.35 at different amplitudes (experimental validation) for PSA/anti PSA capture. c Reo as a function of amplitude. d The figure of merit (FOM) as a function of amplitude
Figure 8c shows Reo as a function of amplitude and Fig. 8d shows the figure of merit (FOM) as a function of amplitude. With increasing amplitude, the flow lines move irregularly, and this enhances the Reo, (the extent of mixing) and as a result, Cs,avg also increases. However, at higher amplitudes, the pressure drop is also higher that leads to the decrease in the FOM value.
In Fig. 9a, b, the effect of amplitude on flow direction and mixing intensity is shown using micro-PIV. We selected those pictures where the substantial changes were visible. As the effect is not much visible, only slight enhancement in the capture can be observed. This section further validates the previously obtained experimental and numerical results of capture for different cases.
4 Conclusion
In this work, we have studied, both numerically and experimentally, the role of the active mixing in facilitating the transport of antigens in heterogeneous microfluidic immunosensors. The experiments on the capture of PSA by anti-PSA were conducted and the numerical simulations were used to verify the results. First, we checked the effect of the pulse flow on capture efficiency, and obtained an enhancement in the capture of the antigen. Subsequently, the study of active mixing parameter (average velocity, frequency and amplitude) on analyte capture was done. With the increase in the average velocity (uavg), the antigen capture increases but the corresponding pressure drop also increases, and FOM attains a maxima at Re ~ 0.6. The analyte capture increases with the frequency also, but the increase in the frequency reduces the reaction time scale and decreases the capture of the analyte. The amplitude has the least effect on the antigen capture. The flow profiles were also seen with micro-PIV for the different cases to compare the temporal variation at various flow conditions. We also proposed two correlations among average the parameters Cs,avg, Re and St. The correlations indicate that dependency coefficient for St is higher than Re, and, thus, indicate that the vortex propagation has a higher effect (i.e., active mixing) on antigen capture efficiency than Re.
References
Abbott MSR, Harvey AP, Perez GV et al (2012) Biological processing in oscillatory baffled reactors: operation, advantages and potential. Interface Focus 3:1–13
Abbott MSR, Harvey AP, Perez GV et al (2014) Reduced power consumption compared to a traditional stirred tank reactor (STR) for enzymatic saccharification of alpha-cellulose using oscillatory baffled reactor (OBR) technology. Chem Eng Res Des 92:1969–1975
Alonso MB, Granados X, Faraudo J et al (2014) Magnetic actuator for the control and mixing of magnetic bead-based reactions on-chip. Anal Bioanal Chem 406:6607–6616
Art MS, Noblitt SD, Krummel AT et al (2018) IR-compatible PDMS microfluidic devices for monitoring of enzyme kinetics. Anal Chim Acta 1021:95–102
Asgharzadehahmadi S, Raman AAA, Parthasarathy R et al (2016) Sonochemical reactors: review on features, advantages and limitations. Renew Sustain Energy Rev 63:302–314
Bange A, Halsall B, Heineman WR (2005) Microfluidic immunosensor systems. Biosens Bioelectr 20:2488–2503
Bhuvana M, Narayanan JS, Dharuman V et al (2013) Gold surface supported spherical liposome–gold nano-particle nano-composite for label free DNA sensing. Biosens Bioelectr 41:802–808
Brown AP, Anson FC (1977) Cyclic and differential pulse voltammetric behavior of reactants confined to the electrode surface. Anal Chem 49:1589–1595
Chauhan R, Singh J, Solanki PR (2016) Label-free piezoelectric immunosensor decorated with gold nanoparticles: kinetic analysis and biosensing application. Sens Actutors B 222:804–814
Chen CK, Cho CC (2008) A combined active/passive scheme for enhancing the mixing efficiency of microfluidic devices. Chem Eng Sci 63:3081–3087
Chen Q, Wang D, Cai G et al (2016) Fast and sensitive detection of foodborne pathogen using electrochemical impedance analysis, urease catalysis and microfluidics. Biosens Bioelectr 86:770–776
Chen S et al (2019) Microfluidic device directly fabricated on screen-printed electrodes for ultrasensitive electrochemical sensing of PSA. Nano Res Lett 14:71
Chepyala R, Panda S (2013) Tunable surface free energies of functionalized molecular layers on Si surfaces for microfluidic immunosensor applications. Appl Surf Sci 271:77–85
Chepyala R, Panda S (2014) Zeta potential and reynolds number correlations for electrolytic solutions in microfluidic immunosensor. Micro Nano 18:1329–1339
Cherkasov N, Denissenko P, Deshmukh S et al (2020) Gas-liquid hydrogenation in continuous flow- The effect of mass transfer and residence time in powder packed-bed and catalyst-coated reactors. Chem Eng J 379:122292
Di CM, Ting YY, Yang DZ et al (2019) Microchannel with stacked microbeads for separation of plasma from whole blood. Chin J Anal Chem 47(5):661–668
From MathWorld– A Wolfram Web Resource. http://mathworld.wolfram.com/PiecewiseFunction.html. Accessed 22 Feb 2020
Fujii T (2002) PDMS-based microfluidic devices for biomedical applications. Micro Elec Eng 61–62:907–914
Fujii T, Sando Y, Higashino K et al (2003) A plug and play microfluidic device. Lab Chip 3:193–197
Glasgow I, Lieber S, Aubry N (2004) Parameters influencing pulsed flow mixing in microchannels. Anal Chem 76:4825–4832
Hart R, Lec R, Noh HM (2010) Enhancement of heterogeneous immunoassays using AC electroosmosis. Sens Actatuars B 147:366–375
Hessel V, Lowe H, Schonfeld F (2005) Micromixers—a review on passive and active mixing principles. Chem Eng Sci 60:2479–2501
Hu G, Gao Y, Li D (2007) Modeling micropatterned antigen-antibody binding kinetics in a microfluidic chip. Biosens Bioelectr 22:1403–1409
Ibii T, Kaieda M, Hatakeyama S et al (2010) Direct immobilization of gold-binding antibody fragments for immunosensor applications. Anal Chem 82:4229–4235
Imran H, Manikandan PN, Prabhu D et al (2019) Ultra selective label free electrochemical detection of cancer prognostic p53-antibody at DNA functionalized grapheme. Sens Biosens Res 23:100261
Kadilak AL, Ying L, Shrestha S et al (2014) Selective deposition of chemically- bonded gold electrodes onto PDMS microchannel side walls. J Electr Anal Chem 727:141–147
Kalita P, Singh J, Singh MK et al (2012) Ring like self-assembled Ni nanoparticles based biosensor for food toxin detection. Appl Phys Lett 100:093702
Kardous F, Fissi LE, Friedt JM et al (2011) Integrated active mixing and biosensing using low frequency vibrating mixer and Love-wave sensor for real time detection of antibody binding event. J Appl Phys 109(094701):1–8
Klink MJ, Iwuoha EI, Ebenso EE (2011) The electro-catalytic and redox-mediator effects of nanostructured PDMA-PSA modified-electrodes as phenol derivative sensors. Int J Electrochem Sci 6:2429–2442
Kumar H, Tawhai MH, Hoffmann EA et al (2011) Steady streaming: a key mixing mechanism in low-Reynolds-number acinar flows. Phys Fluids 23:041902-1–04190222
Lebedev K, Mafe S, Stroeve P (2006) Convection, diffusion and reaction in a surface-based biosensor: modeling of cooperativity and binding site competition on the surface and in the hydrogel. J Colloid Interface Sci 296:527–537
Lee CY, Chang CL, Wang YN et al (2011) Microfluidic mixing: a review. Int J Mol Sci 12:3263–3287
Mandal MM, Kumar V, Nigam KDP (2010) Augmentation of heat transfer performance in coiled flow inverter vis-a-vis conventional heat exchanger. Chem Eng Sci 65:999–1007
Mandal MM, Palka A, Nigam KDP (2011) Liquid_Liquid mixing in coiled flow inverter. Ind Eng Chem Res 50:13230–13235
Mansur EA, Mingxing YE, Yundong W et al (2008) A State-of-the-art review of mixing in microfluidic mixers. Chin J Chem Engg 16(4):503–516
Masngut N, Harvey AP (2012) Intensification of biobutanol production in batch oscillatory baffled bioreactor. Proc Eng 42:1079–1087
Nelson KE, Foley JO, Yager P (2007) Concentration Gradient Immunoassay. 1. An immunoassay based on interdiffusion and surface binding in a microchannel. Anal Chem 79:3542–3548
Nguyen NT, Wu Z (2005) Micromixers—a review. J Micromech Microeng 15:R1–R16
Ni X, Gao S, Pritchard DW (1994) A study of mass transfer in yeast in a pulsed baffled. Bioreactor Biotech Bioeng 45:165–175
Ni X, Gao S, Pritchard DW et al (1995) A comparative study of mass transfer in yeast for a batch pulsed baffled bioreactor and a stirred tank fermenter. Chem Eng Sci 50(13):2127–2136
Rath D, Panda S (2015) Enhanced capture efficiencies of antigens in immunosensors. Chem Eng J 60:657–670
Rath D, Panda S (2016) Correlation of capture efficiency with the geometry, transport, and reaction parameters in heterogeneous immunosensors. Langmuir 32:1410–1418
Rath D, Kumar S, Panda S (2012) Enhancement of antigen–antibody kinetics on nanotextured silicon surfaces in mixed non-flow systems. Mater Sci Eng C 32:2223–2229
Renaudin A, Chabot V, Grondin E et al (2010) Integrated active mixing and biosensing using surface acoustic waves (SAW) and surface plasmon resonance (SPR) on a common substrate. Lab Chip 10:111–115
Reverberi R, Reverberi L (2007) Factors affecting the antigen-antibody reaction. Blood Transfus 5:227–240
Sarvazyan A (2010) Diversity of biomedical applications of acoustic radiation force. Ultrasonics 50:230–234
Singh J, Kalita P, Singh MK et al (2011) Nanostructured nickel oxide-chitosan film for application to cholesterol sensor. Appl Phys Fluid 98:123702
Singh J, Choudhary N, Nigam KDP (2014) The thermal and transport characteristics of nanofluids in a novel three-dimensional device. Can J Chem Eng 92:2185–2201
Solanki PR, Kaushik A, Ansari AA et al (2008) Zinc oxide-chitosan nanobiocomposite for urea sensor. Appl Phys Lett 93:163903
Squires TM, Messinger RJ, Manalis SR (2008) Making it stick: convection, reaction and diffusion in surface-based biosensors. Nat Biol 26:417–426
Srivastava S et al (2011) A self assembled monolayer based microfluidic sensor for ures detection. Nanoscale 3:2971–2977
Subramanian RS Convective mass transfer. Department of Chemical and Biomolecular Engineering, Clarkson University. https://web2.clarkson.edu/projects/subramanian/ch330/notes/Convective%20Mass%20Transfer.pdf Accessed on 3 June 2020
Verma S, Panda S (2020) Correlations of the capture efficiency with the Dean number and its constituents in heterogeneous microfluidic immunosensors. Micro Nano 24:9
Vijayendran RA, Leckband DE (2001) A quantitative assessment of heterogeneity for surface-immobilized proteins. Anal Chem 73:471–480
Wang AW, Kiwan R, White RM et al (1998) A silicon-based ultrasonic immunoassay for detection of breast cancer antigens. Sens Actaturs B 49:13–21
Wu G, Datar RH, Hansen KM et al (2001) Bioassay of prostate-specific antigen (PSA) using microcantilevers. Biotech Nat 19:856–860
Yu C, Kim GB, Clark PM et al (2015) A microfabricated quantum dot-linked immuno-diagnostic assay (µQLIDA) with an electrohydrodynamic mixing element. Sens Actaturs B 209:722–728
Zhang F et al (2015) A Microfluidic love-wave biosensing device for PSA detection based on an aptamer. Beacon Probe Sensors 15:13839–13850
Zhang D et al (2019) Electrochemical aptamer-based microsensor for real-time monitoring of adenosine in vivo. Anal Chimica Acta 1076:55–63
Acknowledgements
Financial support from the Ministry of Electronics and Information Technology, Government of India (Grant Number 2(4)/2014-PEGD (IPIIW)) is acknowledged. The use of the micro-PIV unit (supported by the FIST program of the Department of Science and Technology, Government of India) in the PG Research Laboratory in the Department of Chemical Engineering is acknowledged. Also, the help of Dr. Satyendra Kumar with the processing of the antibody and antigen samples is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, S., Panda, S. Effect of active mixing on capture efficiency in heterogeneous microfluidic immunosensor. Microfluid Nanofluid 24, 58 (2020). https://doi.org/10.1007/s10404-020-02364-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-020-02364-0