Abstract
Hypertensive disorders are quite common, complicating about 10% of pregnancies, while preeclampsia occurs in 2–8% of cases. The most recognized etiopathogenetic factor for the development of preeclampsia is deficient remodeling of the spiral arteries during trophoblastic invasion. Recently, some authors speculated about the “cardiovascular origin of preeclampsia”; in particular, they postulate that placental dysfunction is not the primum movens of preeclampsia, but it could be caused by a failure of the maternal cardiovascular system to adapt to the pregnancy itself. Moreover, several studies have also shown that developing preeclampsia in pregnancy is associated with an increased risk of cardiovascular disease later in life. Due to the importance of this pathology, it would be crucial to have an effective screening in order to implement a prophylaxis; for this purpose, it could be useful to have an accurate and noninvasive device for the assessment of maternal hemodynamic variables. USCOM® (Ultrasonic Cardiac Output Monitor) is a noninvasive Doppler ultrasonic technology which combines accuracy, reproducibility, noninvasiveness, and a fast learning curve. Maternal hemodynamic evaluation is important in order to monitor the changes that the maternal organism encounters, in particular a reduction in blood pressure, a decrease in total peripheral resistances, and an increase in cardiac output, resulting in a hyperdynamic circle. These hemodynamic modifications are lacking in pregnancies complicated by preeclampsia. For these reasons, it is crucial to have a tool that allows these parameters to be easily evaluated in order to identify those women at higher risk of hypertensive complications and more severe outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hypertensive disorders in pregnancy
Hypertensive disorders are quite common, complicating about 10% of pregnancies, while preeclampsia occurs in 2–8% of pregnancies. The classification of hypertensive disorders in pregnancy is currently being disputed among experts. In particular, the controversies concern lack of uniqueness for the terminology used, diagnostics, and consequently the identification of the different types of hypertension. The classification currently adopted was published by the International Society for the Study of Hypertension in Pregnancy (ISSHP) [1] in 2018. Gestational hypertension is characterized by the new onset of blood pressure ≥ 140 mmHg systolic or ≥ 90 mmHg diastolic, at or after 20 weeks’ gestation. It is defined as severe if the systolic blood pressure is ≥ 160 and/or diastolic ≥ 110 mmHg. A manual sphygmomanometer is indicated for the measurements, but if it is not available, automatic devices may also be used. The presence of proteinuria, on the other hand, should be assessed by automated urine examination, and if positive, the albumin/creatinine ratio (PCr Ratio) should be determined, which is positive in the case of values ≥ 30 mg/mmol (0.3 mg/mg). Proteinuria is no longer an essential criterion for diagnosis of preeclampsia, although massive proteinuria (> 5 g/24 h) is associated with severe neonatal outcomes. This classification distinguishes:
-
A.
Hypertension recognized before pregnancy or present in the first 20 weeks known as chronic hypertension: arterial hypertension pre-existing to pregnancy or recognized before 20 weeks. Most cases are primitive, while secondary causes are rare.
-
B.
Hypertension de novo that occurs after 20 weeks of gestation in the absence of the characteristics that define preeclampsia.
-
C.
Preeclampsia (de novo or complicating on chronic hypertension): hypertension with the addition at least one of these characteristics:
-
•
Proteinuria.
-
•
Maternal organ dysfunction including acute renal insult (creatinine ≥ 90 μmol/L; 1 mg/dL) or liver involvement (high transaminases > 40 IU/L) with or without epigastric pain or upper abdominal quadrants, neurological complications (e.g., eclampsia, altered mental state, scotomas or blindness, clonias, stroke, severe headache), or hematological complications (thrombocytopenia, platelets less than 150,000/μL, hemolysis, scattered intravascular clotting).
-
•
Uteroplacental dysfunction (intrauterine growth restriction or fetal death).
-
•
Preeclampsia (PE) superimposed on chronic hypertension (about 25% of women with chronic hypertension, with higher percentages in women with underlying kidney disease): this diagnosis is made when a woman with chronic hypertension develops one of the organ dysfunctions characteristic of preeclampsia. In women with proteinuric kidney disease, an increase in proteinuria in pregnancy is not enough to diagnose overlapping PE and cannot be used as a diagnostic criterion for PE.
Preeclampsia has long been defined as "the disease of theories", for which multiple models were proposed. The most recognized etiopathogenetic factor for the development of this pathological condition is deficient remodeling of the spiral arteries during trophoblastic invasion [2, 3]. The failure of trophoblastic invasion in the spiral arteries, the reduction of maternal blood flow into the intervillous space, and hypoxia, alternating with episodes of reoxygenation of the placenta, determine the release into the maternal circle of fragments of the syncytiotrophoblast with consequent maternal inflammatory response [4]. Early alterations in serum marker concentrations support the theory that the development of preeclampsia takes place during an early stage of pregnancy, leading to the formulation of the “placental origin of preeclampsia”.
Vascular endothelium dysfunction is also considered one of the primum movens of a series of events that occur progressively in preeclamptic women and that seem to explain its clinical heterogeneity [5, 6]. In other words, there is an imbalance between vasoconstrictor and vasodilator factors, such as vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and soluble factor 1 of the VEGF receptor (sFlt-1). SFlt-1 antagonizes the angiogenic action of VEGF and PlGF, and its levels are increased in preeclamptic patients, a feature that is hypothesized to be exploited in the context of screening [7]. Pregnancy-associated protein A (PAPP-A), also dosed in the combined trisomy risk definition test, is reduced in the first trimester in women who subsequently develop preeclampsia or fetal growth restriction.
Recently, some authors speculated about the “cardiovascular origin of preeclampsia” [8]; in particular, they postulate that placental dysfunction is not the primum movens of preeclampsia, but it could be caused by a failure of the maternal cardiovascular system to adapt to pregnancy. In particular, they compared preeclampsia and gestational diabetes: they are both specific conditions of pregnancy resolving after childbirth, but increasing the risk of diabetes and cardiovascular complications later in life. As gestational diabetes is caused by a pancreas unable to face the glycemic increase characteristic of gestation, so preeclampsia could be caused by the failure of the maternal cardiovascular system to adapt to pregnancy.
This hypothesis is reinforced by some data from maternal echocardiography, showing that preeclampsia and cardiovascular diseases share the same risk factors, and that the cardiovascular dysfunction may precede the development of hypertension itself [9]. Therefore, for those authors, the placental underperfusion due to the suboptimal maternal cardiovascular adaptation to pregnancy is the leading cause of preeclampsia. This debate (chicken or egg first) might seem purely speculative, but understanding the underlying causes of the development of hypertensive disorders of pregnancy can change the therapeutic and preventive strategies.
Screening and prophylaxis
Due to the importance of this pathology in obstetric practice, it would be essential to have a screening test available and, once women at risk have been identified, implement an effective prophylaxis. As mentioned, an etiological factor of preeclamptic pathology is the failure of the trophoblastic invasion to transform the uterus-placental circulation into a high flow and low resistance one. By means of color Doppler, it is possible to show the correlation between resistance in the uterine arteries in the second trimester and preeclampsia. Women with an increased Doppler pulsatility index have a higher probability per year than the general population of developing hypertensive disorders and intrauterine growth restriction [10]. However, this test has some intrinsic limits: firstly, it is carried out at 20–24 weeks of gestation, when the placentation process has already occurred and when prevention is no longer feasible. To overcome this, research has focused on early screening of preeclampsia. A first trimester screening using a combination of maternal anamnestic risk of factors, average blood pressure, Doppler of uterine arteries, PAPP-A, and PIGF was proposed by Nicolaides et al. [11]. This approach can identify about 95% of cases of early onset preeclampsia by analyzing these parameters:
-
Pregnancy-associated plasma protein-A (PAPP-A): A syncytiotrophoblastic metalloproteinase, which improves the mitotic function of insulin-like growth factor, playing an important role in placental growth and development. Low PAPP-A values are associated with a higher incidence of early preeclampsia in pregnancies with fetuses with a normal karyotype [11].
-
Placental growth factor (PlGF): A glycoprotein part of the vascular endothelial growth factor family, synthesized by cytotrophoblasts. Multiples of the median (MoM) values of PAPP-A and PlGF are significantly reduced at 11–13 weeks of gestation in women who subsequently develop preeclampsia; on the contrary, the higher the MoM of these metabolites, the more advanced the time of delivery [12].
-
Blood pressure: Even though hypertension is just one of the signs of preeclampsia, it is an important marker of disease.
-
Doppler of the uterine arteries: The pulsatility index (UtPI) is significantly increased in women who develop subsequent preeclampsia, and there is a negative linear correlation between the UtPI and the gestational age at childbirth [13].
Guidelines for the first trimester screening of preeclampsia have recently been developed by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) [14]. The only validated prophylactic measure for its prevention is the use of low-dose aspirin (150 mg/day) [15,16,17] started before 16 weeks of pregnancy for women at increased risk, particularly in case of: previous pre-eclampsia, chronic hypertension, kidney disease, pre-existing diabetes mellitus, antiphospholipid antibody syndrome, multiple pregnancies, obesity and medically assisted procreation. The aim of this early screening is to achieve primary prevention of preeclampsia even in those women without such anamnestic factors but at increased risk at the first trimester evaluation. For this purpose, a large randomized double-blind multicenter trial [16] was conducted in 1,776 women at high risk for the development of early preeclampsia. Of these, 798 received 150 mg/day of aspirin, while the remaining 822 received a placebo, from 11–14 weeks of gestation until 36 weeks. Compliance was excellent, with more than 85% of tablets taken by 80% of participants. In the group of women treated with aspirin, a lower incidence of preeclampsia was observed as compared with women who received only placebo.
Preeclampsia and maternal cardiovascular system
There have been many studies [18, 19] demonstrating that developing preeclampsia in pregnancy is associated with an increased risk of cardiovascular disease (CVD) later in life. In a meta-analysis, McDonald et al. [18] showed that women with a history of preeclampsia/eclampsia were approximately twice as likely to develop cardiovascular events than women with no hypertensive disorders, even after eliminating various confounding factors. In addition, analysis of the data suggests a "dose–response" correlation, with a higher risk in women with previous severe or early preeclampsia, or who have encountered complications such as preterm birth, fetal growth restriction, or fetal death. These women are therefore at risk of early onset CVD: as many as 11 of the 14 studies considered in this meta-analysis demonstrated the presence of these adverse events in relatively young women (≤ 56 years old).
A few decades ago, Sibai [19] defined pregnancy as a screening for the subsequent development of hypertension and diabetes, a kind of cardiovascular and metabolic test that can predict the possible onset of CVD later in life. More recent studies support this view of pregnancy as a "stress test for life", in which women who have vascular and endothelial dysfunction in the placental bed may develop similar alterations in other anatomical districts later in life. For this reason, in recent years there has been a growing interest in the study of maternal hemodynamics and the adaptations that the cardiovascular system must face in pregnancy [20, 21]. This is still a matter of debate, with several studies leading to conflicting or inconclusive results.
Heart rate, peripheral resistances, and systolic function
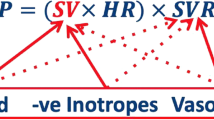
As for heart rate (HR), its increase begins at the first trimester, reaches its peak in the third, and returns to preconception values about 10 weeks after childbirth. Stroke volume (SV) is the amount of blood that is pumped during each heart cycle and varies widely from 60 to 100 ml. It increases about 20–30% in pregnancy, but there is disagreement about the timing at which these changes occur [21]. The simple one-dimensional examination of the left ventricle allows us to calculate the heart flow (cardiac output, CO), although the method validated in pregnancy for this calculation involves measuring the diameter of the efflux section of the left ventricle and the integral of aortic flow using this formula: CO = SV * HR. SV is calculated by the product between the aortic anulus area and the integral of the flow rate through the aortic valve. Once the heart flow rate has been obtained and systolic (PAS) and diastolic (PAD) blood pressure are measured, it is easy to calculate the average blood pressure (PAM) and peripheral vascular resistances (TVR) in dynes·s−1·cm−5: TVR = (PAM / CO) * 80. There is general agreement that CO increases in the first two quarters of pregnancy, but there is considerable discrepancy of opinion about the changes that occur in the third trimester. Peripheral resistances follow a pattern similar to average blood pressure, with an early decrease at about 8 weeks of pregnancy, a nadir at 28 weeks, and then a slight increase until delivery. The most frequently reported values are between 1,400 at conception to about 1000 at the beginning of the third trimester.
Cardiac ventricular geometry
Different authors [22,23,24] have found an increase in the mass of the left ventricle and alterations in the geometry of the same in patients suffering from gestational hypertension; this is a completely surprising finding, given that gestational hypertension and preeclampsia are time-limited events, unlike chronic hypertension. The most common compensatory response of the left ventricle to pressure overload, in fact, is hypertrophy, and in particular concentric remodeling. Even during a physiological pregnancy there is an increase in the mass of the left ventricle [20], but a harmonious balance is maintained between left ventricular cavity and wall thickness.
Diastolic function
Diastolic function is defined as the ability of the ventricle to release, receiving an adequate blood volume by the end of diastole, without an abnormal increase in atrial pressures. Diastole is a complex phenomenon that can be studied by evaluating the mitral valve zone with power Doppler. The “E wave” is the early fill wave and reflects the pressure gradient between the left atrium and the ventricle. The “A wave” represents the late filling, the diastolic component due to atrial contraction. The E/A ratio decreases as gestation progresses; there is, indeed, a decrease in the ventricular release capacity, compensated by an increase in the contribution of atrial contraction. These modifications are the same as those reported for the tissue Doppler technique [21].
Melchiorre et al. [23] assessed the presence of diastolic dysfunction in women with preeclampsia compared to women with uncomplicated pregnancy, through ultrasound and pulsed tissue Doppler, showing that 40% of women with preeclampsia had criteria for diagnosis of global diastolic dysfunction, compared to 14% of women in the control group (P = 0.007). Moreover, even pre-eclamptic women who did not fulfill the diagnosis of global diastolic dysfunction had evidence of regional diastolic dysfunction.
Echocardiographic changes after pregnancy complicated by preeclampsia
The cardiovascular implications of preeclampsia, however, do not end with the birth of the baby and the expulsion of the placenta [25, 26]. After childbirth, altered proprieties of the peripheral vascular system and abnormalities of the left ventricle may persist, especially in patients who presented severe or preterm forms of preeclampsia. Melchiorre et al. [26] assessed 64 women with previous preeclampsia and 78 women with previous uncomplicated pregnancy using transthoracic echocardiography. Asymptomatic left ventricular dysfunction/hypertrophy, moderate to severe, was significantly persistent 1 year after childbirth in patients who experienced preterm preeclampsia (56%) compared to those with term preeclampsia (14%) or uncomplicated controls (8%). Moreover, the authors emphasized the importance of asymptomatic abnormalities of the left ventricle in the stratification of risk for heart disease (stage B heart failure). Most cases of preterm preeclampsia [26] had asymptomatic stage B heart failure in the postpartum and 40% of them developed essential arterial hypertension within 1–2 years after pregnancy. The relative risk of developing hypertension within 2 years after childbirth is 14.5 (95% confidence interval 5.14–40.89, P < 0.0001). The higher prevalence of stage B heart failure in women who developed preterm rather than term preeclampsia or in uncomplicated controls is consistent with the fact that women with previous preterm preeclampsia have a higher risk of subsequently developing congestive heart failure and cardiac ischemic pathologies than women with term preeclampsia or normal pregnancy.
Maternal hemodynamic evaluation by USCOM® device
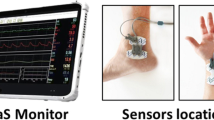
USCOM® (Ultrasonic Cardiac Output Monitor), a noninvasive Doppler ultrasonic technology for the determination of hemodynamic variables, combines accuracy, noninvasiveness, speed of performance, fast learning curve, and reproducibility [27]. It works through a continuous Doppler transducer that measures the rate of aortic or pulmonary blood flow coming out of the heart. Validated internal algorithms calculate the diameter of the aortic and pulmonary valves based on the patient's height and weight. Thus, knowing how big the valve anulus is and how fast the blood flows through it, USCOM® calculates how much blood flows per minute, which is, by definition, the cardiac output. This method is quite accurate, and in the hands of an experienced user, provides precise hemodynamic measurements.
Initially, the device asks the operator to enter personal and anthropometric data of the patient to obtain the estimated diameter of the aortic valve anulus, together with blood pressure. Then, for the evaluation of left systolic function, the small transducer is positioned perpendicular to the blood flow out of the heart, at the level of the aortic window on the jugular. Maximum alignment of the incident beam is important, allowing greater clarity of the received signal, with the patient lying in the supine position and with the backrest reclined by about 30°; if necessary, the patient can be asked to slightly overextend the neck to expose the portion of the jugular better (Fig. 1). The signal recorded is the systolic peak wave coming out of the aorta, preceded by the valve click (Fig. 2). Through the calculation of this integral, it is possible to determine hemodynamic parameters such as:
-
systolic peak velocity (Vpk, m/s);
-
medium pressure gradient through the valve during systole (Pmn, mmHg);
-
heart rate (HR, bpm);
-
normalized ejection time (ET%): time of each heart cycle occupied by the systole;
-
stroke volume, volume of blood pumped from the heart per heart cycle (SV, cm3);
-
stroke volume index: SV indexed by body area (SVI, cm3/m2);
-
cardiac output (CO, l/min);
-
cardiac index: CO indexed by body area (CI, l/min/m2);
-
total vascular peripheral resistances (TVR, dyne s / cm5);
-
inotropic index (INO, Watt/m2).
USCOM® technology has various clinical utilities, especially in emergency settings, intensive care units, during major surgery, or in case of infectious diseases, helping the diagnosis of sepsis [28,29,30,31]. In obstetrics, hemodynamic evaluation is important to monitor the changes that the maternal organism encounters, in particular a reduction in blood pressure, both systolic and diastolic, a reduction in total peripheral resistances, and an increase in the cardiac output, resulting in a hyperdynamic circle. All these hemodynamic changes that we have previously described can be evaluated and monitored longitudinally using USCOM in the various trimesters of pregnancy. In a recent paper [32], the London group generated normograms with the percentile graphs of the main calculated hemodynamic parameters (CO, SV, TVR) by inserting women’s demographic characteristics and gestational age into a special Excel calculator.
This hemodynamic adaptation is lacking in pregnancies complicated by preeclampsia, which are characterized by generalized vasoconstriction, with high TVR and low CO [22]. For these reasons, it is crucial to have a tool that allows evaluation of these parameters easily, with good reproducibility and a fast learning curve.
As shown by several studies, the most accurate independent predictor for developing hypertensive disorders later in pregnancy is demonstrated to be precisely the increase in peripheral resistances (TVR > 1340–1400 dyne s/cm5), with a positive predictive value of 86% and an accuracy of 91% [33]. In a study by Tiralongo et al. [34], 100 women were enrolled during the first trimester of pregnancy, 75 with TVR under 1200 dyne s/cm5 and 25 with TVR greater than 1200. No differences were reported between the two groups in terms of blood pressure at the time of analysis, while in the first group there were higher values of cardiac output and inotropic index. The sample, on the other hand, was too small to affirm that in the higher resistance group there was a higher incidence of preeclampsia during pregnancy. But this intuition was then confirmed retrospectively in a subsequent study [35], where 150 patients were enrolled during the first trimester of pregnancy. Evaluation using USCOM® showed significantly lower cardiac output and lower inotropy index values in patients who subsequently developed preeclampsia later in pregnancy as compared with uncomplicated cases, as well as significantly higher peripheral resistances.
For this purpose, we conducted a prospective observational study [36] with 162 women enrolled during the first-trimester ultrasound in order to evaluate the correlation between maternal hemodynamic parameters detected using USCOM® and ultrasound or biochemical parameters in women during the first-trimester screening of chromosomal abnormalities. We also analyzed the outcomes of pregnancy of those women who delivered in our clinic. We confirmed the correlation between biochemical markers such as PAPP-A and placental function, expressed by the pulsatility index of the uterine arteries and maternal hemodynamics, since the first trimester of pregnancy. Analyzing postpartum data, if we combine the onset of hypertensive disorders and fetal growth restriction, the most predictive parameter is indexed TVR, indicating that the inclusion of maternal hemodynamic evaluation could be useful in the screening protocols of preeclampsia.
In patients with chronic hypertension, adverse outcomes of pregnancy are more common than in the general obstetric population, as reported in a paper by our group [37]. We noticed that women with chronic hypertension had increased TVR, while the cardiac output remained within normal values, able to face the needs of pregnancy. The sudden and acute increase in TVR that we observed in those cases before the decision to deliver, due to bad blood pressure control despite therapy, may precede the alterations in blood tests (decreased platelet count and high liver enzymes). These data may be useful in identifying those at higher risk of developing more severe forms of HELLP/severe preeclampsia.
In two recently published papers [38, 39], the authors found that there were some features of left ventricular structure and function that correlated with pregnancy outcomes in women with chronic hypertension, both before conception and during the first trimester. In particular, altered geometry of the left ventricle with heart remodeling (OR, 5.94; 95% confidence interval, 2.90–12.19), diastolic dysfunction detected at tissue Doppler (OR, 3.22; 95% confidence interval, 1.63–6.37), and altered total vascular resistance (OR, 3.52; 95% confidence interval, 1.78–6.97) were demonstrated to be independent predictors for complications of pregnancy. The morphological and structural alterations of the heart chambers are obviously not demonstrable by a simplified approach such as USCOM®, but these patients could be identified thanks to the TVR values, which various studies indicate as the best predictor of hypertensive complications.
Various authors have recently hypothesized that this device may be useful in setting up a personalized antihypertensive therapy for patients with preeclampsia [40, 41], choosing the best antihypertensive drug according to the hemodynamic profile (prevalence of increased peripheral resistance or reduced output) and monitoring the response to it via USCOM®. Obviously, all of these are fields of research still in development, with numerous multicenter trials still ongoing, but which will undoubtedly change the clinical management of women with hypertensive disorders in the near future.
Some authors have analyzed the potential role of pre-pregnancy hemodynamic evaluation in the prediction of hypertensive disorders, reporting a lower cardiac output in patients who will develop hypertension in comparison with women with uncomplicated pregnancies, but within the normal range [42]. Pregnancy from its onset implies a cardiovascular effort with significant hemodynamic changes, such as an increase in cardiac output and a reduction in peripheral resistance mediated by progesterone. Therefore, the greatest efforts were focused on the first trimester, when it is possible to observe whether these changes are present or if they are lacking, thus leading to an early risk assessment, when it is still possible to implement preventive strategies.
But the field of hypertensive disorders in pregnancy is not the only one in which USCOM® is used. It has been shown that patients with small fetuses for the gestation age presented a hemodynamic profile with lower TVR and higher CO compared with women with fetal growth restrictions due to a deficient placental development [43]. This confirms the similar etiopathogenesis of hypertensive disorders and growth restrictions. Furthermore, women who experience preterm birth also have these same hemodynamic characteristics [44].
A new field that researchers are exploring in recent years is maternal hemodynamics during labor, in order to assess whether worse neonatal outcomes or greater frequency of fetal bradycardias after epidural analgesia may correspond to different hemodynamic profiles [45,46,47].
Conclusions
In pregnancy, the maternal cardiovascular system undergoes profound hemodynamic changes, which are lacking in pregnancies developing hypertension and/or preeclampsia. These differences in hemodynamics parameters can be detected and monitored through USCOM® in order to identify those women at higher risk of more severe outcomes.
References
Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43.
Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970–5.
Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2021;226:S907–27.
Staff AC, Fjeldstad HE, Fosheim IK, et al. Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia. Am J Obstet Gynecol. 2020;226:S895–906.
Karumanchi SA, Maynard SE, Stillman IE, et al. Preeclampsia: a renal perspective. Kidney Int. 2005;67:2101–13.
Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69.
O’Gorman N, Nicolaides KH, Poon LC. The use of ultrasound and other markers for early detection of preeclampsia. Womens Health (Lond). 2016;12:199–207.
Kalafat E, Thilaganathan B. Cardiovascular origins of preeclampsia. Curr Opin Obstet Gynecol. 2017;29:383–9.
Melchiorre K, Giorgione V, Thilaganathan B. The placenta and preeclampsia: villain or victim? Am J Obstet Gynecol. 2021;226:S954–62.
Lovgren TR, Dugoff L, Galan HL. Uterine artery Doppler and prediction of preeclampsia. Clin Obstet Gynecol. 2010;53:888–98.
Poon LC, Nicolaides KH. First-trimester maternal factors and biomarker screening for preeclampsia. Prenat Diagn. 2014;34:618–27.
Verlohren S, Perschel FH, Thilaganathan B, et al. Angiogenic markers and cardiovascular indices in the prediction of hypertensive disorders of pregnancy. Hypertension. 2017;69:1192–7.
Melchiorre K, Wormald B, Leslie K, et al. First-trimester uterine artery Doppler indices in term and preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:133–7.
Sotiriadis A, Hernandez-Andrade E, da Silva CF, et al. ISUOG Practice Guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet Gynecol. 2019;53:7–22.
Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218:287-93.e1.
Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–22.
Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017;50:492–5.
McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–30.
Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011–6.
Melchiorre K, Sharma R, Khalil A, et al. Maternal cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension. 2016;67:754–62.
Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol. 2012;24:413–21.
Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703–14.
Melchiorre K, Sutherland GR, Baltabaeva A, et al. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57:85–93.
Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Curr Opin Obstet Gynecol. 2011;23:440–7.
Ghi T, Degli Esposti D, Montaguti E, et al. Post-partum evaluation of maternal cardiac function after severe preeclampsia. J Matern Fetal Neonatal Med. 2014;27:696–701.
Melchiorre K, Sutherland GR, Liberati M, et al. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709–15.
Mulder E, Basit S, Oben J, et al. Accuracy and precision of USCOM versus transthoracic echocardiography before and during pregnancy. Pregnancy Hypertens. 2019;17:138–43.
Cheng YW, Xu F, Li J. Identification of volume parameters monitored with a noninvasive ultrasonic cardiac output monitor for predicting fluid responsiveness in children after congenital heart disease surgery. Medicine (Baltimore). 2018;97: e12289.
Deep A, Goonasekera CD, Wang Y, et al. Evolution of haemodynamics and outcome of fluid-refractory septic shock in children. Intensive Care Med. 2013;39:1602–9.
Hodgson LE, Venn R, Forni LG, et al. Measuring the cardiac output in acute emergency admissions: use of the non-invasive ultrasonic cardiac output monitor (USCOM) with determination of the learning curve and inter-rater reliability. J Intensive Care Soc. 2016;17:122–8.
Zheng ML, He SR, Liu YM, et al. Measurement of inotropy and systemic oxygen delivery in term, low- and very-low-birth-weight neonates using the Ultrasonic Cardiac Output Monitor (USCOM). J Perinat Med. 2020;48:289–95.
Vinayagam D, Thilaganathan B, Stirrup O, et al. Maternal hemodynamics in normal pregnancy: reference ranges and role of maternal characteristics. Ultrasound Obstet Gynecol. 2018;51:665–71.
Vasapollo B, Novelli GP, Valensise H. Total vascular resistance and left ventricular morphology as screening tools for complications in pregnancy. Hypertension. 2008;51:1020–6.
Tiralongo GM, Lo Presti D, Pisani I, et al. Assessment of total vascular resistance and total body water in normotensive women during the first trimester of pregnancy. A key for the prevention of preeclampsia. Pregnancy Hypertens. 2015;5:193–7.
Gagliardi G, Tiralongo GM, LoPresti D, et al. Screening for pre-eclampsia in the first trimester: role of maternal hemodynamics and bioimpedance in non-obese patients. Ultrasound Obstet Gynecol. 2017;50:584–8.
Montaguti E, Youssef A, Cavalera M, et al. Maternal hemodynamic assessment by USCOM(®) device in the first trimester of pregnancy. J Matern Fetal Neonatal Med. 2021. https://doi.org/10.1080/14767058.2021.1887129.
Montaguti E, Di Donna G, Pilu G. 2021 Usefulness of USCOM® evaluation in women with chronic hypertension who developed severe preeclampsia with low platelets and elevated liver enzymes. J Matern Fetal Neonatal Med. 2021. https://doi.org/10.1080/14767058.2021.1873269.
Valensise H, Farsetti D, Pisani I, et al. (2020) Hemodynamic maladaptation and left ventricular dysfunction in chronic hypertensive patients at the beginning of gestation and pregnancy complications: a case control study. J Matern Fetal Neonatal Med. 2020. https://doi.org/10.1080/14767058.2020.1818206.
Vasapollo B, Novelli GP, Gagliardi G, et al. Pregnancy complications in chronic hypertensive patients are linked to pre-pregnancy maternal cardiac function and structure. Am J Obstet Gynecol. 2020;223:425.
Di Pasquo E, Ghi T, Dall’Asta A, et al. Maternal cardiac parameters can help in differentiating the clinical profile of preeclampsia and in predicting progression from mild to severe forms. Am J Obstet Gynecol. 2019;221:633.
Ferrazzi E, Stampalija T, Monasta L, et al. Maternal hemodynamics: a method to classify hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2018;218:124.
Foo FL, Mahendru AA, Masini G, et al. Association between prepregnancy cardiovascular function and subsequent preeclampsia or fetal growth restriction. Hypertension. 2018;72:442–50.
Di Pasquo E, Ghi T, Dall’Asta A, et al. Hemodynamic findings in normotensive women with small-for-gestational-age and growth-restricted fetuses. Acta Obstet Gynecol Scand. 2020;100:876–83.
Valensise H, Farsetti D, Lo Presti D, et al. Preterm delivery and elevated maternal total vascular resistance: signs of suboptimal cardiovascular adaptation to pregnancy? Ultrasound Obstet Gynecol. 2016;48:491–5.
Kalafat E, Barratt I, Nawaz A, et al. Maternal cardiovascular function and risk of intrapartum fetal compromise in women undergoing induction of labor: pilot study. Ultrasound Obstet Gynecol. 2020;56:233–9.
Valensise H, Lo Presti D, Tiralongo GM, et al. Foetal heart rate deceleration with combined spinal-epidural analgesia during labour: a maternal haemodynamic cardiac study. J Matern Fetal Neonatal Med. 2016;29:1980–6.
Valensise H, Tiralongo GM, Pisani I, et al. Maternal hemodynamics early in labor: a possible link with obstetric risk? Ultrasound Obstet Gynecol. 2018;51:509–13.
Acknowledgements
The authors report no acknowledgements.
Funding
There is no funding sources.
Author information
Authors and Affiliations
Contributions
EM devised the topic, did literature search, and drafted and wrote the paper. GDD participated in writing, literature searching, and arranging of the references. AY provided expertise and participated in writing and editing of the text. GP participated in writing and editing of the text. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
Given the nature of the review article, no ethical committee approval was required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Montaguti, E., Di Donna, G., Youssef, A. et al. Hypertensive disorders and maternal hemodynamic changes in pregnancy: monitoring by USCOM® device. J Med Ultrasonics 49, 405–413 (2022). https://doi.org/10.1007/s10396-022-01225-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-022-01225-3