Abstract
In this paper, we report a microfluidic chip containing a cross-junction channel for the manipulation of UV-photopolymerized microparticles. Hydrodynamic-focusing is used to form a series of using 365 nm UV light to solidify the hydrogel droplets. We were able to control the size of the hydrogel droplets from 75 to 300 μm in diameter by altering the sample and by changing the flow rate ratio of the mineral oil in the center inlet channel to that of the side inlet channels. We found that the size of the emulsions increases with an increase in average velocity of the dispersed phase flow (polymer solution flow). The size of the emulsions decreases with an average velocity increase of the continuous phase flow (mineral oil flow). Experimental data show that the emulsions are very uniform. The developed microfluidic chip has the advantages of ease of fabrication, low cost, and high throughput. The emulsions generated are very uniform and have good regularity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The area of microfluidics and its applications is inherently interdisciplinary and far-reaching. The applications of microfluidics in drug research and biotech industries are a fairly new research field (León and Galván 1995). A ‘lab-on-a-chip’ integrates the necessary devices on a single small chip to perform complicated biological and chemical processes. Many studies have integrated microfluidics and the ‘lab-on-a-chip’ concept to develop a microfluidic chip (Bernhard et al. 2003). These microfluidic chips can move/deliver/mix fluids in a controlled channel (David and Dongqing 2004). In the micro-fluid system, a micro-volume of fluid is very precisely manipulated by micro-fluid controlled technology. These advantages are used in many fields (e.g., analytical chemistry, microelectronics, and biomedical diagnosis).
The core technology behind the design of a biochip is microfluidics. The design of these devices can be classified as continuous flow and non-continuous flow. The continuous flow design depends on pumps, gates, and fluid for droplet manipulation. The non-continuous flow design depends on independent droplet manipulation by various modalities. The behavior of the liquid has been applied successfully in microfluidic diffusion-based separation and other analytical purposes, including the fabrication of various microstructures, and patterning inside microchannels (Weigl and Yager 1999; Gao et al. 2001; Kenis et al. 1999; Zhao et al. 2001).
Applications using the flow behavior of immiscible liquids at the microscale level have been reported. For example, Song et al. (2003) presented a microfluidic system that forms aqueous droplets in a continuous flow of a water-immiscible fluid. The droplets act as microreactors, which rapidly mix reagents and transport them with no dispersion. Zhao et al. (2002) proposed a strategy to control the flow of immiscible liquids in microchannels by patterning surface free energies, which were applied in the fabrication of a semipermeable membrane. Hisamoto et al. (2001) used an immiscible system to develop a new method of performing multi-ion sensing. The proposed immiscible system maintains stable multilayer interfaces for a long distance and completes the ion pair extraction reaction inside the microchannel.
In a previous study, Jeong et al. (2005) proposed a strategy to generate the emulsion with a later exposure to 365 nm UV light. Their chip generated microparticles with a size ranging from 90 to 260 μm. However, microfluidic methods have provided a facile approach for the synthesis and fabrication of monodisperse polymer capsules in the micrometer size range (Zhao et al. 2002; Hisamoto et al. 2001; Jeong et al. 2005; Burns et al. 1985). For example, Lin and colleagues (Huang et al. 2006, 2007) and Zhao et al. (2002) proposed a method for particle generation by used cross-junction microchannels. In this study, we develop a new method of fabricating microfluidic chips that resist acid and could generate droplets. The method we developed is based on (1) a new way to fabricate a PDMS cross-junction microfluidic chip, (2) using hydrodynamic-focusing to form a series of emulsions in a microfluidic chip. (3) These emulsions are transported to a UV exposure area (365 nm, 1.2 mW/cm2; Novacure, Photonic Solutions Inc.) by a Teflon tube, (4) and the hydrogel droplets are then polymerized by continuous UV exposure for 10 s in the Teflon tube. (5) Finally, the polymer microparticles are formed. The developed chip can generate uniform microparticles in the range of 75–300 μm. The generation processes of the microparticles are shown in Fig. 1. The aim of this study was to investigate and compare the size of the emulsions obtained by creating a different flow rate ratio in the side inlet channels then that in the center inlet channel. The developed microfluidic chip has the advantages of ease of fabrication, low cost, high throughput, good uniformity, and simplicity. Applications of our method in the clinical field are expected in the near future.
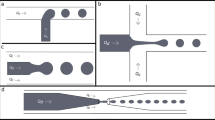
Illustration of the emulsion processes. The microchannel is carried out into the PMMA substrate with a laser micromachining process using a CO2 laser machine. A microchannel chip of PMMA is used as the convex mold, into which the PDMS is injected. The mold is then turned over to fabricate a concave mold of PDMS. The water-in-oil (w/o) emulsions generated in the cross-junction microchannel. The hydrogel droplets are polymerized in situ as a result of exposure to 365 nm UV light
2 Materials and methods
2.1 Materials
The polymerizable sample solution (disperse phase flow, viscosity 9 mPa·s) was combined with 4-hydroxybutyl acrylate (TOKYO KASDI KOGYO), acrylic acid (ACROS ORGANIC), ethyleneglycol dimethacrylate (SHOWA CHEMICAL CO, LTD), and 2,2-dimethoxy-2-phenyl-acetophenone (ACROS ORGANIC). The immiscible solution consisted of mineral oil (viscosity 25 mPa·s; MP Biomedicals, LLC). The microfluidic chip material was polydimethylsiloxane (PDMS).
2.2 Fabrication of the microfluidic chip
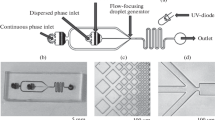
The developed microfluidic chip consists of three inlet ports, a cross-channel, and an observation region. The microfluidic chip was designed in AutoCAD®. It was carried into the PMMA substrate (length/width/depth: 27.0 cm/21.0 cm/1.5 mm) using a laser micromachining process with a CO2 laser machine (LaserPro Venus, GCC, Taiwan). A microchannel chip of PMMA was used as the convex mold, into which the PDMS was then injected. After 40 min at 70°C, the mold was turned over to fabricate a concave mold of PDMS, which was the upper microfluidic chip. Then we injected a thin PDMS layer between the upper and bottom chip to combine them by thermal bonding at 100°C. Finally, we finished the PDMS microfluidic chip. The processes used for fabricating the microfluidic chip are shown in Fig. 2. Using the thermal bonding method, we combined the convex PMMA mold and used it to fabricate a PDMS chip. In this chip, the width of the microchannel is 800 μm and the depth of the microchannel is 1.5 mm.
2.3 Experimental procedure
Figure 3a shows an overview of the experimental setup. The sample flow and the oil flows were injected into the PDMS chip using two syringe pumps (kdScientific KDS 230) controlled by a computer program. In this experiment, the sample flow was hydrodynamically focused between two oil streams, constructing the water-in-oil (w/o) emulsions along the microchannel. The hydrogel droplets in the center channel were then exposed to 365 nm UV light and became the UV-photopolymerization microparticles, as shown in Fig. 3b. The flow rates of the sample channel and the mineral oil channels were adjusted to control the degree of the shear forces and the size of the emulsions.
2.4 Emulsions size measurement
We measured the diameter of the emulsions at the cross-junction channel using an optical microscope (BX60; Olympus, Japan) and then photographed the PDMS channel using a digital camera (DP70; Olympus, Japan).
3 Results and discussion
In this experiment, continuously generated emulsions that solidified in 365 nm UV light at the cross-junction channel were successfully obtained. To generate the water-in-oil emulsions, polymerizable sample solution and mineral oil were employed as the polymer phase fluid and the oil phase fluid, respectively. A polymer-soluble dye (blue ink) was dissolved in the sample solution to facilitate real-time observation, allowing us to observe the polymeric microparticles clearly through the optical microscope.
3.1 Simulation of a cross-flow microfluidic chip
We used CFDRC-ACE single phase flow for simulating the velocity distribution in the microfluidic chip. We drew the same shape as that used in the experimental chip to simulate a 800 μm width. We also set the water inlet (3.89 × 10−3 m/s) and oil inlet (5.57 × 10−2 m/s) in the channel. The constant input and output flow rate conditions at which the maximum fluid velocity occurred at the cross-junction, as shown in Fig. 4. In the simulation results, the sheath force was generated in the cross position, producing emulsions in the chip.
3.2 Formation of polymer emulsions
In a typical procedure, the polymeric solution flowed through the center inlet channel and the oil solution flowed through the side inlet channel, as shown in Fig. 5a. In the observation region, we could see the hydrogel emulsions, which solidified after exposure to 365 nm UV light. We found the oil phase fluid could create a sheath force on the sample phase fluid to form continuous emulsions in the microchannel (the maximum flow velocity position was the same as the result of the simulation), as shown in Fig. 5b, c. The flow rates of the sample phase and oil phase fluids were set to 0.07 and 2.4 mL/min, respectively. The sample phase fluid (blue color) became compressed by a shear force, appearing as an arrow shape and then generating a continuous emulsion of about 200 μm in diameter. The emulsion continuously generated in the cross-junction microchannel is controllable and reproducible using the microfluidic technique. We can manipulate the size of the emulsion by adjusting the sample flow rate and mineral oil flow rate. The hydrogel droplets in the center channel are exposed to 365 nm UV light, becoming UV-photopolymerization microparticles, as shown in Fig. 6.
a Image of the microfluidic chip. b Image of the shearing region. Oil flows from the upper and the lower channels pinch off the sample flow to form micro-droplets. (The arrow indicates the direction of the emulsion generation, scale bar 200 μm.) c The emulsions generated in the microchannel at a mineral oil rate of 2.4 mL/min and a polymer rate of 0.07 mL/min (scale bar 200 μm)
3.3 Influence of the flow rate
The relationship between the size of the emulsion and the speed of the flows is important. In our experiment, we found that the size of the emulsion was easily controlled by changing the flow conditions in the microchannels. The results show a uniform emulsion with a narrow classification of size in the microchannel, demonstrating that a stable correct control can be obtained by using the microfluidic system to generate microparticles. Figure 7a–d show that when the rate of mineral oil flow increases, it can result in smaller polymeric emulsion. The smallest size of polymeric emulsion is 75 μm in a sample flow of 0.07 mL/min and a mineral oil flow rate of 3 mL/min. Figure 8a shows the relationships between the dispersed phase flow rate (polymer solution flow rate) and emulsion size (diameter) under a continuous phase (mineral oil flow rate) at a fixed flow rate. Figure 8b shows the relationship between the continuous phase flow rate (mineral oil flow rate) and emulsion size (diameter) under a dispersed phase at a fixed flow rate. For a given 2.4 mL/min of the continuous phase rate, the emulsion size increases as the average velocity of the dispersed phase increases. When the mineral oil flow rate is set to 1.0 mL/min, the lower dispersed phase flow rate generates smaller sized emulsions, as shown in Fig. 8a. For a given 0.07 mL/min of dispersed phase flow rate, the emulsion size decreases as the average velocity of the continuous phase increases. The same tendency can be observed for 0.07 mL/min dispersed phase flow, as shown in Fig. 8b. Consequently, as the continuous phase flow rate increases, the shear force increases comparatively and the size of the emulsions decrease.
a The relationship between emulsion size and water flow rate under a fixed mineral oil flow rate. The emulsion size increases as the sample flow rate increases. b The relationships between emulsion size and mineral oil flow rate under a fixed sample flow rate. The emulsion size decreases as the mineral oil flow rate increases
If we increase the flow speeds of both the dispersed phase and the continuous phase (the ratio being constant) at the higher pressures in the microchannel, energy efficiency becomes a serious problem. In addition, the emulsion will not be generated when the flow speed ratio of the polymer/mineral oil is above 1:1 or below 1:40. Finally, the polymeric microparticles become UV-photopolymerized microparticles at 365 nm UV light of 1.2 mW/cm2.
4 Conclusion
We demonstrated polymeric microparticles generated by the immiscible property of sample and mineral oil solutions in microchannels and solidified them by 365 nm UV light. The diameter of the microparticles ranged from 75 to 300 μm with a narrow size distribution (<10%). We can control the uniformity of the size of the UV-photopolymerized microparticles by fluid pressure, with no need for a follow-up process to adjust the size. The microfluidic chip we developed is capable of generating relatively uniform microparticles. In addition, the microparticle size can be controlled, producing the chip is a simple and low cost process, and it has a high throughput.
References
Bernhard HW, Ron LB, Catherine RC (2003) Lab-on-a-chip for drug development. Adv Drug Deliv Rev 55:349–477
Burns MA, Kvesitadze GI, Graves DJ (1985) Dried calcium alginate/magnetite spheres: a new support for chromatographic separation and enzyme immobilization. Biotechnol Bioeng 27:137–145
David E, Dongqing L (2004) Integrated microfluidic devices. Anal Chim Acta 507:11–26
Gao J, Xu J, Locascio LE, Lee CS (2001) Integrated microfluidic system enabling protein digestion, peptide separation, and protein identification. Anal Chem 73:2648–2655
Hisamoto H, Horiuchi T, Uchiyama K, Tokeshi M, Hibara A, Kitamori T (2001) On-chip integration of sequential ion sensing system based on intermittent reagent pumping and formation of two-layer flow. Anal Chem 73:5551–5556
Huang KS, Lai TH, Lin YC (2006) Manipulating the generation of Ca-alginate microspheres using microfluidic channels as a carrier of gold nanoparticles. Lab Chip 7:954–957
Huang KS, Lai TH, Lin YC (2007) Using a microfluidic chip and internal gelation reaction for monodisperse calcium alginate microparticles generation. Front Biosci 12:3061–3067
Jeong WJ, Kim JY, Choo J, Lee EK, Han CS, Beebe DJ, Seong GH, Lee SH (2005) Continuous fabrication of biocatalyst immobilized microparticles using photopolymerization and immiscible liquids in microfluidic systems. Langmuir 21:3738–3741
Kenis PJA, Ismagilov RF, Whitesides GM (1999) Microfabrication inside capillaries using multiphase laminar flow patterning. Science 285:83–85
León R, Galván F (1995) Glycerol photoproduction by free and Ca-alginate entrapped cells of Chlamydomonas reinhardtii. J Biotechnol 42:61–67
Song H, Tice JD, Ismagilov RF (2003) A microfluidic system for controlling reaction networks in time. Angew Chem Int Ed Engl 42:768–772
Weigl BH, Yager P (1999) Microfluidic diffusion-based separation and detection. Science 283:346–347
Zhao B, Moore JS, Beebe DJ (2001) Surface-directed liquid flow inside microchannels. Science 291:1023–1026
Zhao B, Viernes NOL, Moore JS, Beebe DJ (2002) Control and applications of immiscible liquids in microchannels. J Am Chem Soc 124:5284–5285
Acknowledgments
The authors would like to thank the Center for Micro/Nano Technology Research, National Cheng Kung University, Tainan, Taiwan, for access to their equipment and for their technical support. Funding from the Ministry of Education and the National Science Council of Taiwan, R.O.C. under contract no. (NSC 95-2221-E-006-006-MY3) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeh, CH., Lin, YC. Using a cross-flow microfluidic chip for monodisperse UV-photopolymerized microparticles. Microfluid Nanofluid 6, 277–283 (2009). https://doi.org/10.1007/s10404-008-0333-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-008-0333-1