Abstract
We quantitatively characterize the perturbed droplet generation in co-flow microfluidics with mechanical vibration by estimating the fluctuations in the flow rate of inner fluid. We show the variation of generation frequency and uniformity of droplets as the frequency and amplitude of vibration. Synchronized droplet generation occurs in certain range of the vibration frequency that is predicted by our model. Besides, we scale the droplet size by incorporating the effects of vibration, inner and outer flows. The vibration is found to be capable of promoting the dripping, suppressing the jetting and even shifting the jetting into the dripping when the jet length is sufficiently short. The delayed dripping–jetting transition is characterized in a phase diagram by taking vibration into consideration. Our results unveil the mechanism of mechanically perturbed droplet generation and provide valuable guidelines for practical applications of vibration-enhanced droplet generation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to the fine control of individual droplets with small fluid volumes, droplet microfluidics attracts wide interests and applications in foods (Augustin and Hemar 2009), pharmaceuticals (Haber 2006; Whitesides 2006), cosmetics (Patravale and Mandawgade 2008) and materials synthesis (Kim et al. 2013; Kong et al. 2013, 2014). These droplets are exploited as micro-reactors for chemical reactions (deMello 2006; Song et al. 2006; Zhang et al. 2010) and biological assays (Dittrich and Manz 2006; Kim et al. 2011) for example single-molecule polymerase chain reaction (PCR) (Nakano et al. 2003), or as carriers for active ingredients such as drugs (Khan et al. 2015; Zhao 2013), proteins (Evans et al. 2009) and cells (Chan et al. 2013; Collins et al. 2015). To facilitate these applications, on-demand control over droplet generation with prescribed size and production frequency is essential.
To date, several microfluidic geometries have been developed to generate droplets: microchannel-terrace (Kobayashi et al. 2001; Sugiura et al. 2002), co-flow (Umbanhowar et al. 2000), flow-focusing (Anna et al. 2003; Anna and Mayer 2006; Garstecki et al. 2004), T-junction (Fan et al. 2010; Wang et al. 2009; Zhang and Wang 2011), and variations of these configurations such as double T-junction (Hong et al. 2010) and V-junction (Ding et al. 2015). All these approaches involve the injection of one immiscible fluid into another, with the injection fluid segregating into droplets due to hydrodynamic instability in several modes: geometry-controlled (Anna and Mayer 2006; Zhu et al. 2015), dripping (Guillot et al. 2007, 2008; Utada et al. 2007), jetting (Guillot et al. 2007, 2008; Utada et al. 2007), tip-streaming (Anna and Mayer 2006) and tip-multi-breaking (Zhu et al. 2015). Droplet size is determined by dynamic parameters such as flow rates and pressure sources, device geometry, and fluid properties including fluid viscosities and interfacial tension. Typically, droplet size is tuned by changing flow rates or driving pressures, but the response time is normally quite long (e.g., several minutes) (Stone et al. 2004; Zhou and Yao 2014), which may limit the practical applications of droplet microfluidics. As droplet size depends on device geometry, to produce small targeted droplets, channels with small dimensions are needed (Lee et al. 2009). Fabrication of such required small channels is often expensive and challenging. Therefore, it is critical to develop other methods with fast response and more flexible control over droplet generation.
Active droplet production methods, aided by external actuation, enable on-demand generation of droplets with prescribed size and production frequency. For oil–water systems, there are three basic means which can manipulate droplet size in an active manner (Collins et al. 2015; Zhou and Yao 2014). The first is to change channel dimensions (Khoshmanesh et al. 2015), for instance by pneumatic valves (Abate et al. 2009; Abate and Weitz 2008). The second lies in controlling fluid properties. For example, variations in interfacial tension induced by local heating (heating also varies fluid viscosities) (Nguyen et al. 2007) and electrical potential (Tan et al. 2014a, b) have been developed to modulate droplet size. The third is to actuate fluid pressure, either by surface acoustic wave (SAW) (Schmid and Franke 2013, 2014) or mechanical vibration (Li et al. 2015). Besides oil–water systems, modulating fluid pressure is also effective in tuning bubble size in gas–liquid systems with high interfacial tension (Chong et al. 2015), and droplet size in aqueous two-phase systems (ATPS) with ultra-low interfacial tension (Moon et al. 2015; Sauret and Shum 2012; Sauret et al. 2012; Ziemecka et al. 2011). However, most active methods involve on-chip integration of additional parts such as microactuators and electrodes, complexing the process of device fabrication. In contrast to them, mechanical vibration only requires an off-chip vibrator perturbing the microtubing, and thus possesses high flexibility to perturb any fluid systems. Although previous work demonstrates the ability and efficiency of mechanical vibration in breaking jets into drops and modulating droplet size (Li et al. 2015; Sauret and Shum 2012; Sauret et al. 2012), a comprehensive and quantitative study remains limited on influences of vibration amplitude and frequency on droplet generation. Moreover, a lack of knowledge on the fluid pressure and flow rate in response to the external vibration limits the physical understanding toward the vibration-modulated droplet generation.
We present a quantitative description of droplet generation in co-flow microfluidic channel subjected to mechanical vibration via determining the vibration-induced fluctuations in the flow rate of inner fluid. We determine the droplet size and generation frequency in the synchronization region where droplet generation frequency is synchronized with the vibration frequency. In this region, droplet size depends on device geometry very weakly, and the vibration is thus the most effective in adjusting droplet size. In addition, we highlight the effects of vibration on suppressing jetting evolution while reinforcing dripping formation, which is of practical interest for producing uniform droplets in a wider range of flow rates. Our results provide fundamental understandings of vibration-induced droplet generation, and would benefit the development of other active and on-demand techniques of droplet production via fluctuations in flow rates exerted by disturbing fluid pressure.
2 Experiments and flow rate estimation
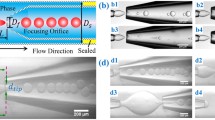
Droplet generation inside a capillary microfluidic device was observed under an inverted microscope (XD101, Nanjing Jiangnan Novel Optics Co. Ltd) on which a high-speed camera (MotionPro®X4, IDT, Taiwan) was mounted to visualize and record the flow process (Fig. 1a). Both inner and outer flow rates (Q in and Q out, respectively) were controlled and measured by syringe pumps (Longer Pump). A mechanical vibrator (Pasco Scientific, Model SF-9324) was connected to and vibrated the microtubing that introduced inner fluid into the device. The microtubing was perturbed in the gravitational direction with a sinusoidal displacement of y(t) = ɛ 0 sin(2πft), where ε 0 and f are vibration amplitude and frequency, respectively (Fig. 1b). The capillary microfluidic device was fabricated by coaxially inserting one tapered cylindrical glass capillary (injection capillary) into another tapered one (collection capillary), as presented in Fig. 1c. The lengths of the tapered region for the injection and collection capillaries were 9.60 and 7.95 mm, respectively. The inserting length of the injection capillary was 860.97 μm. The injection capillary was with inclined angle α ≈ 0° at the tip, outer diameter D in = 107.84 μm and inner diameter D i = 57.91 μm; the collection capillary was with inclined angle α ≈ 1.4° and inner wall diameter D out = 180.14 μm at the tip of the injection capillary (Fig. 1d, and the inner wall diameter for the originally non-tapered capillary was 580 μm).
Perturbed droplet generation in co-flow microfluidics with mechanical vibration. a Schematic of experimental setup. A mechanical vibrator perturbs the microtubing carrying the inner fluid in the gravitational direction with displacement of y(t). b A sinusoidal wave y(t) = ε 0sin(2πft) is generated by the vibrator with frequency f (Hz) and amplitude ε 0 (mm). c Schematic of the channel geometry. Q in and Q out are inner and outer phase flow rates, respectively, both controlled by syringe pumps. Mechanical vibration induces fluctuations in the flow rate of inner fluid, thus modifying constant Q in into transiently fluctuating Q in(t). d Magnification of the microchannel at which droplets are generated. D in and D i are the outer and inner diameters of the injection nozzle, respectively; D out is the inner diameter of the collection capillary at the tip of injection nozzle. The tapered microchannel is with inclined angle α
The outer fluid is silicone oil (viscosity η out = 492.875 mPa s, density ρ out = 0.969 g cm−3), and inner fluid is a mixture of 70 wt% glycerol and 30 wt% distilled water (viscosity η in = 19.07 mPa s, density ρ in = 1.183 g cm−3). The interfacial tension between the two fluids is γ = 30.07 mN m−1. Viscosity and interfacial tension were measured by a viscometer (microVISC™, RheoSense, Inc.) and a ring tensiometer (Surface Tensiometer 20, Cole-Parmer), respectively; the density was measured by quantifying the volume of a known-mass fluid. Droplet size, droplet generation frequency and jet length were measured in the captured videos by using ImageJ and taking their average values over five to ten droplets for the first two and over jet lengths at ten different instants for the last.
The sinusoidal vibration induces periodic pressure fluctuations in inner fluid. Considering the displacement of y(t) = ɛ 0 sin(2πft) in the gravitational direction, we assume a pressure fluctuation of ρ in gɛ 0 sin(2πft) in microtubing at the vibrating location, where g is gravity acceleration. Without perturbation from the vibrator, inner fluid flow rate Q in inside the microtubing is, by using Poiseuille’s law (Batchelor 1967):
where R iw is inner wall radius of the microtubing (R iw = 0.43 mm), ΔP is the pressure drop from the vibrating location to the injection nozzle and L is the length between the two locations (Fig. 1a). Experimentally, L was kept constant at 40 cm. Now, by adding pressure fluctuation term ρ in gɛ 0 sin(2πft) into Eq. (1), we have an estimation of the transient flow rate of inner fluid Q in(t) (Fig. 1c),
with ɛ = πR 4iw ρ in gɛ 0/(8Q in η in L) being the dimensionless amplitude, whose numerical values for various combinations of ε 0 and Q in can be found in Table S1 in Online Resource 6.
Since inner flow rate is transiently fluctuating rather than being steady under the mechanical vibration, local flow field as well as local force balance where droplet breaks up alters. Consequently, both droplet size and generation frequency can be modulated by the vibration.
3 Results and discussion
3.1 Effects of vibration frequency
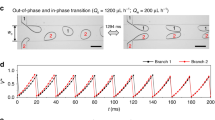
We show that vibration frequency f interferes with or even controls droplet generation in certain cases. In dripping regime without vibration, uniform droplets are generated with constant frequency at fixed flow rates, such as the 25 Hz of natural droplet generation frequency in Fig. 2a. When vibration frequency f is lower than this natural generation frequency, droplet generation is disturbed by vibration, resulting in non-uniform droplets (Fig. 2a, 10 Hz). As vibration frequency increases above the natural frequency, mechanical vibration provides with a robust control over droplet generation. In the range of 20–85 Hz (Fig. 2a), droplet generation frequency f d is synchronized with the vibration frequency f, and monodisperse droplets are produced. However, synchronization collapses when vibration frequency is above a critical value, such as 86 Hz (Fig. 2a). Beyond such a critical value, changing vibration frequency shows a weak impact on droplet generation, including both droplet generation frequency and size (Fig. 2a, for example, 86 and 200 Hz have no difference essentially in droplet generation). In this region, droplet generation frequency decreases from the maximum synchronization frequency, but is still higher than the natural generation frequency. Figure 2b shows the variation of droplet generation frequency with the vibration frequency. The polydispersity of droplets also depends on vibration frequency, as shown in Fig. 2c. Highly polydispersed droplets are generated in the neighborhood of synchronization region, with coefficient of variation (CV, the ratio of the standard deviation to the mean of droplet radius) around 5.5 % (10 and 100 Hz in Fig. 2c), while more monodisperse droplets are formed in synchronization and high-vibration-frequency region (f > 200 Hz), with CV around 2 % (Fig. 2c).
Effects of vibration frequency on droplet generation. a Droplet generation with increasing vibration frequency when Q in = 80 μL h−1, Q out = 1 mL h−1 and ε 0 = 2 mm. The value of vibration frequency f is marked upper right on each snapshot. For f = 0 Hz, droplets are generated naturally with frequency f d = 25 Hz in the dripping mode. For f lower than the natural generation frequency 25 Hz, droplet generation is interfered by the vibration with polydisperse size distribution, such as f = 10 Hz. For f in the range of 20–85 Hz, monodisperse droplets are produced with the generation frequency f d synchronized with vibration frequency f. For f above 85 Hz, f d deviates from f and fluctuates around a constant value. Scale bar 200 μm. b Plot of droplet generation frequency f d versus vibration frequency f under the same condition (a). c Polydispersity of droplet radius versus vibration frequency under the same condition (a). d Log–log plot of droplet radius versus generation frequency with Q in = 80 μL h−1 and ε 0 = 2 mm. A fitting slope is −0.33812, very close to −1/3, as predicted by Eq. (3). e Experimental validation of Eq. (3) with the same Q out (1 mL h−1) but different Q in. By fitting experimental data through origin, a slope of 1.0246 shows good agreement with the prediction from mass conservation in Eq. (3)
Since droplet generation frequency is modulated by vibration (Fig. 2b), the resultant droplet size is consequently vibration-influenced according to mass conservation. The constant average flow rate Q in of inner fluid should be equal to the product of droplet volume and droplet generation frequency. Therefore,
with R being droplet radius. For fixed Q in, R scales with f d as \(R \sim f_{\text{d}}^{ - 1/3}\), as verified in Fig. 2d. To confirm Eq. (3), we vary Q in, and compare the predicted frequency 3Q in/4πR 3 from Eq. (3) with the measured droplet generation frequency f d in Fig. 2e. A linear fitting through origin of the measured frequency agrees well with the prediction by Eq. (3). Based on Eq. (3), we can tune droplet size by changing droplet generation frequency through external vibration. In the synchronization region where droplet generation frequency is precisely correlated with the vibration frequency, especially the vibration is highly effective in accurately controlling droplet size.
3.2 Effects of vibration amplitude
Vibration amplitude can significantly impact the uniformity of droplet size. Figure 3a shows that small amplitude such as ε 0 = 1 and 2 mm retains the monodispersity of resultant droplets, but an increase in ε 0 to 3 mm leads to the generation of many small satellite droplets. The transient inner flow rate Q in(t) accounts for this difference. According to Eq. (2), a large amplitude ε 0 induces a large ε so that Q in(t) < 0 at some time instant t. Therefore, suction of inner fluid back into the injection nozzle occurs during one period of vibration, accompanied with an elongated slender liquid thread which multi-breaks into several satellite drops (see Online Resource 1). In contrast, a small amplitude reduces the value of dimensionless amplitude ε in Q in(t), and thus, the slender thread formation is suppressed which guarantees the generation of uniform droplets (see Online Resource 2). Note that ε > 1 is only the necessary but not sufficient condition for sucking-back of inner fluid into the injection nozzle. Such a sucking-back occurs only when the drainage of fluid from the droplet into the nozzle exceeds some threshold in one period. The drainage, expressed as an integral of Q in(t) over ∆t during which Q in(t) < 0, is proportional to Q in, ε, and 1/f. Accordingly, either increasing Q in and ε, or decreasing f would enhance this sucking-back. On the contrary, even though ε > 1, the sufficiently small Q in and sufficiently high f can still ensure no sucking-back, like the case in Fig. 3a with ε 0 = 2 mm (ε = 1.836), f = 80 Hz and Q in = 80 μL h−1.
Effects of vibration amplitude on droplet generation. a Influence of vibration amplitude on the uniformity of droplet size when f = 80 Hz, Q in = 80 μL h−1 and Q out = 1 mL h−1. Resultant droplets are monodisperse at low amplitude, such as ε 0 = 1 mm (ε = 0.918) and 2 mm (ε = 1.836), but polydisperse at large amplitude such as ε 0 = 3 mm (ε = 2.755). The range of synchronization is also influenced by amplitude, as exampled by the upper two snapshots of ε 0 = 1 mm and 2 mm. b Unique droplet sequence at large vibration amplitude (ε 0 = 3 mm) and with vibration frequency just above the maximum synchronization frequency. Vibration frequency f is marked upper right on each snapshot. Four-, three- and two-droplet sequences are exampled with different vibration frequencies. Scale bars 200 μm
The vibration amplitude affects the droplet generation not only independently but also synergistically with the vibration frequency. As presented in Fig. 3a, while droplet generation frequency is synchronized with the vibration frequency (80 Hz) at ε 0 = 2 mm, synchronization vanishes at ε 0 = 1 mm. The vibration amplitude impacts thus the range of synchronization. Another influence of vibration amplitude emerges when vibration frequency is just above its maximum synchronization frequency. In this situation, the droplet uniformity retains at small amplitude (such as Fig. 2a, 86 Hz), but gives the way to the unique droplet sequences containing ploydisperse droplets at large amplitude. The four-, three- and two-droplet sequences are, for example, generated periodically with ε 0 = 3 mm at different vibration frequencies (Fig. 3b; see Online Resource 3), similar to those generated in the tip-multi-breaking mode, a recently reported new droplet breakup mode (Zhu et al. 2015).
3.3 Synchronization of droplet generation
Having discussed the influences of vibration frequency and amplitude separately, we move our attention to map the overall effects of vibration-induced droplet generation in dripping regime. With fixed vibration amplitude but increasing vibration frequency f the droplet generation undergoes typically transitions among three distinct regions (Fig. 4a): non-synchronization region I, synchronization region II and non-synchronization region III. Region I occurs when vibration frequency f is approximately lower than the natural frequency f n of droplet generation. In this region, the droplet generation originates from the superposition (Driessen et al. 2014) of several instability modes, including the intrinsic Rayleigh–Plateau instability mode of the fluid system and the perturbations caused by external vibration. Consequently, the droplet produced in region I is polydisperse (Fig. 2c, 10 Hz). However, region II produces monodisperse droplets with a synchronized generation frequency. In this region, the external vibration dominates and is highly effective in controlling the generation of droplets. For example, the droplet diameter modulated by vibration in this region II can even be smaller than nozzle diameter D in (Fig. 2a, 85 Hz), which is essentially impossible by purely hydrodynamic shearing in co-flow geometry (Erb et al. 2011; Umbanhowar et al. 2000). Exceeding the maximum synchronization frequency f m the vibration leads the droplet generation into Region III, where the synchronization disappears, and the effect of vibration becomes weaker compared to Region II. The resultant droplet size can either be monodisperse or polydisperse in this region, depending on the vibration amplitude.
Characterization of droplet synchronization. a Schematic of three regions of droplet generation with mechanical vibration. Region I droplet generation interfered by vibration, producing polydisperse droplets. Region II synchronization region, producing monodisperse droplets. Region III vibration decaying region, uniformity of droplets largely depends on amplitude ε 0. b Process of droplet generation in synchronization region. Upmost image, tip growing; middle, neck formation; bottom, after breakup. f = 80 Hz, ε 0 = 2 mm, Q in = 80 μL h−1 and Q out = 1 mL h−1. c Process of droplet generation just above the maximum synchronization frequency. From top to bottom: first image, tip growing; second, inner fluid suction back; third, neck disappearing and tip re-growing; forth, neck formation; last, after breakup. f = 86 Hz, ε 0 = 2 mm, Q in = 80 μL h−1 and Q out = 1 mL h−1. Squares in (b) and (c) highlight the size of inner liquid tip. d Schematic of the tip size as a result of Q in(t) accumulating within half period 1/2f. e Experimental validation of Eq. (4). f Unscaled date of minimum droplet radius R m versus vibration amplitude ε 0 for various Q in and Q out. g Scaling of minimum droplet size with the influence of vibration. The linear fit slope of −0.3048 is consistent with the theoretical prediction from Eq. (5). Scale bars 200 μm
It is thus optimal to generate droplet in synchronization region II for producing uniform droplets effectively. The lower limit of the synchronization frequency f l is approximately equal to the natural frequency f n of droplet generation and is nearly independent of the vibration amplitude ε 0. Theoretically, f n can be determined from Eq. (3), since droplet radius R can be precisely predicted once device geometry, fluid properties, and dynamic parameters are known (Erb et al. 2011; Umbanhowar et al. 2000).
To determine the maximum synchronization frequency f m , we examine and differentiate the processes of droplet breakup inside (Fig. 4b) and beyond (Fig. 4c) the synchronization region in detail. When perturbed at the synchronization frequency (Fig. 4b), the bulbous inner fluid tip first grows to its maximum size due to a positive Q in(t) and then a sudden suction back of inner fluid toward the nozzle accelerates droplet breakup, generating one droplet in one period of vibration (see Online Resource 4). When the vibration frequency is above its maximum synchronization value (Fig. 4c), however, the inner fluid tip cannot grow into a critical breakup size before the suction back of inner fluid, so that droplet is incapable of breakup in one period, and then two or more periods become necessary for the tip to grow into a critical size (see Online Resource 5). Because vibration amplitude influences Q in(t), we expect that f m depends on ε 0, as demonstrated qualitatively in Fig. 3a.
Now, we estimate the maximum synchronization frequency f m by identifying the critical tip size for the breakup. Experimentally, we find that at frequency f m , inner fluid tip approximately grows in the first half period and pinches off in another half period due to the fluctuation term ɛ sin(2πft) in Eq. (2). We can thus estimate the maximum volume of inner fluid tip V drop by integrating Q in(t) over time interval [0, 1/(2f)] (Fig. 4d). Accordingly, \(V_{\text{drop}} \approx \int\limits_{0}^{{\frac{1}{2f}}} {Q_{\text{in}} \left( {1 + \varepsilon \sin (2\pi ft)} \right){\text{d}}t} = \frac{{Q_{\text{in}} }}{2f}\left( {1 + \frac{2\varepsilon }{\pi }} \right)\). To have droplet breakup within one period, V drop must be larger than its critical volume V c , so that \(\frac{{Q_{\text{in}} }}{2f}\left( {1 + \frac{2\varepsilon }{\pi }} \right) \ge V_{c}\). Consequently, the maximum synchronization frequency f m is,
Equation (4) shows that f m increases linearly with the flow rate Q in of inner fluid and the vibration amplitude ε 0, but is inversely proportional to the critical volume V c . Although the exact value of V c is not available at present, it should decrease with the outer fluid capillary number Ca out = 4η out Q out/(γπD 2out ). Accordingly, f m increases with Ca out. To validate Eq. (4), we plot f m /Q in versus ε for constant Q out in Fig. 4e, which shows a reasonably linear relationship between the two variables.
Based on the mass conservation in Eq. (3), we have \(f_{m} /Q_{in} \sim R_{m}^{ - 3}\). Meanwhile, Eq. (4) and Fig. 4e show that f m /Q in ∼ ɛ. Consequently, a scaling for the minimum droplet size R m is R m ∼ ɛ −1/3 given constant flow rate Q out of outer fluid. From previous study (Christopher and Anna 2007; Umbanhowar et al. 2000), the droplet radius R scales with Ca out as R/R in ∼ [1 + 1/(3Ca out)] in the dripping regime, where R in is the radius of injection nozzle (R in = 0.5D in). Finally, we obtain:
As shown in Fig. 4f, the minimum droplet radius R m depends noticeably on inner flow rate Q in, outer flow rate Q out and vibration amplitude ε 0 for different data sets. When scaled as R m /{R in[1 + 1/(3Ca out)]}, however, all the data collapse onto a single curve with a fitting slope of −0.3048 versus the dimensionless amplitude ε in Fig. 4g, a slope very close to −1/3 from Eq. (5).
3.4 Delaying dripping–jetting transition
Besides dripping, we study the jet formation modulated by vibration. The same inner and outer fluids are used in jetting experiments as those in dripping experiments. By increasing the inner flow rate Q in while fixing outer flow rate Q out, we observe a dripping to jetting transition. The mechanical vibration, however, suppresses the jet by compressing its length (Fig. 5a), similar to that in ATPS (Moon et al. 2015; Sauret et al. 2012; Ziemecka et al. 2011). Figure 5b shows the variation of the intact jet length (the length from injection nozzle to the rear of an unbroken jet, see Fig. 5a, 60 Hz) with the vibration frequency. Three distinguished regions, similar to those for the dripping presented in Fig. 4a, can be identified according to the variation of the jet length. When the frequency is low, the jet length varies in a disordered manner, attributed to the interference from the vibration. For a modest frequency, the jet length decreases with the vibration frequency, and the droplet generation is synchronized with the vibration frequency (shadow region in Fig. 5b). If the frequency is high, the effect of vibration on the jet length becomes weaker but still appreciable (Fig. 5a, 140 Hz), due to a decayed vibration amplitude at high frequency.
Delayed dripping to jetting transition modulated by vibration. a Variation of jet length (L jet) with vibration frequency f. With increasing f, the jet length first decreases (f = 60, and 120 Hz), and then increases (f = 140 Hz), but vibration still reduces the jet length in this case. When jet length is short enough, jetting transforms into dripping (f = 120 Hz), producing monodisperse droplets. Q in = 1.3 mL h−1, Q out = 1 mL h−1, ε 0 = 3 mm. Scale bar 200 μm. b Plot of dimensionless jet length L jet /R in versus dimensionless vibration frequency Ω. The shadow region is synchronization region similar to that in dripping case. c Log–log plot of L jet /R in versus Ω, with a fitting slope of −0.9884, confirming the theoretical prediction from Eq. (6). d Boundary of dripping–jetting transition at different vibration amplitudes. Transition occurs at larger inner flow rate Q in corresponding to larger vibration amplitude ε 0. Monodisperse droplets can thus be produced in a wider range of flow conditions by exerting mechanical vibration. e Phase diagram in the (Q in(1–0.2ε 0), Q out) plane showing the dripping–jetting transition. The scaled data collapse onto a single transition boundary, which shows a satisfactory agreement with the theoretical prediction from Guillot et al. (2007)
To characterize the shrinking of jet length with increasing frequency in synchronization region, we consider the temporal perturbations caused by vibration growing on the jet (radius R jet) in terms of R jet = R 0(1 − Πe 2πft), with R 0 being the unperturbed jet radius and Π (a positive real number) being the amplitude of growing perturbations. When the jet breaks up, Πe 2πft = 1. The breakup time is thus t = − ln Π/(2πf), inversely proportional to the vibration frequency f. Now, we approximate the intact jet length L jet as the product of jet injection velocity u in = 4Q in/(πD 2in ) and its breakup time t to be: L jet = −u in ln Π/(2πf). We thus obtain the dimensionless jet length L jet/R in as a function of dimensionless frequency Ω = 2πR in f/u in in the form of
Equation (6) shows that the jet length decreases with the vibration frequency in a hyperbolic form, resembling the breakup length of forced jets in air (García et al. 2014; Meier et al. 1992). Replotting the data in the synchronization region (shadow region in Fig. 5b) into a log–log plane, the data have a fitting slope of −0.9884, an excellent agreement with the prediction from Eq. (6), as shown in Fig. 5c.
Besides compressing the jet length, the vibration can lead to the transition from the jetting into the dripping at appropriate frequency (Fig. 5a, 120 Hz), accompanied with uniform droplets formation in synchronization region. Conventionally, an increase in inner phase flow rate contributes to a transition from the dripping to the jetting (Utada et al. 2007), but larger inner flow rate is required to transform the dripping to the jetting with vibration applied (Fig. 5d). This enables monodisperse droplet production in a wider range of flow rates compared with the case without vibration. The delay in dripping–jetting transition becomes stronger for a larger value of amplitude ε 0 (Fig. 5d). Experimentally, a scaling of inner flow rate Q in into Q in (1 − 0.2 ɛ 0) could collapse all the data sets with the same boundary of dripping to jetting transition in phase diagram Fig. 5e. Our dripping–jetting transition boundary agrees with the theoretical prediction (Guillot et al. 2007, 2008) (see Online Resource 6 for “determining dripping-jetting transition boundary”). The existing deviation from the theoretical prediction may originate from the channel geometry. Because the analytical solution of the transition boundary considers a cylindrical co-flow microchannel, in our experiments the round microcapillary is tapered to have an inclined angle α ≈ 1.4°.
4 Concluding remarks
In summary, we have examined influences of mechanical vibration on droplet formation in co-flow microfluidics. Dynamically, vibrating microtubing of inner fluid induces fluctuations in its flow rate, thereby changing the local force balance and varying the droplet formation. Technically, the vibration is highly effective in controlling droplet breakup in synchronization region, where the frequency of droplet generation is synchronized with the vibration frequency, and droplet size can thus be modulated in a wide range. This offers an effective way of overcoming the long-standing limitation that the droplet size depends strongly on the device geometry. We then quantitatively characterize the maximum synchronization frequency and minimum droplet size by estimating the fluctuating flow rate of inner fluid. Moreover, the mechanical vibration can delay the jet formation and enable the dripping to occur in a wider range of flow rates. This is important for generating uniform droplets, because the droplet size is unwantedly polydisperse in jetting regime but ideally monodisperse in dripping regime. Our quantitative characterizations of vibration-modulated droplet generation in both dripping and jetting regimes provide possibility to control droplet formation in a more efficient and precise manner for practical applications. Besides, we believe our results would benefit understanding the mechanisms of droplet generation in other perturbed systems that also involve flow rate fluctuations, such as those perturbed by piezoelectric transducers (Ziemecka et al. 2011) or pneumatic valves (Moon et al. 2015).
References
Abate AR, Weitz DA (2008) Single-layer membrane valves for elastomeric microfluidic devices. Appl Phys Lett 92:243509
Abate AR, Romanowsky MB, Agresti JJ, Weitz DA (2009) Valve-based flow focusing for drop formation. Appl Phys Lett 94:023503
Anna SL, Mayer HC (2006) Microscale tipstreaming in a microfluidic flow focusing device. Phys Fluids 18:121512
Anna SL, Bontoux N, Stone HA (2003) Formation of dispersions using “flow focusing” in microchannels. Appl Phys Lett 82:364
Augustin MA, Hemar Y (2009) Nano-and micro-structured assemblies for encapsulation of food ingredients. Chem Soc Rev 38:902–912
Batchelor G (1967) An introduction to fluid dynamics. Cambridge University Press, Cambridge
Chan HF, Zhang Y, Ho Y-P, Chiu Y-L, Jung Y, Leong KW (2013) Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci Rep. doi:10.1038/srep03462
Chong ZZ, Tor SB, Loh NH, Wong TN, Gañán-Calvo AM, Tan SH, Nguyen N-T (2015) Acoustofluidic control of bubble size in microfluidic flow-focusing configuration. Lab Chip 15:996–999
Christopher G, Anna S (2007) Microfluidic methods for generating continuous droplet streams. J Phys D Appl Phys 40:R319–R336
Collins DJ, Neild A, deMello A, Liu A-Q, Ai Y (2015) The Poisson distribution and beyond: methods for microfluidic droplet production and single cell encapsulation. Lab Chip 15:3439–3459
deMello AJ (2006) Control and detection of chemical reactions in microfluidic systems. Nature 442:394–402
Ding Y, i Solvas XC, deMello A (2015) “V-junction”: a novel structure for high-speed generation of bespoke droplet flows. Analyst 140:414–421
Dittrich PS, Manz A (2006) Lab-on-a-chip: microfluidics in drug discovery. Nat Rev Drug Discov 5:210–218
Driessen T, Sleutel P, Dijksman F, Jeurissen R, Lohse D (2014) Control of jet breakup by a superposition of two Rayleigh–Plateau-unstable modes. J Fluid Mech 749:275–296
Erb RM, Obrist D, Chen PW, Studer J, Studart AR (2011) Predicting sizes of droplets made by microfluidic flow-induced dripping. Soft Matter 7:8757–8761
Evans HM, Surenjav E, Priest C, Herminghaus S, Seemann R, Pfohl T (2009) In situ formation, manipulation, and imaging of droplet-encapsulated fibrin networks. Lab Chip 9:1933–1941
Fan J, Zhang YX, Wang LQ (2010) Formation of nanoliter bubbles in microfluidic T-junctions. NANO 5:175–184
García F, González H, Castrejón-Pita J, Castrejón-Pita A (2014) The breakup length of harmonically stimulated capillary jets. Appl Phys Lett 105:094104
Garstecki P, Gitlin I, DiLuzio W, Whitesides GM, Kumacheva E, Stone HA (2004) Formation of monodisperse bubbles in a microfluidic flow-focusing device. Appl Phys Lett 85:2649–2651
Guillot P, Colin A, Utada AS, Ajdari A (2007) Stability of a jet in confined pressure-driven biphasic flows at low Reynolds numbers. Phys Rev Lett 99:104502
Guillot P, Colin A, Ajdari A (2008) Stability of a jet in confined pressure-driven biphasic flows at low Reynolds number in various geometries. Phys Rev E 78:016307
Haber C (2006) Microfluidics in commercial applications; an industry perspective. Lab Chip 6:1118–1121
Hong J, Choi M, Edel JB, deMello AJ (2010) Passive self-synchronized two-droplet generation. Lab Chip 10:2702–2709
Khan IU, Stolch L, Serra CA, Anton N, Akasov R, Vandamme TF (2015) Microfluidic conceived pH sensitive core–shell particles for dual drug delivery. Int J Pharm 478:78–87
Khoshmanesh K, Almansouri A, Albloushi H, Yi P, Soffe R, Kalantar-zadeh K (2015) A multi-functional bubble-based microfluidic system. Sci Rep. doi:10.1038/srep09942
Kim SH, Shim JW, Yang SM (2011) Microfluidic multicolor encoding of microspheres with nanoscopic surface complexity for multiplex immunoassays. Angew Chem Int Ed 50:1171–1174
Kim JH, Jeon TY, Choi TM, Shim TS, Kim S-H, Yang S-M (2013) Droplet microfluidics for producing functional microparticles. Langmuir 30:1473–1488
Kobayashi I, Nakajima M, Nabetani H, Kikuchi Y, Shohno A, Satoh K (2001) Preparation of micron-scale monodisperse oil-in-water microspheres by microchannel emulsification. J Am Oil Chem Soc 78:797–802
Kong TT, Liu Z, Song Y, Wang LQ, Shum HC (2013) Engineering polymeric composite particles by emulsion-templating: thermodynamics versus kinetics. Soft Matter 9:9780–9784
Kong TT, Wang LQ, Wyss HM, Shum HC (2014) Capillary micromechanics for core–shell particles. Soft Matter 10:3271–3276
Lee W, Walker LM, Anna SL (2009) Role of geometry and fluid properties in droplet and thread formation processes in planar flow focusing. Phys Fluids 21:032103
Li J, Mittal N, Mak SY, Song Y, Shum HC (2015) Perturbation-induced droplets for manipulating droplet structure and configuration in microfluidics. J Micromech Microeng 25:084009
Meier G, Klöpper A, Grabitz G (1992) The influence of kinematic waves on jet break down. Exp Fluids 12:173–180
Moon B-U, Jones SG, Hwang DK, Tsai SSH (2015) Microfluidic generation of aqueous two-phase system (ATPS) droplets by controlled pulsating inlet pressures. Lab Chip 15:2437–2444
Nakano M, Komatsu J, Matsuura S-i, Takashima K, Katsura S, Mizuno A (2003) Single-molecule PCR using water-in-oil emulsion. J Biotechnol 102:117–124
Nguyen N-T et al (2007) Thermally mediated droplet formation in microchannels. Appl Phys Lett 91:084102
Patravale VB, Mandawgade SD (2008) Novel cosmetic delivery systems: an application update. Int J Cosmet Sci 30:19–33
Sauret A, Shum HC (2012) Forced generation of simple and double emulsions in all-aqueous systems. Appl Phys Lett 100:154106
Sauret A, Spandagos C, Shum HC (2012) Fluctuation-induced dynamics of multiphase liquid jets with ultra-low interfacial tension. Lab Chip 12:3380–3386
Schmid L, Franke T (2013) SAW-controlled drop size for flow focusing. Lab Chip 13:1691–1694
Schmid L, Franke T (2014) Acoustic modulation of droplet size in a T-junction. Appl Phys Lett 104:133501
Song H, Chen DL, Ismagilov RF (2006) Reactions in droplets in microfluidic channels. Angew Chem Int Ed 45:7336–7356
Stone HA, Stroock AD, Ajdari A (2004) Engineering flows in small devices: microfluidics toward a lab-on-a-chip. Annu Rev Fluid Mech 36:381–411
Sugiura S, Nakajima M, Seki M (2002) Prediction of droplet diameter for microchannel emulsification. Langmuir 18:3854–3859
Tan SH, Maes F, Semin B, Vrignon J, Baret J-C (2014a) The microfluidic jukebox. Sci Rep. doi:10.1038/srep04787
Tan SH, Semin B, Baret J-C (2014b) Microfluidic flow-focusing in ac electric fields. Lab Chip 14:1099–1106
Umbanhowar PB, Prasad V, Weitz DA (2000) Monodisperse emulsion generation via drop break off in a coflowing stream. Langmuir 16:347–351
Utada AS, Fernandez-Nieves A, Stone HA, Weitz DA (2007) Dripping to jetting transitions in coflowing liquid streams. Phys Rev Lett 99:094502
Wang LQ, Zhang YX, Cheng L (2009) Magic microfluidic T-junctions: valving and bubbling. Chaos Soliton Fractals 39:1530–1537
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442:368–373
Zhang YX, Wang LQ (2011) Nanoliter-droplet breakup in confined T-shaped junctions. Curr Nanosci 7:471–479
Zhang YX, Jiang W, Wang LQ (2010) Microfluidic synthesis of copper nanofluids. Microfluid Nanofluid 9:727–735
Zhao C-X (2013) Multiphase flow microfluidics for the production of single or multiple emulsions for drug delivery. Adv Drug Deliv Rev 65:1420–1446
Zhou H, Yao S (2014) A facile on-demand droplet microfluidic system for lab-on-a-chip applications. Microfluid Nanofluid 16:667–675
Zhu PA, Kong TT, Kang ZX, Tian XW, Wang LQ (2015) Tip-multi-breaking in capillary microfluidic devices. Sci Rep. doi:10.1038/srep11102
Ziemecka I, van Steijn V, Koper GJM, Rosso M, Brizard AM, van Esch JH, Kreutzer MT (2011) Monodisperse hydrogel microspheres by forced droplet formation in aqueous two-phase systems. Lab Chip 11:620–624
Acknowledgments
The authors wish to thank Dr. Ho Cheung Shum for the generous use of mechanical vibrator. The financial support from the Research Grants Council of Hong Kong (GRF 17211115, GRF17207914, GRF HKU717613E, GRF HKU718111E) and the University of Hong Kong (URC 201511159108, 201411159074 and 201311159187) is gratefully acknowledged. The work is also supported in part by the Zhejiang Provincial, Hangzhou Municipal and Lin’an County Governments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 966 kb)
Supplementary material 2 (MOV 1136 kb)
Supplementary material 3 (MOV 1335 kb)
Supplementary material 4 (MOV 777 kb)
Supplementary material 5 (MOV 1700 kb)
Rights and permissions
About this article
Cite this article
Zhu, P., Tang, X. & Wang, L. Droplet generation in co-flow microfluidic channels with vibration. Microfluid Nanofluid 20, 47 (2016). https://doi.org/10.1007/s10404-016-1717-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-016-1717-2