Abstract
This article presents a microfluidic device (so called concentrator) for rapid and efficient concentration of micro/nanoparticles using direct current dielectrophoresis (DC DEP) in continuous fluid flow. The concentrator is composed of a series of microchannels constructed with PDMS-insulating microstructures to focus efficiently the electric field in the flow direction to provide high field strength and gradient. Multiple trapping regions are formed within the concentrator. The location of particle trapping depends on the strength of the electric field applied. Under the experimental conditions, both streaming movement and DEP trapping of particles simultaneously take place within the concentrator at different regions. The former occurs upstream and is responsible for continuous transport of the particles, whereas the latter occurs downstream and rapidly traps the particles delivered from upstream. The observation agrees with the distribution of the simulated electric field and DEP force. The performance of the device is demonstrated by successfully and effectively concentrating fluorescent nanoparticles. At the sufficiently high electric field, the device demonstrates a trapping efficiency of 100%, which means downstream DEP traps and concentrates all (100%) the incoming particles from upstream. The trapping efficiency of the device is further studied by measuring the fluorescence intensity of concentrated particles in the channel. Typically, the fluorescence intensity becomes saturated in Trap 1 by applying the voltage (400 V) for >2 min, demonstrating that rapid concentration of the nanoparticles (107 particles/ml) is achieved in the device. The microfluidic concentrator described can be implemented in applications where rapid concentration of targets is needed such as concentrating cells for sample preparation and concentrating molecular biomarkers for detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In many biomedical and analytical applications, it is essential to concentrate or extract micro/nanoparticles, such as cells, bacteria, viruses, and DNA, from the background matrix. The form of these particles in high concentration facilitates the subsequent analytical and processing steps. For example, current methods in microbial analysis of water quality require subpopulations (e.g. E. coli) sampled in detectable levels of concentration (Greenberg et al. 2005), which is usually done with overnight culturing and benchtop sample processing. In the process of gene hybridization, rates can be accelerated by increasing the concentration of single-stranded DNA (Hames and Higgins 1995). In many of the fluorescence-based bioassays such as fluorescence immunoassay (Visor and Schulman 2006), labeled targets can be pre-concentrated to promote the subsequent detection and analytical procedures.
In recent years, more and more biological and chemical assays are conducted in microscale devices with the benefit from the rapid development of micro total analysis systems (μ-TAS) or labs-on-a-chip (LOC) (Reyes et al. 2002; Lee and Lee 2004). As one of the major components of LOC systems, on-chip sample preparation such as separation and concentration of analytes presents a challenging task, and is usually required prior to subsequent analytical processes. Traditional benchtop methods of concentrating samples by centrifuging and subsequently removing the supernatant are not amenable to the format of microchips. A number of methods have been reported concerning on-chip microfluidic concentration and manipulation of micro/nanoparticles such as dielectrophoresis (Durr et al. 2003; Chen et al. 2006; Lapizco-Encinas et al. 2004; Zhu and Xuan 2009), optical tweezers (Sheetz 1998), hydrodynamic filtration (Yamadaa and Seki 2005), and ultrasonic wave (Nilsson et al. 2004). They are readily integrated into microdevices by patterning micro/nanometal electrodes (in the case of dielectrophoresis), microchannels, or using remote manipulation with laser or ultrasound. In many cases, a microfluidic device that is capable of concentrating or separating particles in a continuous fluid flow other than a stationary flow is of particular interest, due to its potential capacity to process relatively large sample volume in microscale devices and produce high throughput.
Dielectrophoresis (DEP), the motion of a particle in a nonuniform electric field due to the unbalanced electrostatic forces on the particle’s induced dipole (Jones 1995; Pohl 1978), has been widely used in various microfluidic devices for concentration, manipulation, separation, sorting, and transport of particles such as beads, bacteria, and cells (Durr et al. 2003; Chen et al. 2006; Lapizco-Encinas et al. 2004; Chen and Du 2007; Kralj et al. 2006; Pethig 1996; Li and Bashir 2002). The majority of these applications employed AC electric fields generated by closely spaced microelectrode arrays that were generally constructed with MEMS (microelectromechanical systems)-based microfabrication techniques. AC fields promote lower electrode polarization and electrophoretic effects. However, AC DEP faces certain issues that limit its applications, such as electrode fouling and electric field decay above the microelectrodes. An alternative to AC DEP is the insulator-based DEP (iDEP) or DC DEP (Lapizco-Encinas et al. 2004; Kang et al. 2007; Chou et al. 2002), in which no metal microelectrodes are embedded in the chip and DC electric fields are applied from an external electrode pair. This simplifies the fabrication of microdevices by eliminating the metal deposition processes. Insulator structures are robust and chemically inert. Effects such as electrochemical reactions and electrolysis observed in AC DEP are less likely to appear in iDEP. Cummings and Singh (Cummings and Singh 2003) observed two flow regimes in insulator-based DEP: (1) ‘streaming DEP’ where streams of highly concentrated and rarified particles were created between the insulating posts at relatively low electric fields, and (2) ‘trapping DEP’ where particles were trapped around the insulating posts at higher electric fields. The mechanics behind the phenomenon is the competition between electrokinetic (electrophoresis and electroosmosis) and dielectrophoretic forces (Cummings and Singh 2003). DC DEP has found numerous applications, including particle separation (Kang et al. 2007), focusing (Zhu and Xuan 2009), and trapping (Chou et al. 2002). In most of the reports (Lapizco-Encinas et al. 2004; Kang et al. 2007; Chou et al. 2002), trapping of particles using DC-DEP required a relatively high voltages (or electric fields) to induce DEP trapping. In addition, trapping/concentration efficiency and time response were not fully addressed and discussed. A delicate yet simple design of insulator microstructure based on DC-DEP for on-chip sample concentration in a rapid and efficient format is desirable for applications in lab-on-a-chip devices.
This article describes an insulating microstructure (so called concentrator) for rapid, efficient concentration of micro/nanoparticles using DC dielectrophoresis in a continuous fluid flow. The concentrator is designed to focus highly and therefore ‘amplify’ the electric field and field gradient in specific areas. Typically, the distribution of the electric field in the concentrator is as follows: the field upstream is relatively lower and electrokinetic effect is dominant, whereas the field downstream is higher and DEP is dominant. The objective is to focus and therefore strengthen the externally applied electric field at defined areas within the microfluidic device so that a lower external electric field is sufficient to trap the particles. In the current configuration, upstream electrokinetic flow is responsible for continuous transport of particles, while DEP is responsible for trapping of the particles downstream. Upon the application of a voltage, the generated electric field is focused in the direction of fluid flow. Particles are first trapped at field-focused areas located downstream, while EOF continuously transports and delivers the particles from upstream. At the sufficiently high electric field, the device demonstrates a trapping efficiency of 100%, which means downstream DEP traps and concentrates all (100%) the incoming particles from upstream. The concentration is rapid and can be achieved within few minutes. The described setup is capable of rapidly concentrating and collecting nanoparticles from continuous flow that is driven by electroosmosis. It can be implemented in applications where rapid concentration of targets is needed such as concentrating cells for sample preparation and concentrating molecular biomarkers for detection.
2 Materials and methods
2.1 The insulating microstructure
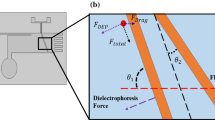
In the case of DC DEP, the nonuniformity of electric field is generally created by embedded obstacles such as a specifically arranged array of insulators in a microchannel. Insulators of delicate design give rise to useful distribution of the electric field as well as the resulting electrostatic forces that are associated with the field, such as DEP, electrophoretic, and electro-osmotic effects. In this study, the proposed microdevice is composed of a special insulating structure constructed in a channel for the purpose of field focusing (Fig. 1). Figure 1a depicts a field-focusing unit constructed by electrically insulating materials such as PDMS, glass, and SU-8. An electric field is generally applied externally from the two electrodes (anode and cathode respectively) located at opposite ends of the channel. Upon the application of the field, a nonuniform distribution is generated along the insulators, as indicated by the field lines. Depending on the patterns of the insulators, the field is relatively concentrated in certain areas. ‘Field-focused’ areas are of higher nonuniformity compared to the “unfocused” areas and therefore are more preferable for DEP trapping. Derived from the design in Fig. 1a, a more effective insulating structure consisting of a series of focusing units (Fig. 1a) is shown in Fig. 1b, which is referred to as a ‘tree system’. The tree system enables multilevel-focusing operations in the direction of flow (from the left to the right) at consecutive regions (see Sect. 3.2). From the entry on the left to the exit on the right, the electric field gradually converges. This results in interesting movement of particles in the fluid flow due to the combined effects of various forces.
2.2 Theoretical background
Suspended particles in the electric field experience a number of significant forces including DEP, electrophoretic, and electroosmotic effects. The superposition of electrophoretic and electroosmotic transport is generally termed electrokinetic (EK) flow. The resulting motion of the particle is determined by the superposed electrokinetic velocity (Ramosy et al. 1998)
where \( \mu_{\text{ek}} \), \( \mu_{\text{eo}} \), and \( \mu_{\text{ep}} \) are the electrokinetic, electroosmotic, and electrophoretic mobility, respectively. E is the electric field. Equation 1 indicates that the EK motion of the particles is linearly proportional to the local electric field. Under electrokinetic flow, particles flow along the electric field lines, and no concentration of particles takes place.
On the other hand, dielectrophoretic velocity, \( u_{\text{dep}} \), which is induced by the dielectrophoretic force in a nonuniform field, is proportional to the gradient of the electric field square (Jones 1995)
where \( \mu_{\text{dep}} \) is the dielectrophoretic mobility.
For insulating spherical particles in a nonuniform electric field, the DEP force is proportional to the term \( \nabla |{\mathbf{E}}|^{2} \) (Jones 1995). Therefore, \( u_{\text{dep}} \) can be rewritten as
where \( \mu^{\prime}_{\text{dep}} \) is proportional to \( \mu_{\text{dep}} \).
Equations 2 and 3 indicate that DEP is a nonlinear effect, and is dependent on the nonuniformity of the electric field. In contrast to electrokinetic flow, dielectrophoretic motion follows the electric field gradient, and the transport can result in focusing, concentration, or trapping of particles in nonuniform fields. Field gradients are readily produced either by embedding-spaced metal electrodes in the case of AC dielectrophoresis or by insulating microstructures within the channel exposed to an external electric field. Depending on the polarizability of the particles and suspending medium, positive DEP (drawing particles to field maximum) or negative DEP (repelling particles from field maximum) takes place (Jones 1995). For polystyrene particles used in this study, the particles experienced negative DEP when they were suspended in deionized water of conductivity 1.0–10 mS/m which was controlled by adding phosphate buffer solution (PBS) into DI water.
The above equations imply that the resulting particle movement depends on the relative strengths of electrokinetic and dielectrophoretic effects. The former tends to produce streamwise movement of particles, while the latter tends to trap and concentrate particles dielectrophoretically. By specially designing the microchannels, the electric field can be extremely nonuniform at certain areas, such as geometry corners and junctions. DEP force becomes dominant around these areas and counteracts the effect from electrokinetic force, giving rise to trapping or concentration of particles. In this study, we proposed a field focusing structure (Fig. 1) in which rapid electric field gradients are created to readily induce DEP trapping. Electrokinetic flow is responsible for particle transport, whereas dielectrophoresis is responsible for local particle trapping.
2.3 Device fabrication and experimental setup
The microfluidic device containing the insulating microstructures (Fig. 1b) was fabricated using conventional microfabrication techniques (Fig. 2). The insulating microstructures formed with polydimethylsiloxane (PDMS) was manufactured by casting from a microfabricated silicon master. In order to fabricate the master, a layer (10 μm thick) of photoresist (AZ9260, Clariant, Somerville, NJ) was spun and patterned on a 4-inch wafer with a photolithography process (Fig. 2a). The patterned photoresist served as a mask for the subsequent process of deep reactive ion etching (DRIE). In order to form the complementary patterns in the master, an ICP (inductively coupled plasma) deep RIE process was performed (Surface Technology Systems plc, Newport, UK). The etching rate was about 3 μm/min with a gas recipe of 115-sccm SF6 + 13-sccm 150 O2 + 100-sccm C4F8 at a cycling of 8 s passivation and 13 s etching. The features of 20–50 μm deep were fabricated (Fig. 2b, c). The master surface was then passivated with gas C4F8 for 5 min. The purpose of passivation was to reduce the adhesion of PDMS to the master surface and, in turn, facilitate the subsequent PDMS peeling-off step. PDMS mixture (Sylgard® 184 Silicone Elastomert, Dow Corning Corporation, Midland, MI) was then poured onto the master, and incubated at room temperature overnight (Fig. 2d). The cured PDMS film was peeled off from the master, and the insulating microstructures were formed on the PDMS film (Fig. 2e). In order to construct an enclosed microfluidic device containing the microstructures and microchannels, the PDMS film was then bonded to a glass slide (Fig. 2f).
Figure 3a depicts a photograph of the PDMS-insulating microstructure fabricated with the process described above. Microchannels formed with PDMS house the electroosmotic flow from the left to the right. The microchannels were 40 μm wide and 20–50 μm deep. In the flow direction, the microchannels merge into subsequent ones, terminating at a single outlet downstream. In the proposed structure, four concentration regions (indicated as ‘Region I’–‘Region IV’) are formed within the microchannels. Depending on the applications, more or less regions can be produced by redesigning the structure. These regions have been designed to concentrate effectively particles at various conditions by focusing the electric field (see Sect. 3). Figure 3b shows a schematic diagram of the completed microfluidic chip containing the microfabricated insulators, and the inlet/outlet reservoirs. In order to generate the electric field, a DC voltage was applied to the two Pt electrodes that were vertically placed in the inlet and outlet reservoirs, respectively. The distance between the electrodes was 25 mm. In experiments, the solution containing the particles was introduced into the inlet reservoir with a pipette. The liquid then automatically filled the microchannels due to the capillary action. The motion of particles was imaged using an epifluorescent microscope (Nikon TE2000-S, Nikon Corporation, Tokyo, Japan).
3 Results and discussion
3.1 Simulation
In order to investigate the electric field distribution and the field-focusing effect of the concentrator, the electric field was simulated with a finite element software (Femlab 3.2, COMSOL Inc., Burlington, MA, USA). In the simulation, a model was built for the setup of Fig. 3b, and a DC voltage was applied to the electrode at the reservoirs of the main channel (Fig. 3b). The conductivity of the solution was set to 1.0 mS/m. The electric field is governed by the Laplace equation
where \( \phi \) is the electric potential. The above equation was subjected to the boundary condition \( {\mathbf{E}} \cdot {\mathbf{n}} = 0 \) as the channel walls were electrically nonconducting where n denotes the unit vector normal to the channel wall. The electric field is determined by \( {\mathbf{E}} = - \nabla \phi \).
Figure 4a depicts the magnitude of the simulated electric field (|E|) distribution in the microchannels, when a voltage of 1000 V was applied to the electrodes. Depending on the distribution of the field, the microstructure is divided into five regions as indicated with ‘IV’, ‘III’, ‘II’, ‘I’, and ‘0’ from the left to the right in the figure. From Regions IV–0, the electric field strength increases gradually. The insulating concentrator was designed in this way to focus the electric field in the direction of the fluid flow (from the left to the right). Figure 4b depicts a plot of the magnitude of \( \nabla |E|^{2} \) in Region I and Trap 1. According to Eq. 3, the DEP velocity is proportional to \( \nabla |E|^{2} \). We can see that the maximum DEP effects occur around the geometry corners in Trap 1. Negative DEP repels the particles from these corners, counteracts electrokinetic effects, and results in trapping and concentration of particles in the trap.
a A plot of electric field (E) distribution in the concentrator. In the simulation, a voltage of 1000 V was applied to the electrodes. The microchannels are divided into five regions: Regions 0, I, II, III, and IV. Four traps are also indicated in the plot. b The magnitude of \( \nabla |E|^{2} \) in Region I and Trap 1. The maximum values are around the corners of the channel geometry in Trap 1
In order to further study how quantities such as electric field and DEP force vary in the flow direction, plotting paths A–B and C–D were defined, respectively, along the centers of the microchannels and close to (2 μm away from) the channel walls, as indicated with dashed lines A–B and C–D in Fig. 4. The paths were chosen because these were one of the actual moving paths for the particles and fluids. The value of the quantities along the path was calculated and plotted as a function of the distance from the start position ‘A’ (for path A–B) and ‘C’ (for path C–D) along the path. Figure 5a, b depict plots of the magnitude of the electric field along the path A–B and C–D, respectively. The plot shows a number of ‘plateaus’, which correspond to the values in various regions. As expected, the values of these plateaus increases stepwise from the left to the right (from Region IV to Region 0), until the maximum at the outlet. The features of the plateaus give rise to the stepwise concentration of particles in the device as described in Sect. 3.2. The rapid changes between plateaus in Fig. 5b are from the vicinity of the corners of the microchennels, where the electric field is highly nonuniform. It should be noted that the electrokinetic effect also increases stepwise along the path since it is proportional to the electric field (see Eq. 1). In addition, the above system presents a high throughput which is crucial for lab-on-a-chip devices. Because of the field-focusing operations in the current design, the electric field within the microchannels increases stepwise from upstream to downstream (from the left to the right), and so does the EOF driven flowrate. The flowrate reaches its maximum at the final exit channel. Despite the single exit channel, the overall throughput of the system is high since the flowrate increases in the field-focusing direction.
The magnitude of electric field plotted along the path A–B (a) and path C–D (b) in the channel (indicated in Fig. 4). The ‘position’ refers to the relative distance to the start point ‘A’ or ‘C’ along the path. The field strength increases stepwise from Regions IV to 0. c, d The magnitude of \( \nabla |E|^{2} \) along the paths A–B and C–D, respectively. The peaks correspond to the traps and outlet in the concentrator. The magnitude of \( \nabla |E|^{2} \) is higher along path C–D (close to channel walls) compared to that along path A–B (at channel center). Path C–D is 2 μm away from the channel walls. In the simulation, a voltage of 1000 V was applied to the electrodes
Figure 5c, d depicts plots of the magnitude of \( \nabla |E|^{2} \) along the paths A–B and C–D, respectively. The peaks correspond, respectively, to the values of the traps and outlet as indicated in Fig. 4. Similarly, the values of these peaks increased rapidly from ‘Trap 4’ to ‘Trap 1’. The magnitude of \( \nabla |E|^{2} \) is higher along path C–D (close to channel walls) compared to that along path A–B (at channel center), demonstrating a better trapping capacity in the vicinity of corners. As discussed in Sect. 2.2, the electrokinetic effect is linearly proportional to the electric field while the DEP force is nonlinearly proportional to the field. At high electric fields, DEP will become dominant over the electrokinetic effect and trapping of particles occurs. Figure 5 implies that particles will be first trapped in ‘Trap 1’, and then in ‘Trap 2’ and ‘Trap 3’, and finally in ‘Trap 4’, as the field increases. However, there will be no trapping at the outlet since the flow is from the left to the right. It should be noted that the magnitude of \( \nabla |E|^{2} \) is nonzero (Fig. 5c, d) at those ‘plateaus’ along the paths where the electric field is nearly constant (Fig. 5a, b). The plateaus indicate that the electric field is uniform in each region in the channel direction (e.g., in the X direction in Fig. 4a). However, the field is nonuniform across the channel (e.g., in the Y direction in Fig. 4a). Therefore, the corresponding gradient of the electric field \( \nabla |E|^{2} \) is nonzero while the electric field |E| is constant in the X direction, as shown in Fig. 5c, d.
3.2 Experiments
The performance of the microfluidic concentrator was tested with green fluorescent polystyrene spheres of 930 nm in diameter (Duke Scientific Co., CA, USA). The microspheres emit green light (508 nm) when they are excited by blue light (468 nm). Before use, the particles were resuspended in deionized water and the conductivity of the medium was adjusted by adding phosphate buffer solution (PBS, Fisher Scientific, NJ). The conductivity was measured using a conductivity meter with graphite sensor electrodes (Dist3WP, Hanna Instruments Inc., RI). Solutions of 1.0–10.0 mS/m were used in the experiments. The concentration of particles was approximately about 2 × 109 particles/ml. In order to begin, suspension of fluorescent microspheres was injected into the inlet reservoir of the microfluidic chip with a pipette. A DC voltage was then applied to the platinum wire electrodes placed in the two reservoirs. In order to investigate the effect of electric field strength on the particle motion, the voltage applied on the two electrodes was increased incrementally from 50 to 1000 V with a high-voltage power supply (PS350, Stanford Research) and the particle movement was observed and recorded. The corresponding mean electric field was ~20 to 400 V/cm.
Figures 6 and 7 show the observed particle behavior within the microchannels of the insulating structure for increasing electric fields. Dark areas are the solid insulators and bright areas are the microchannels filled with fluid. At a low voltage of 100 V, streaming movement of particles was observed (Fig. 6). Streamlines of particles driven by the EOF were observed within the microchannels. Particles entered from the left of the channels, traversed the whole structure, and then exited at the right outlet. No obvious concentration of particles was observed under this condition. In this low electric field regime, drag forces on the particles from electrokinetic flow dominated the holding effects of DEP, and thus resulted in the streaming movement along the microchannels.
Particles moved streamwise within the microchannels under low electric fields applied (<40 V/cm). In this low field domain, the electrokinetic effects dominated over DEP and resulted into streaming movements of particles. Dark areas are the PDMS structures, and bright (green) areas are the microchannels filled with fluid flow. The flow direction is from the left to the right
Concentration of green fluorescent submicron polystyrene particles in the insulting microstructures. a–d The concentration at different regions as the applied voltage increased. a Particles were mainly trapped in Region I when the applied voltage was 300 V; b all particles were trapped and no particles exited the outlet when applied voltage was increased to 400 V; c particles were trapped in Region II when the applied voltage was increased 500 V; d particles were trapped in Regions III and IV when the voltage was increased to 600 V and higher. Dark areas are the PDMS structures, bright (green) areas are the microchannels formed by the PDMS. The flow direction is from the left to the right
As the applied voltage increased to ~300 V, trapping of particles began to occur downstream (Trap 1 and Region I) in the concentrator (Fig. 7a). Due to the focused electric field in this region, the DEP force became high enough to overcome the electrokinetic effects, resulting in the trapping of particles in this region. Because of the trapping, fewer particles exited the outlet. On the other hand, particles still moved streamwise in the upper regions (the left part of the concentrator). The co-existence of both streaming movement and trapping DEP highly improved the concentration efficiency: the streaming movement was responsible for continuously transporting the particles from upstream, while DEP was responsible for trapping the delivered particles downstream. As the voltage further increased to 400 V, the loss of particles at the outlet disappeared, meaning that the concentrator trapped all the incoming particles delivered by the flow (Fig. 7b). The device demonstrated a 100% trapping efficiency under this condition. In addition, the particles were highly condensed as indicated by the intensity of the fluorescence. Similarly, trapping DEP began to occur in Trap 2 (Region II) as the applied voltage increased to 500 V (Fig. 7c) and occurred in Trap 3/Region III and Trap 4/Region IV, respectively, as the applied voltage increased to 600 V or higher (Fig. 7d). At this point, the concentrator trapped all the particles entered from the inlet and packed them into a dense format within the microchannels. In order to collect the concentrated particles, the residual solution in the reservoirs was first discarded. The applied voltage was then turned off, and a pressure source (generated with a syringe) was applied from the inlet to propel the particles to the outlet reservoir. The solution collected from the concentrator was observed to contain particles in high concentration.
We observed that the early concentrated particles had an effect to enhance the trapping of incoming particles from upstream. A lower applied voltage was needed for successful trapping once obvious trapping established downstream such as in the Region I. This was due to the fact that the trapped particles affected the distribution of the electric field in the vicinity. The electric field close to the trapped particles and the channel walls was highly nonuniform, and, therefore, DEP effect was more significant at these areas. We also investigated the effects of the trapped particles on the flow rate. As time passed, more and more particles were trapped and concentrated. Trapped particles first filled up the microchannels at Region I, and then started to fill up the microchannels at the next region (Fig. 7). These particles ‘packed’ in the downstream channels would reduce the overall flow rate of the system. When Region I was filled with trapped particles, observation showed that the flow rate at Region IV was ~80% of that when Region I was unfilled. The above observation was based on the comparison of the videos taken at the time = 0 s when particles started to be trapped at Region I and the time = 180 s when particles filled up Region I. However, there was no risk that the concentrated particles would block the channel, due to the fact that particles were more concentrated and focused in the central areas of the microchannels (around the path A–B in Fig. 4). For applications in biological samples, the concentration of the sample is usually much lower compared to that of the nanoparticles (~2×109 particles/ml) used in the above tests. Therefore, the effect of the trapped particles on the overall flow rate of the system will be much less significant.
In order to quantitatively study the trapping efficiency of the device, the fluorescence intensity of the trapped particles was measured. Two sets of measurements were carried out. In the first set of experiments, fluorescent nanoparticles were first concentrated in the device, and then the relative fluorescence intensity along the centers of the microchannes (path A–B in Fig. 4) was studied. This measurement was to study the trapping capacity in different regions and traps. The second test was to study the time response of the device, by measuring the fluorescence intensity of traps as a function of time when the device was energized. In the above measurement, fluorescent nanoparticle suspension of ~107 particles/ml was used. In order to measure the relative fluorescence intensity, images were first taken with a fluorescence microscope, and then the images were analyzed with a software (ImageJ 1.41, National Institutes of Health, USA).
Figure 8a depicts the measured relative fluorescence intensity (RFU) plotted along the path A–B in the channel (indicated in Fig. 4). In the experiment, a voltage of 400 V was applied for about 1 min, and then an image was taken and measured. The intensity accounts for the number of fluorescent nanoparticles trapped locally. The two visible peaks corresponded to the values in Trap 1 and Trap 2, respectively. The area of the peak indicated the concentration and number of the trapped particles. It can be seen that Trap 1 concentrated the majority of the particles, and Trap 2 concentrated a very limited amount of particles, while there were no obvious trapping in Traps 3 and 4 under this condition. This is consistent with the observation in Fig. 7.
Measurement of fluorescence intensity. a Relative fluorescence intensity (RFU) plotted along the path A–B in the channel (indicated in Fig. 4). The intensity reflects the number of fluorescent nanoparticles trapped locally. b Relative fluorescence intensity in each trap plotted as a function of flowing time. The average intensity in the trap (defined by the circles in Fig. 4) was used. A series of images were taken every 2-s, and the fluorescence intensity was analyzed with a software
Figure 8b depicts the relative fluorescence intensity in each trap plotted as a function of flowing time (voltage applying time). The average intensity in the trap (areas defined by the circles in Fig. 4) was used. In the experiment, a voltage of 400 V was applied to the electrodes and a series of images were taken every 2-s, and the relative fluorescence intensity in each trap was analyzed. The intensity in Trap 1 rapidly increased versus time as more and more particles were concentrated in that area. It reached its maximum in about 2 min, indicating that Trap 1 was saturated with nanoparticles after the voltage was applied for ~2 min and rapid concentration was achieved. The fluorescence intensity in Trap 2 increased relatively slowly since much less particles were concentrated there. The fluorescence intensity in Trap 3 (and Trap 4, not shown) was nearly constant at the background valve, indicating that no particles were concentrated.
The threshold voltage V th, at which particle trapping starts to take place for each trap was measured. Figure 9 depicts the measured threshold voltage for each trap region. In general, the threshold voltage increased exponentially from Trap 1–4. In summary, electrokinetic effect was dominant and particles moved streamwise when the applied voltage was smaller than V 1 (V < V 1); particles trapped in Region I when the applied voltage was greater than V 1 but smaller than V 2 (V 1 < V < V 2); particles trapped in both Regions I and II when the applied voltage was greater than V 2 but smaller than V 3 (V 2 < V < V 3); particles trapped in Regions I–III when the applied voltage was greater than V 3 but smaller than V 4 (V 3 < V < V 4); and particles trapped in Regions I–IV when the applied voltage was greater than V 4 (V > V 4). In practice, a working voltage can be selected in a proper range for particle trapping in defined traps and regions.
4 Conclusions and outlook
A microfluidic device for rapid and efficient trapping of micro/nanoparticles based on DC dielectrophoresis has been presented. The concentrator was composed of a series of microchannels formed by PDMS-insulating microstructures. The applied fields were focused stepwise within the microchannels. Particles moved streamwise at low electric fields, and were trapped at higher electric fields. As the electric field increased, concentration of nanoparticles began to occur at different regions. Both streaming movement and trapping DEP simultaneously occurred. The concentration was rapid and efficient as the streaming movement delivered particles from upstream and DEP trapped the delivered particles downstream. Because of the field-focusing feature of the device, it required lower voltage applied for efficient trapping and concentration. The trapping efficiency was high and rapid concentration was achievable. At the sufficiently high voltage (V > 400 V), the device trapped all the incoming particles. The proposed concentrator design can be reconfigured into a format with more or less trapping regions, depending on the requirements of applications. Since the regions and traps have different threshold voltages, the microdevice can also be used for separation of particles based on the trapping at multiple locations. This study is ongoing and more results will be reported in the future. Furthermore, the microfluidic concentrator can be implemented in applications where concentration of targets are needed, such as the concentration of cells for sample preparation, and the concentration of molecular biomarkers for biological assays.
References
Chen DF, Du H (2007) A dielectrophoretic barrier-based microsystem for separation of microparticles. Microfluid Nanofluid 3:603–610
Chen DF, Du H, Li WH (2006) A 3D paired microelectrode array for accumulation and separation of microparticles. J Micromech Microeng 16:1162–1169
Chou C-F, Tegenfeldt JO, Bakajin O, Chan SS, Cox EC, Darnton N, Duke T, Austin RH (2002) Electrodeless dielectrophoresis of single- and double-stranded DNA. Biophys J 83:2170–2179
Cummings EB, Singh AK (2003) Dielectrophoresis in microchips containing arrays of insulating posts: theoretical and experimental results. Anal Chem 75:4724–4731
Durr M, Kentsch J, Muller T, Schnelle T, Stelzle M (2003) Microdevices for manipulation and accumulation of micro- and nanoparticles by dielectrophoresis. Eletrophoresis 24:722–731
Greenberg AE, Clesceri LS, Eaton AD (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, American Water Works Association, and Water Environment Federation
Hames BD, Higgins SJ (1995) Gene probes: a practical approach. Oxford Oxford University Press
Jones TB (1995) Electromechanics of particles. Cambridge University Press, Cambridge
Kang Y, Li D, Kalams SA, Eid JE (2007) DC-dielectrophoretic separation of biological cells by size. Biomed Microdevices 10:243–249
Kralj JG, Lis MTW, Schmidt MA, Jensen KF (2006) Continuous dielectrophoretic size-based particle sorting. Anal Chem 7:5019–5025
Lapizco-Encinas BH, Simmons BA, Cummings EB, Fintschenko Y (2004) Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis 25:1695–1704
Lee SJ, Lee SY (2004) Micro total analysis system (μ-TAS) in biotechnology. Appl Microbiol Biotechnol 64:289–299
Li HB, Bashir R (2002) Dielectrophoretic separation and manipulation of live and heat-treated cells of Listeria on microfabricated devices with interdigitated electrodes. Sens Actuators B 86:215–221
Nilsson A, Petersson F, Jonsson H, Laurell T (2004) Acoustic control of suspended particles in micro fluidic chips. Lab Chip 4:131
Pethig R (1996) Dielectrophoresis: using inhomogeneous AC electrical fields to separate and manipulate cells. Crit Rev Biotechnol 16:331–348
Pohl HA (1978) Dielectrophoresis. Cambridge University Press, Cambridge
Ramosy HMA, Greenz NG, Castellanosy A (1998) Ac electrokinetics: a review of forces in microelectrode structures. J Phys D: Appl Phys 31:2338–2353
Reyes DR, Lossifidis D, Auroux P-A, Manz A (2002) Micro total analysis systems. 1. Introduction, theory, and technology. Anal Chem 74:2623–2636
Sheetz MP (1998) Laser tweezers in cell biology. Academic Press, New York
Visor GC, Schulman SG (2006) Fluorescence immunoassay. J Pharm Sci 70:469–475
Yamadaa M, Seki M (2005) Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 5:1233–1239
Zhu J, Xuan X (2009) Dielectrophoretic focusing of particles in a microchannel constriction using DC-biased AC flectric fields. Electrophoresis 30:2668–2675
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, D., Du, H. A microfluidic device for rapid concentration of particles in continuous flow by DC dielectrophoresis. Microfluid Nanofluid 9, 281–291 (2010). https://doi.org/10.1007/s10404-009-0545-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-009-0545-z