Abstract
Hollow poly (vinylpyrrolidone) (PVP) + TiO2 and polypyrrole (core)/PVP (sheath) nanofibers were successfully electrospun using hydrodynamic fluid focusing. Utilizing a two-dimensional fluid focusing technique previously applied to aqueous solutions, intersecting microchannels cast in (poly)dimethylsiloxane were utilized to dynamically center core fluids in immiscible sheath fluids prior to electrospinning at the channel outlet. Advantages of using microfluidic channel networks for the electrospinning of composite nanofibers include spatiotemporal control over input reagents, ease of fabrication and the ability to focus the core stream into sheath layer without the need of complex co-annular nozzles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The motivation to develop new and potentially more robust manufacture techniques for hollow and core/sheath nanofibers is driven by the increasing number of functional “nano” materials being developed, extending from medical applications such as controlled-release drug delivery (Wei et al. 2002; Zhang et al. 2006) and tissue culture (Zhao et al. 2007) to novel textiles for protective clothing (Han et al 2006). Most of the reported fabrication methods reported to date employ self-assembly (Wan et al. 2003), template synthesis (Bognitzki et al. 2000; Hou et al. 2002; Steinhart et al. 2002, 2004) or co-axial electrospinning (Li et al. 2004, 2005; Loscertales et al. 2004; McCann et al. 2005; Song et al. 2005; Sun et al. 2003; Yu et al. 2004; Zhang et al. 2006). Of these, co-axial electrospinning using concentrically aligned spinnerettes consisting of tubes or syringes has attracted the greatest attention in past decade, as it provides a straightforward method of generating long (cm scale) nanoscale core/sheath or hollow fibers. This approach, though repeatable, requires a significant amount of time and materials to fabricate each manifold and properly align the nozzles (Bazilevsky et al. 2007). Moreover, clogging of the spinnerettes requires frequent cleaning or replacement. In an effort to simplify the co-axial process, Bazilevsky et al. (2007) recently reported on co-electrospinning from a single nozzle using emulsion, producing long, but radially inhomogeneous core-shell fibers on the order of 0.5–5 μm. In this manuscript, we present an alternative approach to co-electrospinning using hydrodynamic fluid focusing within a poly(dimethylsiloxane) (PDMS) microfluidic manifold. Eliminating the need for concentric spinnerettes, a co-axial stream of immiscible core and sheath solutions is generated in a square microchannel that persists until it is electrospun at the channel outlet. The microfluidic devices, which are fabricated using standard soft lithography techniques (Xia and Whitesides 1998), offer several advantages as electrospinning manifolds, including flexible design constraints and tight control over microchannel dimensions. While clogging has been observed in the flow-focusing devices, the ability to rapidly prototype the devices makes them easy to replace.
The microfluidic 2D-flow focusing devices were molded in PDMS elastomer (Fig. 1) using a modification of a microchannel architecture previously applied to the focusing of miscible aqueous solutions (Simonnet and Groisman 2005). The master mold for the device, consisting of dual height SU-8 microchannels patterned on a silicon wafer, was fabricated using standard lithographic protocols. Two masks were required for the total process, and were scaled up by 1.7% to compensate for bulk PDMS shrinkage. Both masks were printed in high resolution on transparency stock, with the second transferred to a chrome plate for easier alignment. After initial solvent cleaning and evaporation, 3 in. diameter silicon wafers were coated with SU-8 2015 photoresist (MicroChem,Newton, MA, USA) spin-coated at 2,000 rpm, 30 s, to a film thickness of 21 μm. Evaporation of the photoresist solvent was achieved using the manufacturer-prescribed “soft bake” of 1 min at 65°C, followed by 2 min at 95°C. This layer was then exposed through the first mask with ultraviolet light for 80 s, which was placed in contact with the wafer. This step was followed by a post-expose bake of 1 min at 65°C, and 3 min at 95°C. The second layer of photoresist followed a similar procedure. SU-8 2050 was coated at 2,000 rpm, 30 s, to a film thickness of 75 μm, with soft bake times of 3 and 9 min at 65 and 95°C, respectively. The second mask was aligned to the first pattern with the aid of alignment reticules. UV exposure was performed for 2.5 min, followed by a post-expose bake for 1 min at 65°C and 7 min at 95°C. Both layers of photoresist were then developed in polymonoacetate simultaneously, without any subsequent baking of the photoresist.
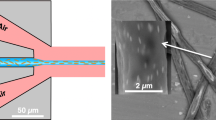
a Top–down schematic representation of the microchannel layout in the PDMS 2D flow-focusing device. The sheath fluid is introduced through inlets A, C and D, while the core fluid is introduced through inlet B. b Illustration of the 2D flow-focusing process in the microfluidic device. Core fluid enters the central microchannel at the A–B junction, is initially focused in the vertical direction at the A–C junction, and subsequently focused laterally into a single stream at the A–D junction prior to exiting the device
All of the microchannels are 100 μm (w), with the exception of B and C, which taper to 50 μm (w) at the junction with channel A. The mold has two channel heights: 75 μm for A and D, and 21 μm for B and C. From the patterned master mold, single layer microfluidic devices were fabricated by pouring a ∼5 mm thick layer of PDMS (Sylgard 184, Dow Corning) in a ratio of 5:1 part A:B on the silane-treated mold and cured at 80°C for 30 min. After the primary cure, the PDMS negative replicas were released from the silicon masters and the fluid inlet holes were punched with (20G) stainless steel luer stubs (McMaster Carr). For final assembly, sealing the bottom of the microchannels, the device was bonded to an unpatterned PDMS layer (∼1 mm) using plasma bonding (Harrick Scientific PDC-001, 20 s, 600 mTorr).
Flow focusing of the core and sheath fluids for the electrospun nanofibers is achieved within the devices by exploiting the two different channel heights. Pressurized sheath fluid is introduced into channels A, C, and D using syringe pumps (Model 11, Harvard Apparatus), while pressurized core fluid is fed into channel B (Fig. 1a). At the A–B channel junction, the core phase in B flows into the bottom of channel A as two separate parallel streams. As the stratified core fluid passes through the A–C junction, sheath fluid entering orthogonally from C lifts the core fluid, focusing it primarily in the z-direction. Absence of lift off in z-direction or incomplete lift off results into co-laminar flows of sheath and core fluids, producing stratified nanofibers with a Janus-type morphology. Finally, at the A–D junction, impinging sheath fluid from D focuses the core fluid in the y-direction, merging the two streams (Fig. 1b).
To initiate electrospinning from the central microchannel outlet, the steel tube connecting the syringe output to the sheath flow inlet (A) is connected to the positive terminal of a high-voltage power supply (ES30P-10W, Gamma High Voltage), with the ground attached to an aluminum plate positioned ∼15 cm from the device outlet. After the sheath and core flow rates are set, the supply voltage is increased to 15 kV, initiating the formation of a bi-component Taylor cone at the spinneret outlet and a stable co-axial jet elongated by the applied field (Fig. 2a). Viewing the device edge on at the outlet, flow focusing of the core phase in the sheath fluid is observed (Fig. 2b).
a Side view of the microchannel outlet during the electrospinning of hollow poly(vinylpyrrolidone) (PVP) + TiO2 nanofibers. At a supply voltage of 15 kV, a bi-component Taylor cone at the spinneret outlet if formed, with a stable co-axial jet elongated by the applied field. b Normal view of focused core fluid within the sheath fluid at droplet formed at the microchannel outlet, in the absence of electric potential. The small sphere visible in the core (left, center) is a projection of the microchannel outlet behind the droplet
Hollow composite nanofibers were electrospun with the microfluidic manifolds using a sheath solution, poly(vinylpyrrole) (PVP) + Ti (OiPr)4, flowed through channels A, C and D, while the core solution, heavy mineral oil (Sigma), was introduced into channel B. The optimized volumetric feed rates from the syringe pumps for the sheath and core solutions were 15 and 2 μl/min, respectively. For the sheath phase, 25% (w/v) Ti(OiPr)4 in ethanol + acetic acid (1:1 v/v) was added to a 6% (w/v) solution of PVP (Mol.wt. 1,300,000, Acros Organics) in ethanol, inducing stabilization the nanofiber sheath by gelation. Hollow composite nanofibers of PVP + TiO2 were synthesized from the PVP + Ti(OiPr)4/heavy mineral oil nanofibers by extracting the mineral oil core with octane. Moisture in the air rapidly hydrolyzes the Ti(OiPr)4 in the sheath to TiO2. Figure 3a and b show SEM and TEM images of hollow PVP nanofibers fabricated from the hydrodynamic fluid focusing electrospinning source respectively. The electrospun fibers have outer diameters on the order of 150–300 nm (d ave ∼250 nm).
To demonstrate the versatility of the platform for electrospinning nanofibers with a core-sheath morphology, the oil core was replaced with a conducting polymeric phase [PVP (6% w/v), pyrrole (0.1 M) and ferric chloride [(FeCl3) 2% w/v] in ethanol + DMF (1:1 v/v), while PVP (4% w/v) in ethanol + DMF (1:1 v/v)] was used as the sheath solution. The applied core and sheath solution volumetric flow rates were identical to those applied to the electrospinning of hollow fibers. SEM and TEM images of the PPy/PVP core/sheath nanofibers are presented in Fig. 3c and d, respectively. The TEM image (Fig. 3d) shows that polypyrrole is encapsulated as a uniform continuous phase in each PVP nanofiber. The PPy/PVP nanofibers were larger than the hollow PVP + TiO2 fibers, with diameters ranging from 400 to 700 nm (d ave ∼500 nm).
Proper balancing of the volumetric flow rates of the core and sheath fluids is critical to electrospin composite nanofibers using 2D microfluidic flow focusing. Two parameters form an envelope for operating conditions: sufficient sheath fluid pressure to separate the core phase from the hydrophobic PDMS walls and, as an upper bound, maintaining the Capillary number of the focused core stream below unity to prevent destabilization and subsequent rupture into discrete droplets (Guillot et al. 2007; Utada et al. 2007). Under experimental operating conditions, using equivalent volumetric flow rates for the sheath fluids entering through channels A, C, and D resulted in stable flow focusing conditions with sheath flow rates of ∼10–20 μl/min per channel with a fixed core channel (B) flow rate of 2 μl/min for both core solutions (mineral oil and conducting polymer). For sheath polymer flow rates of <10 μl/min per channel, incomplete or no lift off of the core solutions from the microchannel wall at the A–C junction was observed. In general, altering the balance between the individual volumetric flow rates of the sheath fluids (A, C, D) destabilized flow focusing in the central microchannel. If the flow rates for channels C or D were increased relative to A, incomplete lift off or flow stagnation at the A–C junction, or backflow of the core oil solution towards inlet A was observed. Increasing the flow rate of A relative to C and D impedes the focusing the oil core into a single stream at the A–D focusing junction. While the device operation is quite robust, permitting electrospinning over a range of sheath flow rates for a fixed core feed rate, excessive sheath/core flow rate ratios also destabilized the focused core stream. At total sheath/core volumetric flow ratios in excess of ∼40:1 for the experimental solutions (∼27 μl/min for sheath fluid flowing through each of the channels A, C, D; 2 μl/min for the core fluid in channel B), the focused core became unstable, breaking into discrete droplets prior to exiting the microfluidic devices.
In conclusion, we have demonstrated that a single cast 2D-hydrodynamic fluid focusing elastomeric device can be successfully used as a source to electrospin hollow and core/sheath nanofibers. The extension of microfluidic flow focusing to nanofiber electrospinning opens up possibilities for the nanomanufacture of novel, multi-laminate structures, analogous to what has been achieved over the past decade using multi-phase microfluidic devices for particle synthesis (Yi et al. 2003; De Geest et al. 2005; Dendukuri et al. 2007). Moreover, the ability to focus complex, multiphase fluids in 2D in microchannels offers an interesting platform for fluid dynamics modeling. With the ability to precisely control physical parameters such as microchannel geometry, fluid composition and flow rates, the easy-to-fabricate 2D-flow focusing devices can readily be utilized to analyze the jetting or dripping behavior of complex, multiphase flows.
References
Bazilevsky AV, Yarin AL, Megaridis CM (2007) Co-electrospinning of core-shell fibers using a single-nozzle technique. Langmuir 23(5):2311–2314
Bognitzki M, Hou HQ, Ishaque M, Frese T, Hellwig M, Schwarte C, Schaper A, Wendorff JH, Greiner A (2000) Polymer, metal, and hybrid nano- and mesotubes by coating degradable polymer template fibers. Adv Mater 12(9):637–640
De Geest BG, Urbanski JP, Thorsen T, De Smedt SC, Demeester J (2005) Monodisperse microgel synthesis inside microfluidic devices. Langmuir 21(23):10275–10279
Dendukuri D, Hatton TA, Doyle PS (2007) Synthesis and self-assembly of amphiphilic polymeric microparticles. Langmuir 23(8):4669–4674
Guillot P, Colin A, Utada AS, Ajdari A (2007) Stability of a jet in confined pressure-driven biphasic flows at low Reynolds numbers. Phys Rev Lett 99, Art No 104502
Han XJ, Huang ZM, He CL, Liu L, Han XJ, Wu QS (2006) Coaxial electrospinning of PC(shell)/PU(core) composite nanofibers for textile application. Polym Composites 27(4):381–387
Hou HQ, Jun Z, Reuning A, Schaper A, Wendorff JH, Greiner A (2002) Poly(p-xylylene) nanotubes by coating and removal of ultrathin polymer template fibers. Macromolecules 35(7):2429–2431
Li D, Babel A, Jenekhe SA, Xia YN (2004) Nanofibers of conjugated polymers prepared by electrospinning with a two-capillary spinneret. Adv Mater 16(22):2062–2066
Li D, McCann JT, Xia YN (2005) Use of electrospinning to directly fabricate hollow nanofibers with functionalized inner and outer surfaces. Small 1(1):83–86
Loscertales IG, Barrero A, Marquez M, Spretz R, Velarde-Ortiz R, Larsen G (2004) Electrically forced coaxial nanojets for one-step hollow nanofiber design. J Am Chem Soc 126(17):5376–5377
McCann JT, Li D, Xia YN (2005) Electrospinning of nanofibers with core-sheath, hollow, or porous structures. J Mater Chem 15(7):735–738
Simonnet C, Groisman A (2005) Two-dimensional flow focusing in a simple microfluidic device. Phys Rev Lett 87, Art No 114104
Song T, Zhang YZ, Zhou TJ, Lim CT, Ramakrishna S, Liu B (2005) Encapsulation of self-assembled FePt magnetic nanoparticles in PCL nanofibers by coaxial electrospinning. Chem Phys Lett 415(4–6):317–322
Steinhart M, Wendorff JH, Greiner A, Wehrspohn RB, Nielsch K, Schilling J, Choi J, Gosele U (2002) Polymer nanotubes by wetting of ordered porous templates. Science 296(5575):1997
Steinhart M, Wehrspohn RB, Gosele U, Wendorff JH (2004) Nanotubes by template wetting: a modular assembly system. Angew Chem Int Ed 43(11):1334–1344
Sun ZC, Zussman E, Yarin AL, Wendorff JH, Greiner A (2003) Compound core-shell polymer nanofibers by co-electrospinning. Adv Mater 15(22):1929–1932
Utada AS, Fernando-Nieves A, Stone HA, Weitz DA (2007) Dripping to jet transitions in coflowing liquid streams. Phys Rev Lett 99, Art No 094502
Wan MX, Wei ZX, Zhang ZM, Zhang LJ, Huang K, Yang YS (2003) Studies on nanostructures of conducting polymers via self-assembly method. Synth Metals 135(1–3):175–176
Wei M, Kang B, Sung C, Mead J (2002) Core-sheath structure in electrospun nanofibers from polymer blends. Macromol Mater Eng 291(11):1307–1314
Xia Y, Whitesides GM (1998) Soft Lithography. Angew Chem Int Ed 37:551–575
Yi G-R, Thorsen T, Manoharan VN, Hwang MJ, Pine DJ, Quake SR, Yang S-M (2003) Generation of uniform colloidal assemblies in soft-microfluidic devices. Adv Mater 15(15):1300–1304
Yu JH, Fridrikh SV, Rutledge GC (2004) Production of submicrometer diameter fibers by two-fluid electrospinning. Adv Mater 16(17):1562–1566
Zhang Y, Wang X, Feng Y, Li J, Lim C, Ramakrishna S (2006) Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(epsilon-caprolactone) nanofibers for sustained release. Biomacromol 7(4):1049–1057
Zhao PC, Jiang HL, Pan H, Zhu KJ, Chen W (2007) Biodegradable fibrous scaffolds composed of gelatin coated poly(epsilon-caprolactone) prepared by coaxial electrospinning. J Biomed Mater Res A 83A(2):372–382
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srivastava, Y., Rhodes, C., Marquez, M. et al. Electrospinning hollow and core/sheath nanofibers using hydrodynamic fluid focusing. Microfluid Nanofluid 5, 455–458 (2008). https://doi.org/10.1007/s10404-008-0285-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-008-0285-5