Abstract
Purpose
Blood flow reduction after initiation of lenvatinib therapy may not always indicate tumor necrosis. This study aimed to compare the blood flow detectability of contrast-enhanced ultrasonography (CEUS), contrast-enhanced computed tomography (CT), and contrast-enhanced magnetic resonance imaging (MRI) in hepatocellular carcinoma (HCC) during lenvatinib therapy.
Methods
A total of 12 cases underwent CEUS and contrast-enhanced CT/MRI within 2 weeks during lenvatinib therapy. Vascularity on CEUS and CT/MRI was compared.
Results
At the time of CEUS examination, the median period from the start of lenvatinib was 227 ± 210 (31–570) days. CEUS showed hyperenhancement in eight cases (66.7%), hypoenhancement in two cases (16.7%), and no enhancement in one case (8.3%), while CT/MRI showed hyperenhancement in one case (8.3%), ring enhancement in three cases (25.0%), and hypoenhancement in eight cases (66.7%) (p = 0.007). Transarterial chemoembolization (n = 3), radiofrequency ablation (n = 2), and stereotactic body radiation therapy (n = 2) were performed after blood flow detection by CEUS.
Conclusions
The viability of the HCC should be confirmed using CEUS when contrast-enhanced CT/MRI reveals lesion hypoenhancement during lenvatinib therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) generally has a poor prognosis, particularly when it is unresectable [1]. Hypervascularity is one of the features of HCC, based on which therapeutic options are selected, such as transarterial chemoembolization (TACE) or angiogenesis inhibitors. Lenvatinib is a tyrosine kinase inhibitor (TKI) that acts against vascular endothelial growth factor receptors (VEGFR) 1–3, fibroblast growth factor receptors (FGFR) 1–4, platelet-derived growth factor receptor-alpha (PDGFRα), RET, and KIT [2, 3]. Decreased HCC vascularity was observed on contrast-enhanced computed tomography (CT) in a clinical setting after the administration of lenvatinib [4, 5].
Two previous studies using CT or magnetic resonance imaging (MRI) demonstrated that a reduction in blood flow at 2–4 weeks after initiation of lenvatinib therapy predicted a favorable outcome [4, 5]. However, this hemodynamic change may not always be indicative of tumor necrosis or treatment effects [6]. Contrast-enhanced ultrasonography (CEUS) has also been used to detect hemodynamic changes on day 7 after initiation of treatment in previous studies [7, 8]. The sensitivity of CEUS for detecting blood flow in HCC is higher than that of CT and MRI [9, 10]. Long-term hemodynamic changes during sorafenib administration have previously been investigated using CEUS [11] and perfusion CT [12]; however, those during lenvatinib therapy to date remain unelucidated. It is important to select the appropriate imaging modality for follow-up during lenvatinib therapy.
This study aimed to compare the blood flow detectability of CEUS, contrast-enhanced CT, and contrast-enhanced MRI in HCC during lenvatinib therapy.
Materials and methods
Patients
This retrospective study included nine cases enrolled between June 2018 and February 2021. The inclusion criteria were patients with unresectable HCC who underwent both CEUS and contrast-enhanced CT/MRI within 2 weeks during lenvatinib therapy. CEUS was performed in 19 cases during lenvatinib therapy; seven cases that did not undergo contrast-enhanced CT/MRI within 2 weeks were excluded.
Clinical data, including serological data, were obtained from the hospital records in all cases. The study was approved by the Institutional Review Board of our institution (RK-200908-04). Given the retrospective nature of this study, informed consent was waived, because the data were obtained using a standard clinical care protocol.
Lenvatinib therapy
Lenvatinib was administered at 12 mg/day or 8 mg/day. The dose was determined based on the body weight of the patient. Lenvatinib was discontinued when a severe adverse event occurred and the Child–Pugh score was > 7. If patients recovered from an adverse event or worsening hepatic functional reserve, lenvatinib therapy was resumed. Imaging tests, including contrast-enhanced CT/MRI/ultrasonography, were performed every 6–12 weeks.
CEUS procedure
CEUS was performed in the decubitus position by an ultrasonographer (N. M) who is a Board Certified Fellow of the Japan Society of Ultrasonics in Medicine, and has 18 years of experience. CEUS was performed using the Arietta 850 ultrasound platform with a C252 transducer (Hitachi, Tokyo, Japan) and the Aplio i700 with a PVT-375BT transducer (Canon Medical Systems, Tochigi, Japan). For a patient with multiple HCCs, a sonographer selected the most appropriate nodule for the imaging tests.
A 0.5-ml bolus of perfluorobutane microbubbles (GE Healthcare Pharma, Tokyo, Japan) was injected into the antecubital vein via a 22-gauge peripheral intravenous cannula, followed by a 10-ml saline flush. The mechanical index was set at 0.1–0.2. CEUS was performed at a rate of 15 frames/s with a dynamic range of 45 dB. The receiver gain and image depth were optimized for each patient at baseline examination. The transit focus was set at the bottom of the target lesion.
The vascularity of the target lesion was evaluated at the early arterial phase, defined as that between 15 and 40 s after the contrast injection.
Contrast-enhanced CT (CECT) procedure
CT scans were performed using a multidetector-row 16-, 64-, or 320-slice raw scanner (Light-Speed, GE Healthcare, Piscataway, NJ, USA; Somatom, Siemens Healthcare, Germany; or Aquilion ONE, Canon Medical Systems, Tokyo, Japan), with a collimation of 2.5–5 mm, section thickness of 3–5 mm, and reconstruction of 2.5–3 mm in the decubitus position under fasting conditions. Scanning was performed using a 15.0- or 53.0-helical pitch, a table feed speed of 0.75 mm or 0.5 mm per rotation, and a voltage of 120 kV. A total of 100–120 mL of contrast medium (350 mg I/mL iomeprol; Iomeron® 350 syringe, Eisai, Tokyo, Japan) was injected intravenously at a rate of 3.0–3.5 mL/s via a 20-gauge peripheral intravenous cannula. The arterial phase was performed 37 s after the initiation of contrast injection. Sonographers scanned at one cross-section that showed the maximum diameter of the target lesion for 30 s, after which they moved the transducer from end to end of the lesion and observed the vascularity. The criteria to decide the target lesion is as follows. First, lesions located (1) deeper than 10 cm from the skin and (2) near the shadow of the lung or the rib were excluded. Second, the lesion of larger size was selected.
Contrast-enhanced MRI (CEMRI) procedure
MRI was performed using a 1.5-T MR scanner (Achiva 1.5 T Nova and Ingenia 1.5 T; Philips Japan, Tokyo, Japan) with an eight-channel system and an eight-channel phased-array coil, or a 3.0-T MR scanner (Discovery MR750w; GE Healthcare, Piscataway, NJ, USA) with a 32-channel system (maximum gradient strength: 44 mT/m, peak slew rate: 200 T/m/s) and a 32-channel phased-array coil. A dose of 0.1 mL/kg of contrast medium (Primovist®, Bayer Schering Pharma, Berlin, Germany) was injected intravenously at a rate of 1 mL/s, followed by a 20-mL saline flush via a 22-gauge peripheral intravenous cannula. Fat-saturated gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced three-dimensional-gradient-recalled echo T1-weighted images were obtained in the appropriately triggered arterial phase.
Subjective evaluation of vascularity
Vascularity was evaluated subjectively by two hepatologists (N.M. and M.K.) with 20 and 12 years of experience, respectively. The vascularity of the target lesion was determined in comparison with the surrounding liver parenchyma in the arterial phase, and classified into four groups as follows: hyper, ring, hypo, and no enhancement. If the evaluators had different opinions regarding classification of enhancement, they discussed the case and reached an agreement. The arterial phase of CEUS was defined as that between 15 and 40 s after injection of the contrast medium.
Quantitative evaluation of vascularity
Image analyses were performed by two hepatologists (N.M. and M.K.). The oval range of interest (ROI) was set in the HCC as large as possible, and in the hepatic parenchyma at the same cross-section while avoiding vessels. Ultrasonographic images were exported to a hard disk and analyzed with ImageJ software. The mean gray value in the ROI at 30 s after contrast medium injection was calculated. The mean signal intensity in the ROI at the arterial phase was measured with Synapse (FUJIFILM Medical, Tokyo, Japan).
The enhancement ratio was calculated using the following formula:
Statistical analysis
McNemar’s chi-squared test was used for statistical analysis, as shown in Fig. 2. Statistical significance was set at p < 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of the R commander that is designed to add statistical functions frequently used in biostatistics [13].
Results
Change in the vascularity of HCC during lenvatinib therapy
At baseline, CT/MRI showed hyperenhancement in 10 cases and isoenhancement in one case. The target lesion newly occurred at 4 months after the initiation of lenvatinib in one case. The median change ratio of the size of the target lesion at 2, 3, 6, 10, 12, and 17 months after the start of lenvatinib therapy was 0.81 (0.67–1.05), 0.79 (0.63–1.00), 1.50 (0.75–2.25), 1.25 (1.05–2.00), 1.54 (1.21–1.88), and 2.33 (1.17–3.50), respectively. Finally, hypoenhancement was observed in eight cases, ring enhancement was observed in two cases, and iso- and hyperenhancement were observed in one case each (Fig. 1).
At baseline, CT/MRI showed hyperenhancement in 10 cases and isoenhancement in one case. The target lesion newly occurred at 4 months after the initiation of lenvatinib in one case. The median change ratio of the size of the target lesion at 2, 3, 6, 10, 12, and 17 months after the start of lenvatinib therapy was 0.81 (0.67–1.05), 0.79 (0.63–1.00), 1.50 (0.75–2.25), 1.25 (1.05–2.00), 1.54 (1.21–1.88), and 2.33 (1.17–3.50), respectively. Finally, hypoenhancement was observed in eight cases, ring enhancement was observed in two cases, and iso- and hyperenhancement were observed in one case each
Comparison of findings at the arterial phase between CEUS and CECT and CEMRI
At the time of CEUS examination, the median period from the start of lenvatinib was 227 ± 210 (31–570) days. CEUS showed hyperenhancement in eight cases (66.7%), hypoenhancement in two cases (16.7%), and no enhancement in one case (8.3%), while CT/MRI showed hyperenhancement in one case (8.3%), ring enhancement in three cases (25.0%), and hypoenhancement in eight cases (66.7%) (p = 0.007) (Table 1, Figs. 2, 3, 4, 5).
CEUS showed hyperenhancement in eight cases (75.0%), hypoenhancement in two cases (16.7%), and no enhancement in one case (8.3%), while CT/MRI showed hyperenhancement in one case (8.3%), isoenhancement in one case (8.3%), ring enhancement in two cases (16.7%), and hypoenhancement in seven cases (58.3%) (p = 0.007)
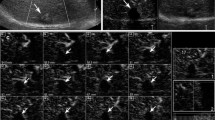
Case 1. CECT showed ring enhancement (a) and CEUS showed hyperenhancement (b). Case 2. CEMRI showed hypoenhancement (c), CEUS showed hyperenhancement (d), and angiography showed tumor stains (e). Case 3. Both CECT (f) and CEUS (g) showed hyperenhancement, and angiography showed tumor stain (h). Case 4. CEMRI showed hypoenhancement (i) and CEUS showed hyperenhancement (j)
Case 5. CEMRI showed ring enhancement (k) and CEUS showed hyperenhancement (l). Case 6. CEMRI showed ring enhancement (m), CEUS showed hyperenhancement (n), and angiography showed tumor stains. B-TACE was then performed (o). Case 7. CECT showed isoenhancement (p) and CEUS showed hyper enhancement (q). Case 8 previously underwent TACE. CECT showed hypoenhancement (r) and CEUS showed hyperenhancement (s)
Case 9. CECT showed hypoenhancement (t) and CEUS showed hyperenhancement (u). Case 10. Both CECT (v) and CEUS (w) showed hypoenhancement. Case 11 previously underwent TACE. Both CECT (x) and CEUS (y) showed hypoenhancement. Case 12 previously underwent TACE and SBRT. CECT showed hypoenhancement (z) and CEUS showed no enhancement (aa)
The enhancement ratio was 1.57 (0.13–8.25) for CEUS and 0.79 (0.44–1.12) for CECT/MRI (p = 0.052) (Fig. 6).
Sequential therapy for HCC after CEUS
Balloon-occluded TACE (B-TACE) was performed in three cases. One patient was treated with transarterial embolization and radiofrequency ablation (RFA). Angiography was performed in four cases and showed tumor stains in all cases. The treatment efficacy was complete response (CR), stable disease (SD), and progressive disease (PD) in one case each. Stereotactic body radiation therapy was performed in two cases. RFA was performed in two cases, including the aforementioned patient, who underwent RFA immediately after transarterial embolization. The target lesions were completely ablated using RFA; there was no local recurrence in either case.
Discussion
We found that most of the cases with HCC showed that the lesions were hypervascular at baseline, which gradually changed to hypoenhancement on CECT/CEMRI during lenvatinib therapy; both CEUS and angiography revealed hyperenhancement of the lesions in these cases.
CEUS can predict the efficacy of chemotherapy in various types of malignancies, such as HCC [7, 8, 14,15,16,17], metastatic renal cancer [18], gastric cancer [19], and lymphoma [20]. It is suitable for evaluation of response to treatment, because it can be performed repeatedly and has high detectability for blood flow [9, 10]. The discrepancy in findings between CEUS and CECT/CEMRI in the arterial phase may be due to differences in sensitivity to blood flow. In the previous literature, treatment response evaluation was performed early after initiation of chemotherapy to predict outcomes [7, 8, 14,15,16,17]. To overcome the lack of objectivity in ultrasonography, a time-intensity curve (TIC) was used for quantification of blood flow in most of these studies. However, recording the TIC at the same cross-section and depth, and in the same condition from baseline to after the initiation of therapy, is time-consuming. In contrast, CEUS was used to confirm the viability of HCC at a relatively late stage after initiation of lenvatinib therapy in the present study. This method is simple and only requires one ultrasound examination. It is widely accepted for clinical use.
Lenvatinib has a strong inhibitory effect on angiogenesis in tumors because of its ability to block VEGFR 1–3. This may lead to decreased vascularity in HCC [4, 5, 7, 8]. Regorafenib also acts against VEGFR 1–3. Suppression of tumor vascularity was previously observed with contrast-enhanced CT, and a decrease in microvascular density was proven by CD31 immunohistochemical staining [21]. Decreased blood flow within an HCC lesion is more frequently observed with lenvatinib therapy than with sorafenib therapy [4, 5, 22]. This phenomenon occurs 1–4 weeks after initiating lenvatinib therapy [4, 5, 7, 8]. While imaging examination of HCC vascularity early in the course of treatment is performed to predict the outcome, a decrease in blood flow does not necessarily indicate tumor necrosis [6], because HCC tumors usually increase in size after withdrawal of lenvatinib, as observed in the present study (Fig. 2). Thus, confirming the viability of HCC is important in determining treatment planning. The vascularity of a lesion is important for determining whether TACE should be performed. CEUS is a superior imaging modality to CECT/CEMRI for this purpose.
The differences in the enhancement-related findings from CEUS and CECT/CEMRI during lenvatinib administration may be explained by a few mechanisms. CEUS has a higher sensitivity for blood flow in HCC than CECT/CEMRI [10]. Furthermore, CEUS has a high temporal resolution and can help detect intratumoral blood flow even if the tumor shows early washout [23]. A previous study using a rat model showed necrosis, hypoxia, decreased microvessel density, and a decrease in CEMRI parameters after administration of sorafenib, while CEUS parameters did not decrease [24]. Decreased permeability of HCC was also observed in a clinical study using perfusion CT after bevacizumab [25] and sorafenib administration [26]. CEUS may be slightly influenced by changes in the permeability of HCC [24].
This study has several limitations. First, the sample size was small. Only a few patients were treated with lenvatinib at our institution. Second, HCC located deep in the liver may be invisible using ultrasonography. Fortunately, all lesions were easily observed using ultrasonography in this study. In most cases of intermediate- or advanced-stage HCC, the lesions are relatively large. Furthermore, while detection of blood flow using CEUS is relatively easy, obtaining a stable TIC is difficult because of the movements caused by the heartbeat, particularly in the left lobe of the liver. Further large-scale studies are required to validate our findings with respect to other chemotherapies.
Conclusion
Lenvatinib decreases the vascularity of HCC; however, this does not necessarily indicate complete remission. The viability of the HCC should be confirmed using CEUS when CECT/CEMRI reveals hypoenhancement during lenvatinib therapy.
References
Kudo M, Izumi N, Sakamoto M, Liver Cancer Study Group of Japan, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer. 2016;5:190–7.
Zschäbitz S, Grüllich C, Lenvantinib A. Tyrosine kinase inhibitor of VEGFR-3, FGFR 1–4, PDGFRα, KIT and RET. Recent Results Cancer Res. 2018;211:187–98.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Hiraoka A, Kumada T, Kariyama K, Real-life Practice Experts for HCC (RELPEC) Study Group and the HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan), et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol Res. 2019;49:111–7.
Kunimoto H, Shakado S, Tanaka T, et al. Reduction in tumor stain at 2 weeks after treatment initiation is a predictor of the efficacy of lenvatinib in patients with unresectable hepatocellular carcinoma. Oncology. 2020;98:779–86.
Koulakian H, Allaham W, Vilgrain V, et al. Non-measurable infiltrative HCC: is post-contrast attenuation on CT a sign of tumor response? Eur Radiol. 2019;29:4389–99.
Kuroda H, Abe T, Fujiwara Y, et al. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2019;25:2365–72.
Kamachi N, Nakano M, Okamura S, et al. Evaluating the therapeutic effect of lenvatinib against advanced hepatocellular carcinoma by measuring blood flow changes using contrast-enhanced ultrasound. Cancer Rep (Hoboken). 2021;5:e1471.
Numata K, Fukuda H, Miwa H, et al. Contrast-enhanced ultrasonography findings using a perflubutane-based contrast agent in patients with early hepatocellular carcinoma. Eur J Radiol. 2014;83:95–102.
Sugimori K, Numata K, Okada M, et al. Central vascular structures as a characteristic finding of regenerative nodules using hepatobiliary phase gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced MRI and arterial dominant phase contrast-enhanced US. J Med Ultrason. 2017;44:89.
Moschouris H, Malagari K, Gkoutzios P, et al. Intermediate and advanced hepatocellular carcinoma treated with the antiangiogenic agent sorafenib. Evaluation with unenhanced and contrast-enhanced ultrasonography. Med Ultrason. 2012;14:87–94.
Kaufmann S, Thaiss WM, Schulze M, et al. Prognostic value of perfusion CT in hepatocellular carcinoma treatment with sorafenib: comparison with mRECIST in longitudinal follow-up. Acta Radiol. 2018;59:765–72.
Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification–preliminary results. Radiology. 2011;258:291–300.
Zocco MA, Garcovich M, Lupascu A, et al. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014–21.
Sugimoto K, Moriyasu F, Saito K, et al. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33:605–15.
Shiozawa K, Watanabe M, Ikehara T, et al. Evaluation of sorafenib for advanced hepatocellular carcinoma with low α-fetoprotein in arrival time parametric imaging using contrast-enhanced ultrasonography. J Med Ultrason. 2017;44:101–7.
Lamuraglia M, Escudier B, Chami L, et al. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer. 2006;42:2472–9.
Ang J, Hu L, Huang PT, et al. Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy. World J Gastroenterol. 2012;18:7026–32.
Kumagawa M, Matsumoto N, Miura K, et al. Correlation between alterations in blood flow of malignant lymphomas after induction chemotherapies and clinical outcomes: a pilot study utilising contrast-enhanced ultrasonography for early interim evaluation of lymphoma treatment. Clin Radiol. 2021;S0009–9260:00089–91.
Cyran CC, Kazmierczak PM, Hirner H, et al. Regorafenib effects on human colon carcinoma xenografts monitored by dynamic contrast-enhanced computed tomography with immunohistochemical validation. PLoS ONE. 2013;8: e76009.
Kim MJ, Choi JI, Lee JS, et al. Computed tomography findings of sorafenib-treated hepatic tumors in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1201–6.
Liu LP, Dong BW, Yu XL, et al. Focal hypoechoic tumors of Fatty liver: characterization of conventional and contrast-enhanced ultrasonography. J Ultrasound Med. 2009;28:1133–42.
Muñoz NM, Minhaj AA, Maldonado KL, et al. Comparison of dynamic contrast-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for evaluation of the effects of sorafenib in a rat model of hepatocellular carcinoma. Magn Reson Imaging. 2019;57:156–64.
Zhu AX, Holalkere NS, Muzikansky A, et al. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120–5.
Coolens C, Driscoll B, Moseley J, et al. Feasibility of 4D perfusion CT imaging for the assessment of liver treatment response following SBRT and sorafenib. Adv Radiat Oncol. 2016;1:194–203.
Acknowledgements
The authors thank Editage (https://www.editage.jp/) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Owing to the retrospective nature of this study, informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Matsumoto, N., Ogawa, M., Kaneko, M. et al. Contrast-enhanced ultrasonography for blood flow detection in hepatocellular carcinoma during lenvatinib therapy. J Med Ultrasonics 49, 425–432 (2022). https://doi.org/10.1007/s10396-022-01204-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-022-01204-8