Abstract
Hierarchical assemblies of nanostructured building blocks on conducting substrates are significant for construction of functional devices. Microfluidics is powerful but less exploited tool for spatial organization or growth of functionally sophisticated nanostructures with precise control. In this paper, we introduce a simple but unique strategy for the hydrothermal synthesis and patterned assembly of ZnO nanostructures within microchannels by soft lithography technique. Optical/antireflection properties of such hierarchically structured nanostructures are studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One dimensional inorganic nanostructures such as nano-wires, tubes, rods, belts, etc. have drawn prime attention due to their significance in understanding fundamental physical concepts and constructing nanoscale electronic and optoelectronic devices (Duan et al. 2001; Huang et al. 2001; Yang et al. 2010). However, next generation devices will most likely require precisely engineered multiscale architectures of materials; hence, there is a rising demand to obtain functional materials with well defined and controllable structures (Qu et al. 2011). Fabrication of functional nanostructures within well-defined micropatterns can synergize the advantages of both unique properties of nanomaterials and diverse applications of micropatterning of materials (Menard et al. 2007; Fan et al. 2006). Among functional nanomaterials vertically aligned ZnO nanostructures have a variety of applications in electronics as well as optoelectronic and electromechanical nanodevices, such as solar cells (Law et al. 2005), field emission devices (Wang et al. 2006a), UV lasers (Govender et al. 2002), light emitting diodes (Park and Yi 2004), piezo-nanogenerators (Wang and Song 2006) and nanosensors (Wang et al. 2004; Han et al. 2012). 1D ZnO nanostructures have been synthesized by several strategies as chemical or physical vapor deposition, molecular beam epitaxy (MBE), pulsed laser deposition or sputtering techniques (Djurisic et al. 2012). In comparison to these techniques wet chemical processes are low cost and scalable methods (Xu and Wang 2011). However, to realize the novel nanoscale device architectures the suitability of a fabrication technique for integration with higher level structures is as important as its capability of producing well-controlled nanostructures.
In an effort to integrate the ZnO nanostructures into a more regular form to enhance the performance of the nanodevices, a variety of techniques have been employed to fabricate patterned vertically aligned ZnO nanostructure arrays, including photolithography (Greyson et al. 2004), nanosphere lithography (Zeng et al. 2009), interference lithography (Kim et al. 2007), laser writing (Ong et al. 2012), electron beam lithography (EBL) (Ng et al. 2004) and nanoimpriniting (Jung and Lee 2011). Mostly a seed layer is patterned with these techniques and then used to grow patterned arrays of ZnO nanostructures separately. These require clean rooms and expensive equipments and are low throughput techniques.
Soft lithography is a low cost and experimentally convenient technology for patterning micro or nanoscale structures (Qin et al. 2010). Microcontact printing of a self assembled monolayer (SAM) has been employed in ZnO nanostructure patterning processes with chemically modified surfaces (Wang et al. 2007; Kang et al. 2011). The use of SAM patterning is clever but restricted by the limitations on compatible substrate materials. Microfluidics-based, surface-adherent synthesis offers a route towards simultaneous spatial patterning and growth of functional nanomaterials (Wang et al. 2006b; Thangawng et al. 2009; Dittrich et al. 2006). Crystals and nanoparticles of superior quality are synthesized in microchannels due to the excellent handling of small fluid volumes of nano- and even picolitres (Yen et al. 2005). However, microfluidic device is also a powerful tool to align inorganic nanostructures on substrate. There are only few reports on use of microfluidics to organize nanostructures with increased complexity/hierarchy. Indeed, in some cases, complex nanowire arrays are fabricated on substrates using fluid flows within the channel but nanostructures are synthesized ex-situ and then horizontally aligned within the channels using fluid flow (Kim et al. 2011; Cui and Lieber 2001; Kenis et al. 2000). In an effort to grow and pattern vertical arrays of nanostructures, a patterned seed template was fabricated by zinc acetate solution flowing though a microfluidic channel and then growth of vertical ZnO nanowires was realized from the seed using thermal chemical vapor deposition on a silicon substrate at high temperatures (Lee et al. 2006). An alternative, more simple strategy is to grow nanostructure arrays directly from Zinc metal substrates. The direct growth of ZnO arrays on zinc substrates was realized by the surface oxidation of zinc foil in solution at room temperature or under hydrothermal conditions (Wang et al. 2008). If Zn foil can be replaced with Zn plating layer, the growth of ZnO nanowire arrays could be extended to various substrates. By exploiting this concept we have successfully achieved micropatterned seedless vertical growth of functional ZnO nanostructures by means of soft microfluidic channels. The photoluminescence and antireflection characteristics of the hierarchically structured ZnO nanowire arrays are studied. Method reported here has the virtue of simplicity, cost efficiency and high throughput which can facilitate fabrication of complex device architectures.
2 Experimental
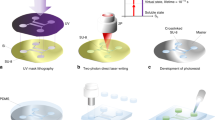
2.1 Fabrication of elastomeric patterned mold
Silicon masters with desired microchannels are fabricated using photolithography. In order to fabricate silicon masters, Si wafers with native oxide layers were cleaned by sonication in acetone and ethanol for 15 min. They were subsequently rinsed with ethanol and dried with nitrogen flux. A photoresist (AZ 9260 Photoresist, Clarion corp.) was applied to the wafer, which was subsequently spun at 3,000 rpm for 30 s, and then the wafers were baked on a hot plate (110 °C) for 1 min 30 s. The resist was patterned by a mask aligner (SussMicroTech Lithography GmBH) and developed with AZ 300 MIF. To realize the patterns within the silicon for the master, ICP-RIE plasma etching system (SPTS Technologies, UK) was used. The Bosch process, consisting of pulsed SF6/C4F8 deep reactive ion etching enables anisotropic etching of silicon through the photoresist mask. After the definition of the patterns within the bulk of the silicon wafer, the photoresist was removed by several baths in acetone and isopropyl alcohol. The patterns were then coated with a 60-nm thick C4F8 fluoropolymer. Polydimethylsiloxane (PDMS) molds with micropatterns were fabricated with Sylgard 184 (Dow Corning) by molding against Si masters. These molds can be used repeatedly several times.
2.2 Microfluidic growth of patterned ZnO nanorod arrays
For the preparation of patterned ZnO nanostructure arrays, zinc substrates (zinc wafer cut into 1 × 1 cm2) were cleaned by sonication in ethanol, propanol and deionised water and dried in air. PDMS molds with micro patterns are kept in conformal contact with zinc substrate. This assembly was supported by Teflon thread tying to keep both substrate and PDMS mold in firm contact with each other forming microchannels throughout the reaction. This microchannel assembly is then placed in 100 ml of 15 % aqueous dimethylformamide solution (v/v). The reaction system was then maintained at a temperature of 85–90 ºC for 12 h. After completion of reaction, microfluidic device is disassembled and Zn substrates with patterned ZnO nanostructures on it are removed, rinsed with absolute ethanol and dried in air for further characterization.
2.3 Characterization
The morphology of all the samples is examined by scanning electron microscopy (Quanta 200 3D, FEI). The X-ray diffraction measurements are carried out on a Philips PW 1830 instrument. HRTEM images and SAED patterns are obtained on a Tecnai F30 FEG machine operated at 300 kV. Raman analyses of all the samples were performed on an HR 800 Raman spectrometer (Jobin–Yvon, Horiba, France) using 632.8 nm green laser (NRS1500W).
The reflectance spectra of the ZnO nanorods are measured using Jasco UV–Vis spectrophotometer (V 570 UV–VIS–NIR). The photoluminescence properties are studied at room temperature on Fluorolog HoribaJobinYvon fluorescence spectrophotometer.
3 Results and discussion
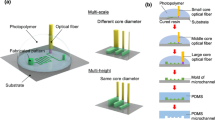
Scheme 1 is the schematic representation of device assembly and subsequent microfluidic growth of ZnO nanostructures inside microchannels. Three walls of these microfluidic channels are composed of soft elastomeric mold, whereas the fourth wall or base of the microchannels acts as substrate on which and material by which ZnO nanostructures are grown. ZnO nanostructure arrays are grown by chemical oxidation of Zn wafer in 15 % dimethyl formamide (DMF) aqueous solution at 90 oC. Naturally dissolved oxygen in water oxidizes metallic zinc slowly due to the formation of passive oxide layer. The oxidation process of metallic zinc is accelerated by DMF. The mechanism for the formation of ZnO nanostructures is as below:
Zn2+ ions are continuously released in the aqueous DMF solution leading to the precipitation of zinc hydroxide on the Zn surface. Zinc hydroxide embryonic clusters grew into nuclei on the surface which then transformed into zinc oxide.
Figure 1 shows SEM images of PDMS molds with patterned micropillars forming microchannels of varied profiles. Mold 1 refers to line pattern with pillars of width 36 µm forming channels of width 70 µm and height of each pillar is 45 µm, mold 2 is a serpentine pattern with continuous pillar of width 60 µm and height of 45 µm forming continuous channels of width 30 µm, mold 3 is having rectangular pillar pattern with dimensions as length 70 µm, width 20 µm, horizontal distance between two respective rectangles is 70 µm and vertical distance between the same is 20 µm, and height 43 µm and mold 4 is having square pillar pattern with dimensions length 50 µm, width 50 µm, horizontal and vertical distance between two respective squares is 100 µm and height 20 µm.
Morphological characterization of patterned ZnO nanorod arrays grown on Zn substrates was done by SEM. The SEM images show that vertically aligned ZnO nanorods are grown with high density within geometrically defined micropatterns. Figure 2a, c, e, g shows the ZnO nanorod arrays grown in microchannels formed by elastomeric Mold 1, Mold 2, Mold 3, Mold 4 and referred as Substrate 1, Substrate 2, Substrate 3 and Substrate 4, respectively. Figure 2b, d, f, h shows magnified images of each pattern with images in inset showing the density of ZnO nanorod arrays on each patterned surface. Figure 3a, b show high resolution SEM images of ZnO nanorods grown in microfluidic channels. These vertically aligned nanorods are observed to have diameter of 80 ± 10 nm and length of 4 ± 1 µm at given reaction conditions. These nanostructure arrays grown in different types of microfluidic channels mostly exhibit uniformity in terms of nanorod dimensions and density but in some cases, it is observed that the nanostructures grown at the edges of the channel walls or pillar walls are notably higher than the nanostructures in the centre of the channels. This can be attributed to the edge effect due to fluid flow in very small channels (Manbachi et al. 2008). Parabolic flow profile of fluids in microchannels results in slow flow of reactant liquids near the channel walls giving more time to them to interact with the active substrate (Zn). Hence, nanostructure growth is accelerated near channel walls making those nanostructures longer as compared to the central nanostructures. Figure 4a, b shows SEM images of ZnO nanostructures grown at the edges of channels. It is observed predominantly in case of ZnO nanostructures grown in straight and serpentine microfluidic channels. Intricate nanostructures can be derived by carefully monitoring reaction conditions, pattern designs and dimensions.
The crystal structure and the orientation of the as grown ZnO nanorods were revealed by the XRD investigation. Figure 5 shows the XRD patterns of the microfluidically prepared ZnO nanorods substrates. All of the diffraction peaks can be indexed to wurtzite structured ZnO (space group P63mc; a = 0.3249 nm and c = 0.5206 nm) (JCPDS card no. 35-1451). XRD patterns obtained from all ZnO nanorods substrates show strong peak at 2θ = 34.4º attributed to the ZnO (002) crystal plane, indicating a preferential orientation of the nanorods along the c-axes perpendicular to the Zn substrate. The enhanced (002) diffraction peaks are consistent with the SEM images that shows the oriented growth of the ZnO nanorods along the c-axes (Lin et al. 2006). The appearance of the weak (102) and (103) reflections of ZnO was probably caused by the uneven surface of the Zn wafer.
Raman scattering measurements were carried out on as prepared ZnO nanorods substrates to investigate the lattice vibrational properties at room temperature. He–Ne laser (λexe = 632.8 nm) is used as the excitation source. Figure 6 shows the Raman spectra of the ZnO nanorods grown in different microfluidic channels. The Raman spectrums of the as grown ZnO nanorods show four prominent peaks at 100, 332, 382, and 438 cm−1.The peak at 100 (low-E2) and 438 cm−1 (high E2), a non-polar optical phonons, are clearly visible in all the ZnO nanorods substrates which are common in Wurtzite type of ZnO nanorods (Sieber et al. 2009). Intense and dominant peak is observed at 438 cm−1 which labeled as E2 indicates the good crystal quality and is a characteristic of Wurtzite (hexagonal) phase of ZnO. The weak and broad peak at 332 cm−1 (E2H–E2L) is a second order E2 Raman mode while the peak positioned at 382 cm−1 can be assigned as A1 (TO) mode (Liu et al. 2009). The intensity of other peaks is much weaker than E2 peak suggests that the ZnO nanorods have fewer defects.
The atomic structure of the individual ZnO nanorod is investigated using a high-resolution transmission electron microscopy (HRTEM). Figure 7a, b shows a TEM and HRTEM images of a single nanorod grown within microchannel. The HRTEM investigation shows crystalline ZnO nanorod growth along the ZnO [0001] direction. Also, the diffraction pattern shown in the inset confirms that the nanorods have a single crystalline growth along ZnO [0001] direction.
ZnO is widely studied material for various types of photovoltaic cells. It has high transparency due to the wide band gap and appropriate refractive index (n ≈ 2 at 600 nm). Optical and electrical losses are primary factors that influence photovoltaic conversion efficiency. In particular, the optical loss attributed to the reflection loss of the incoming light. By incorporating a novel nano surface textures with multiple internal reflections, low surface reflection and high light absorption can be achieved and the efficiency of photovoltaic devices can be significantly enhanced. Micropatterned assembly of ZnO nanostructures resulting in two scale hierarchy can further minimize the reflection loss. In order to verify this logic, we studied the reflectance of all microfluidically grown patterned ZnO nanostructures. The UV–Vis reflectance spectra of all the micropatterned ZnO nanostructures substrates are shown in Fig. 8. Reflectance of unpatterned ZnO nanostructures (Substrate 5) and bare zinc wafer with native thin ZnO layer on its surface (Substrate 6) are taken for reference. Obviously zinc wafer with native thin ZnO layer shows maximum reflection than all other substrates having ZnO nanorods grown over. ZnO nanorods grown in microfluidic channels with square or rectangular pillar patterns are shown to have minimum reflectance even less than unpatterned substrate having ZnO nanostructures all over its surface and support the logic of reduced reflectance with increased hierarchy. However, nanostructures grown in serpentine and straight line channels show more reflectance than unpatterned surface. This may be because they are having more exposed unstructured area and the less density of the nanorod patterns. It should be possible to minimize reflectance in case channel dimensions are reduced, and subsequently pattern density be increased.
As ZnO is an important material in optoelectronic research (Sieber et al. 2010), the optical quality of ZnO nanorods on Zn substrate is evaluated by the room temperature photoluminescence using 325 nm Xe lamp as the excitation source. Figure 9 shows the RT-PL spectra obtained from the ZnO nanostructures. ZnO nanorods from all patterned substrates exhibit a strong UV emission in the range of 382–387 nm along with the weak violet emission band at 427 nm and broad green emission band in the visible range from 503 to 578 nm. The strong UV emission is the characteristic emission of ZnO and attributed to the radiative recombination of electrons in the conduction band and holes in the valence band. The weak violet emission peak at about 427 nm may be due to the existence of the oxygen depletion interface traps into ZnO film. Zeng et al. (2006), previously reported that, violet emission is attributed to the electronic transition from the defect level to the valence band. Among the six types of defects in ZnO lattice, Vo, Zni and Zno are donors, whereas VZn, Oi and OZn are acceptors. Zeng et al. (2010), also proposed a mechanism for the violet emission, if there is excitation of electron to a sub-band of the conduction band, they can first relax to Zni state through a non-radiative transition and then move to the valence band. This mechanism suggest that, for violet emission two excitation modes, with Eg ≤ Eex and EZni ≤ Eex < Eg, are effective. Whereas it is well known that the broad green emission in the visible region is related with defects such as, ionized oxygen vacancies, antisite oxygen, oxygen interstitials, zinc vacancies and surface defects (Gao et al. 2005). UV emission in all ZnO nanorod substrates is stronger than visible emissions which suggest the high crystalline quality of ZnO nanorods.
4 Conclusion
In summary, a robust, inexpensive approach to produce hierarchically structured ZnO nanomaterials is obtained by means of simple microdevice. The resulting micropatterned nanostructures exhibit excellent optical and antireflective properties. This structural hierarchy can reduce optical loss due to reflectance in photovoltaic cells and subsequently enhance energy conversion efficiency. In order to make this approach more general; films of precursor material can be deposited on desired substrate and nanomaterials that are produced by solution process methods can be assembled hierarchically with ease. It was observed that fluid flow inside microchannels affects growth of nanostructures at the edges; innovative nanostructures can be synthesized inside microfluidic channels by controlling and modulating fluid flow. This method is high throughput and scalable as batch processing is also possible.
References
Cui Y, Lieber CM (2001) Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 291:851–853
Dittrich PS, Heule M, Renaud P, Manz A (2006) On-chip extrusion of lipid vesicles and tubes through microsized apertures. Lab Chip 6:488–493
Djurisic AB, Chem X, Leung YH, Ng AMC (2012) ZnO nanostructures: growth, properties and applications. J Mater Chem 22:6526–6535
Duan XF, Huang Y, Cui Y, Wang JF, Lieber CM (2001) Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature 409:66–69
Fan HJ, Werner P, Zacharias M (2006) Semiconductor nanowires: from self-organization to patterned growth. Small 2:700–717
Gao X, Li X, Yu W (2005) Flowerlike ZnO nanostructures via hexamethylenetetramine-assisted thermolysis of zinc-ethylenediamine complex. J Phys Chem B 109:1155–1161
Govender K, Boyle DS, O’Brien P, Binks D, West D, Coleman D (2002) Room-temperature lasing observed from ZnO nanocolumns grown by aqueous solution deposition. Adv Mater 14:1221–1224
Greyson EC, Babayan Y, Odom TW (2004) Directed growth of ordered arrays of small-diameter ZnO nanowires. Adv Mater 16:1348–1352
Han X, Zhou X, Jiang Y, Xie Z (2012) The preparation of spiral, ZnO nanostructures by top–down wet-chemical etching and their related properties. J Mater Chem 22:10924–10928
Huang MH, Mao S, Feick H, Yan H, Wu Y, Kind H, Weber E, Russo R, Yang P (2001) Room-temperature ultraviolet nanowire nanolasers. Science 292:1897–1899
Jung MH, Lee H (2011) Selective patterning of ZnO nanorods on silicon substrates using nanoimprint lithography. Nanoscale Res Lett 6:159
Kang HW, Yeo J, Hwang JO, Hong S, Lee P, Han SY, Lee JH, Rho YS, Kim SO, Ko SH, Sung HJ (2011) Simple ZnO nanowires patterned growth by microcontact printing for high performance field emission device. J Phys Chem C 115:11435–11441
Kenis PJA, Ismagilov RF, Takayama S, Whitesides GM, Li S, White HS (2000) Fabrication inside microchannels using fluid flow. Acc Chem Res 33:841–847
Kim DS, Ji R, Fan HJ, Bertram F, Scholz R, Dadgar A, Nielsch K, Krost A, Christen J, Gosele U, Zacharias M (2007) Laser-interference lithography tailored for highly symmetrically arranged ZnO nanowire arrays. Small 3:76–80
Kim J, Lib Z, Park I (2011) Direct synthesis and integration of functional nanostructures in microfluidic devices. Lab Chip 11:1946–1951
Law M, Greene LE, Johnson JC, Saykally R, Yang P (2005) Nanowire dye-sensitized solar cells. Nat Mater 4:455–459
Lee SH, Lee HJ, Oh D, Lee SW, Goto H, Buckmaster R, Yasukawa T, Matsue T, Hong SK, Ko HC et al (2006) Control of the ZnO nanowires nucleation site using microfluidic channels. J Phys Chem B 110:3856–3859
Lin YR, Yang SS, Tsai SY, Hsu HC, Wu ST, Chen IC (2006) Visible photoluminescence of ultrathin ZnO nanowire at room temperature. Cryst Growth Des 6:1951–1955
Liu H, Piret G, Sieber B, Laureyns J, Roussel P, Xu W, Boukherroub R, Szunerits S (2009) Electrochemical impedance spectroscopy of ZnO nanostructures. Electrochem Commun 11:945–949
Manbachi A, Shrivastava S, Cioffi M, Chung BG, Moretti M, Demirci U, Yliperttulaa M, Khademhosseini A (2008) Microcirculation within grooved substrates regulates cell positioning and cell docking inside microfluidic channels. Lab Chip 8:747–754
Menard E, Rogers JA (2007) Stamping techniques for micro- and nanofabrication. In: Bhushan B (ed) Springer handbook of nanotechnology. Springer, Berlin, pp 279–298
Ng HT, Han J, Yamada T, Nguyen P, Chen YP, Meyyappan M (2004) Single crystal nanowire vertical surround-gate field-effect transistor. Nano Lett 4:1247–1252
Ong WL, Low QX, Huang W, Van Kan JA, Ho GW (2012) Patterned growth of vertically-aligned ZnO nanorods on a flexible platform for feasible transparent and conformable electronics applications. J Mater Chem 22:8518–8524
Park WI, Yi GC (2004) Electroluminescence in n-ZnO nanorod arrays vertically grown on p-GaN. Adv Mater 16:87–90
Qin D, Xia Y, Whitesides GM (2010) Soft lithography for micro- and nanoscale patterning. Nat Protoc 5:491–502
Qu L, Vaia RA, Dai L (2011) Multilevel, multicomponent microarchitectures of vertically-aligned carbon nanotubes for diverse applications. ACS Nano 5:994–1002
Sieber B, Liu H, Piret G, Laureyns J, Roussel P, Gelloz B, Szunerits S, Boukherroub R (2009) Synthesis and luminescence properties of (N-doped) ZnO nanostructures from a dimethylformamide aqueous solution. J Phys Chem C 113:13643–13650
Sieber B, Addad A, Szunerits S, Boukherroub R (2010) Stacking faults-induced quenching of the UV luminescence in ZnO. J Phys Chem Lett 1:3033–3038
Thangawng L, Howell PB, Richards JJ, Erickson JS, Ligler FS (2009) A simple sheath-flow microfluidic device for micro/nanomanufacturing: fabrication of hydrodynamically shaped polymer fibers. Lab Chip 9:3126–3130
Wang ZL, Song JH (2006) Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 312:242–246
Wang X, Summers CJ, Wang ZL (2004) Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett 4:423–426
Wang WZ, Zeng BQ, Yang J, Poudel B, Huang JY, Naughton MJ, Ren ZF (2006a) Aligned ultralong ZnO nanobelts and their enhanced field emission. Adv Mater 18:3275–3278
Wang J, Bunimovich YL, Sui G, Sawas S, Wang J, Guo Y, Heath JR, Tseng HR (2006) Electrochemical fabrication of conducting polymer nanowires in an integrated microfluidic system. Chem Commun 3075–3077
Wang H, Wong ASW, Ho GW (2007) Facile solution route to vertically aligned, selective growth of ZnO nanostructure arrays. Langmuir 23:11960–11963
Wang Y, Li X, Lu G, Quan X, Chen G (2008) Highly oriented 1-D ZnO nanorod arrays on zinc foil: direct growth from substrate, optical properties and photocatalytic activities. J Phys Chem C 112:7332–7336
Xu S, Wang ZL (2011) One-dimensional ZnO nanostructures: solution growth and functional properties. Nano Res 4:1013–1098
Yang PD, Yan RX, Fardy M (2010) Semiconductor nanowire: what’s next? Nano Lett 10:1529–1536
Yen BKH, Gunther A, Schmidt MA, Jensen KF, Bawendi MGA (2005) A microfabricated gas–liquid segmented flow reactor for high-temperature synthesis: the case of CdSe quantum dots. Angew Chem Int Ed 44:5447–5451
Zeng H, Cai W, Hu J, Duan G, Liu P (2006) Violet photoluminescence from shell layer of Zn/ZnO core-shell nanoparticles induced by laser ablation. Appl Phys Lett 88:171910–171913
Zeng H, Xu X, Bando Y, Gautam UK, Zhai T, Fang X, Liu B, Golberg D (2009) Template deformation-tailored ZnO nanorod/nanowire arrays: full growth control and optimization of field-emission. Adv Funct Mat 19:3165–3172
Zeng H, Duan G, Li Y, Yang S, Xu X, Cai W (2010) Blue luminescence of ZnO nanoparticles based on non-equilibrium processes: defect origins and emission controls. Adv Funct Mat 20:561–572
Acknowledgments
We thank Council of Scientific and Industrial Research (CSIR) of India for financially supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehare, R.S., Devarapalli, R.R., Yenchalwar, S.G. et al. Microfluidic spatial growth of vertically aligned ZnO nanostructures by soft lithography for antireflective patterning. Microfluid Nanofluid 15, 1–9 (2013). https://doi.org/10.1007/s10404-012-1119-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-012-1119-z