Abstract
Purpose
Attenuation imaging (ATI) is a new noninvasive ultrasound technique for assessing steatosis grade (S). However, validated region-of-interest (ROI) sampling strategies are not currently available. We investigated the diagnostic performance of various ATI-ROI positions for determining histopathologic S in patients with nonalcoholic fatty liver disease (NAFLD).
Methods
This retrospective study included 105 patients with biopsy-proven NAFLD. All attenuation coefficient (AC, dB/cm/MHz) measurements were obtained by the same hepatologist using a commercially available ultrasound system on the same day as liver biopsy. Mean (± standard deviation) age and body mass index of the patients were 53 (± 18) years and 27.1 (± 4.1) kg/m2, respectively. The numbers of patients with steatosis affecting < 5%, 5–33%, 33–66%, and > 66% of hepatocytes were 8, 50, 29, and 18, respectively. The ATI-ROI was placed at three different positions for AC measurement using a dedicated workstation: the upper edge of the area ROI, twice the depth of the liver capsule, and the lower edge of the area ROI. Diagnostic performance was evaluated using the area under the receiver-operating characteristic curve (AUC).
Results
The AUCs of AC at the three ATI-ROI positions were 0.734 (95% confidence interval [CI]: 0.470–0.998), 0.750 (0.639–0.861), and 0.878 (0.788–0.968) for S ≥ 1; 0.503 (0.392–0.615), 0.824 (0.741–0.907), and 0.809 (0.724–0.895) for S ≥ 2; and 0.606 (0.486–0.726), 0.849 (0.767–0.932), and 0.737 (0.626–0.848) for S = 3, respectively.
Conclusion
For accurate steatosis grade assessment, the ATI-ROI should not be placed at the upper edge of the area ROI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) represents the most common chronic liver disease around the world [1] and is largely influenced by the occurrence of metabolic syndrome and obesity [2]. Nonalcoholic steatohepatitis (NASH) is considered the more aggressive part of the NAFLD spectrum, leading to more rapid progression from fibrosis to cirrhosis and hepatocellular carcinoma, which are established risk factors for liver-related death [3].
Hepatic steatosis, which is the accumulation of fat within hepatocytes, is the key manifestation of NAFLD. This condition is pathologically diagnosed when at least 5% of hepatocytes contain fat deposition [4]. It has been demonstrated that substantial steatosis causes progression to fibrosis in patients with NAFLD [5], and that the quantity of liver fat is associated with the risk of cardiovascular disease in patients with NAFLD [6]. Thus, noninvasive quantitative assessment of steatosis is a clinical necessity for the diagnosis and follow-up of patients with NAFLD/NASH.
The ultrasound (US) beam is more attenuated by liver steatosis compared to normal liver. Using this physical property, the degree of attenuation (i.e., attenuation coefficient [AC]) can be used to quantitatively assess liver steatosis [7]. Recently, a commercial-based US scanner (Aplio i-series) was equipped with software based on quantification of attenuation of the US beam (Attenuation Imaging [ATI]; Canon Medical Systems, Tochigi, Japan), which can calculate AC and assess the degree of liver steatosis in daily clinical practice. Tada et al. [8] evaluated the diagnostic performance of ATI, with histopathologic findings as the reference standard, and reported that the area under the receiver-operating characteristic curve (AUC) was 0.85 for steatosis grade S1 (5%–33% of hepatocytes containing fat), 0.91 for steatosis grade S2 (33%–66% of hepatocytes containing fat), and 0.91 for steatosis grade S3 (> 66% of hepatocytes containing fat).
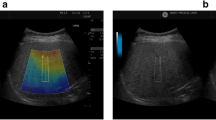
The AC is calculated in a 2 × 4 cm fan-shaped measurement region-of-interest (ROI) within the sampling box (Fig. 1). The ATI system can filter out large vessels or strong artifacts within the sampling box, which can enhance the reliability of AC measurements. During AC measurement, the operator can freely move both the measurement ROI and the sampling box so that they can avoid artifacts such as strong orange areas that usually appear in the upper edge of the sampling box, or dark blue areas posterior to blood vessels [9]. However, this may cause some confusion as to how deep the measurement ROI should be placed, because the deeper the ROI position is, the smaller the AC value becomes (Fig. 1). Thus, establishment of a standardized approach for ROI-based AC measurement is warranted.
Attenuation image of a 34-year-old man with biopsy-proven nonalcoholic steatohepatitis. The steatosis grade is 3. a The yellow sampling region-of-interest (ROI) is placed at the upper edge of the area ROI. The attenuation coefficient (AC) is 1.09 dB/cm/MHz. b The yellow sampling ROI is placed at twice the depth of the liver capsule. The AC is 0.99 dB/cm/MHz. c The yellow sampling ROI is placed at the lower edge of the area ROI. The AC is 0.58 dB/cm/MHz
The present study was, therefore, conducted to evaluate the diagnostic performance of the AC values obtained with ATI at different ROI positions in assessing the histopathologic steatosis grade, and to determine the most appropriate ATI-ROI position.
Materials and methods
Subjects
This study involved the retrospective analysis of 105 liver ATI datasets collected between April 2017 and March 2019, which were previously acquired for studies at Tokyo Medical University [10], in which six patients were removed from the analyses due to the inability to use row data. All patients had biopsy-proven NAFLD/NASH. All ATI examinations were performed on the same day as liver biopsy. The study was approved by our institutional review board with waiver of informed consent. The mean (± standard deviation) values for age and body mass index of the patients were 53 (± 18) years and 27.1 (± 4.1) kg/m2, respectively. Eighty-five (81.0%) of the 105 patients were diagnosed with NASH. The baseline demographic, biochemical, histopathologic, and ATI data of the patients are summarized in Table 1.
ATI examination
All ATI examinations were performed by the same physician with more than 15 years of experience in liver US, using a diagnostic US scanner (Aplio i800; Canon Medical Systems) with a 3.5-MHz convex transducer (PVI-475BX; Canon Medical Systems). Each examination was conducted where liver biopsy was likely to be performed. First, ATI mode was initiated, and the examination was then performed via an intercostal space while the patient held his or her breath. A fan-shaped sampling box was positioned in the liver parenchyma at the upper edge of the liver capsule. Then, a 2 × 4 cm fan-shaped ROI (which is the default setting for measurement) was placed within the sampling box. Detailed information concerning ATI examination methods is described elsewhere [8, 11]. The AC value (dB/cm/MHz) was displayed at the lower left corner, and the reliability of the results was also displayed in terms of the R2 value, with R2 values categorized as poor (R2 < 0.80), good (R2 = 0.81–0.89), or excellent (R2 ≥ 0.90). Based on information provided by the manufacturer, AC values with R2 ≥ 0.80 were considered valid measurements. The ATI examination was performed until five valid measurements were obtained and recorded. The depth of the liver capsule, which was defined as the distance from the skin surface to the liver capsule, was also measured. These data were transferred to a dedicated workstation (Vitrea; Canon Medical Systems) for offline analysis.
Image analysis
To evaluate the diagnostic performance of the ATI AC values obtained at different ROI positions in assessing the histopathologic steatosis grade and to determine the most reliable ATI-ROI position, one representative ATI image that did not contain artifacts such as large blood vessels in the sampling ROI was selected from among the five acquired ATI images. Then, using the ATI raw data, the ATI-ROI was placed at three different positions for AC measurement at the dedicated workstation: the upper edge of the area ROI, twice the depth of the liver capsule, and the lower edge of the area ROI (Fig. 1). Each measured AC value was recorded for analysis. All these procedures were performed by a single technician with more than 10 years of experiences in liver US, who was blinded to the clinical and histopathologic information.
Histopathologic evaluation
Immediately after acquisition of ATI data, part of which were used in this study, US-guided percutaneous liver biopsy was performed using an 18- or 16-gauge core needle biopsy kit (CorVocet; Merit Medical Systems, Inc., Utah, USA) according to the standard protocol. Liver biopsy was performed at a location as close as possible to the area in which the ATI examination was performed. Detailed information is described elsewhere [10]. In brief, histopathologic scoring was performed using the Clinical Research Network in NASH scoring system [12], and the diagnosis of NASH was based on the classification described by Matteoni et al. [13].
Statistical analysis
To summarize the data background, continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as absolute figures with percentages. The differences in AC between steatosis grades were each assessed using the Kruskal–Wallis test. Pairwise Wilcoxon tests were also used for univariate comparisons between the groups. In this analysis, multiple testing corrections were not performed because this was an exploratory analysis requiring validation in an external and/or independent cohort. The overall performance of the AC values obtained at the three different ATI-ROI positions for the assessment of steatosis grade was estimated using the AUC together with the 95% confidence interval (CI). AUCs were compared using the Delong test. Multivariate linear regression analysis was used to identify significant determinant factors for AC at each of the three different ROI positions. All statistical analyses were performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan). Values of P < 0.05 were considered statistically significant, and CIs were reported at the 95% level.
Results
AC values obtained at three different ATI-ROI positions
Upper edge of the area ROI
Boxplots of AC versus steatosis grade are shown in Fig. 2a. The AC values showed no significant differences between steatosis grades S0, S1, S2, and S3 (Kruskal–Wallis test: P = 0.0504; Wilcoxon test: P = 0.0340 between S0 and S1, P = 0.0387 between S0 and S2, and no significant differences in other comparisons). The AUCs of the AC values for the detection of hepatic steatosis are detailed in Table 2. The median values and interquartile ranges in parentheses for S0, S1, S2, and S3 were 1.38 (0.84, 1.67), 0.95 (0.84, 1.05), 0.95 (0.83, 1.03), and 1.00 (0.94, 1.06), respectively. Diagnostic performance was an AUC of 0.734 (95% CI: 0.470–0.998) for steatosis grade ≥ S1, an AUC of 0.503 (95% CI: 0.392–0.615) for ≥ S2, and an AUC of 0.606 (95% CI: 0.486–0.726) for S3.
a Boxplots of attenuation coefficient (AC) measured at the upper edge of the area ROI versus steatosis grade. AC shows no significant differences between steatosis grades S0, S1, S2, and S3 (Kruskal–Wallis test: P = .0504; Wilcoxon test: P = .0340 between S0 and S1, P = .0387 between S0 and S2, and no significant differences in other comparisons). b Boxplots of AC measured at twice the depth of the liver capsule versus steatosis grade. AC shows significant differences between steatosis grades S0, S1, S2, and S3 (Kruskal–Wallis test: P < .0001; Wilcoxon test: P = .3848 between S0 and S1, P = .0221 between S2 and S3, P = .0020 between S0 and S2, P = .0003 between S1 and S2, and P < .0001 in other comparisons). c Boxplots of AC measured at the lower edge of the area ROI versus steatosis grade. AC shows significant differences between steatosis grades S0, S1, S2, and S3 (Kruskal–Wallis test: P < .0001; Wilcoxon test: P = .6853 between S2 and S3, P = .0061 between S0 and S1, P = .0002 between S0 and S2, P = .0002 between S0 and S3, P = .0003 between S1 and S2, and P < .0001 in other comparisons)
Twice the depth of the liver capsule
The AC values showed significant differences between steatosis grades S0, S1, S2, and S3 (Kruskal–Wallis test: P < 0.0001; Wilcoxon test: P = 0.3848 between S0 and S1, P = 0.0221 between S2 and S3, P = 0.0020 between S0 and S2, P = 0.0003 between S1 and S2, and P < 0.0001 in other comparisons) (Fig. 2b). The median values and interquartile ranges in parentheses for S0, S1, S2, and S3 were 0.68 (0.61, 0.71), 0.69 (0.62, 0.79), 0.84 (0.73, 0.90), and 0.93 (0.83, 1.01), respectively. Diagnostic performance was an AUC of 0.750 (95% CI: 0.639–0.861) for steatosis grade ≥ S1, an AUC of 0.824 (95% CI: 0.741–0.907) for ≥ S2, and an AUC of 0.849 (95% CI: 0.767–0.932) for S3 (Table 2).
Lower edge of the area ROI
The AC values showed significant differences between steatosis grades S0, S1, S2, and S3 (Kruskal–Wallis test: P < 0.0001; Wilcoxon test: P = 0.6853 between S2 and S3, P = 0.0061 between S0 and S1, P = 0.0002 between S0 and S2, P = 0.0002 between S0 and S3, P = 0.0003 between S1 and S2, and P < 0.0001 in other comparisons) (Fig. 2c). The median values and interquartile ranges in parentheses for S0, S1, S2, and S3 were 0.465 (0.315, 0.533), 0.560 (0.485, 0.673), 0.740 (0.635, 0.795), and 0.735 (0.668, 0.818), respectively. Diagnostic performance was an AUC of 0.878 (95% CI: 0.788–0.968) for steatosis grade ≥ S1, an AUC of 0.809 (95% CI: 0.724–0.895) for ≥ S2, and an AUC of 0.737 (95% CI: 0.626–0.848) for S3 (Table 2).
Comparison of diagnostic performance of AC in detecting each steatosis grade according to ATI-ROI position
For steatosis grade ≥ S1, no significant differences in AUC were observed between the three ATI-ROI positions. For steatosis grade ≥ S2, the AUCs at twice the depth of the liver capsule (0.824) and at the lower edge of the area ROI (0.809) were significantly higher than the AUC at the upper edge of the area ROI (0.503) (P < 0.0001), but no other significant differences were observed. For steatosis grade S3, the AUC at twice the depth of the liver capsule (0.849) was significantly higher than the AUC at the upper edge of the area ROI (0.606) (P < 0.0001), but no other significant differences were observed. Details are shown in Table 2.
Factors affecting AC values obtained at three different ATI-ROI positions
Multivariate liner regression analysis revealed that hepatic steatosis was significantly associated with ACs at both twice the depth of the liver capsule (P = 0.0002) and the lower edge of the area ROI (P = 0.0001), and age was significantly associated with ACs at the lower edge of the area ROI (P = 0.0010). There were no significant factors associated with ACs at the lower edge of the area ROI (Tables 3, 4, 5).
Discussion
The objective of this study was to determine the most appropriate ROI position for ATI for assessing the histopathologic steatosis grade. Three different ATI-ROI positions were evaluated: the upper edge of the area ROI, twice the depth of the liver capsule, and the lower edge of the area ROI. The results showed that placing the ATI-ROI at the upper edge of the area ROI should definitely be avoided due to the lower AUC as compared to the other positions.
A pronounced dark orange area, which usually appears in the upper edge of the ROI, should not be included in the measurement ROI because it has been deemed to be an artifact (e.g., reverberation artifact). Although ATI can calculate ACs, when automatically filtering out pronounced artifacts such as large vessels, the system cannot avoid such reverberation artifact if the measurement ROI includes the artifact because the system cannot distinguish reverberation artifacts and high attenuation due to fat content. Thus, using the US system’s trackball, the operator should intentionally avoid the pronounced dark orange area, as supported by our results.
The ATI-ROI position that is most appropriate (at twice the depth of the liver capsule or at the lower edge of the area ROI) may depend on the individual patient. The results showed that for steatosis grade ≥ S1, the AUC at the lower edge of the area ROI (0.878) tended to be higher than the AUC at twice the depth of the liver capsule (0.750). On the other hand, for steatosis grade S3, the AUC at twice the depth of the liver capsule (0.849) tended to be higher than the AUC at the lower edge of the area ROI (0.737). Based on these results, for patients suspected to have severe steatosis, the ATI-ROI should be placed at twice the depth of the liver capsule, and for patients suspected to have mild steatosis, the ATI-ROI should be placed at the lower edge of the area ROI. However, it should be noted that when comparing measurement results obtained on different days in the same patient, we should strictly place the ROI at the same level in the liver.
Sugimoto et al. [10] reported the diagnostic performance of ATI for the assessment of steatosis grade. In the study, they placed the ATI measurement ROI at least 1.5 cm below the liver capsule to avoid reverberation artifacts, but unfortunately there was no rigid rule on how to place it. They showed a boxplot of AC versus steatosis grade, in which there was a statistically significant different between S0 and S3 (P < 0.001) and between S0 and S1 (P = 0.01), but there was no statistically significant difference between S2 and S3 (P = 0.34). In contrast, when the ROI was placed at twice the depth of the liver capsule in our study, there was a statistically significant difference between S2 and S3 (P = 0.0221).
Moreover, in the previous studies using ATI, although the cutoff AC values for steatosis grades ≥ S1 and ≥ S2 were relatively consistent, i.e., steatosis grade ≥ S1 (0.67 to 0.64 dB/cm/MHz) and ≥ S2 (0.72 to 0.70 dB/cm/MHz), the cutoff AC values for steatosis grade S3 were inconsistent (0.86–0.73 dB/cm/MHz) [10, 14]. The main reason for this discrepancy is thought to be the lack of strict guidelines concerning ROI placement. Our findings suggest that as the steatosis grade increases, the AC values exhibit greater fluctuation from shallow to deep areas. Thus, those results also suggest that to differentiate between S2 and S3, i.e., relatively high steatosis grades, we should place the ROI at twice the depth of the liver capsule.
We also investigated several factors that were associated with ACs at each of the three ROI positions and determined that hepatic steatosis was significantly associated with ACs at both twice the depth of the liver capsule (P = 0.0002) and the lower edge of the area ROI (P = 0.0001). In contrast, there were no significant factors associated with ACs at the upper edge of the area ROI, which also strongly suggests ACs at the upper edge of the area ROI were reliable. In addition, Jeon et al. [15] stated that not only the degree of steatosis assessed at MRI-PDFF but also the stage of fibrosis at MRE were significant determinant factors in the ACs measured with ATI, which was not in line with our results. Although the precise reason is not clear, the discrepancy may be caused by the difference in the patient population and the difference in the distribution of intrahepatic fibrosis. However, unlike their study, our data are based on the histological proof, which may be the advantage of the present study.
There are several limitations in the present study. First, the study was retrospective in nature and included a relatively small number of patients, especially S0, all of whom were Japanese. Thus, this might have influenced the accuracy when distinguishing between S0 and ≥ S1. It is known that ethnicity is correlated with a variety of pathologies. Further studies involving larger numbers of patients from a variety of ethnic groups are warranted. Second, AC measurements were performed using one image for each patient, without taking into account the variability that may arise when consecutive measurements are obtained. Third, fat deposition in the liver is not necessarily homogeneous, which may have affected our results. However, we performed liver biopsy at homogeneous fat deposition sites as strictly as possible in this series and performed the ATI examination. Finally, we did not evaluate the reproducibility (inter-observer agreement) and repeatability (intra-observer agreement) of the different ROI positions for measuring AC with ATI. However, it has been reported that the intra-observer reproducibility, assessed by means of the intra-class correlation coefficient (ICC), ranges from 0.81 to 0.98, whereas the inter-observer reproducibility ranges from 0.79 to 0.92 [15, 16].
Conclusion
In conclusion, this study evaluated the diagnostic performance of the AC values obtained with ATI at three different ROI positions in the liver. Clinicians who perform ATI examinations to assess steatosis grade can improve the diagnostic performance of AC by avoiding the upper edge of the area ROI and placing the ATI-ROI as consistently as possible.
References
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
VanWagner LB, Rinella ME. Extrahepatic manifestations of nonalcoholic fatty liver disease. Curr Hepatol Rep. 2016;15:75–85.
Sayiner M, Koenig A, Henry L, et al. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20:205–14.
European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Ajmera V, Park CC, Caussy C, et al. Magnetic resonance imaging proton density fat fraction associates with progression of fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155:307-10.e2.
Arulanandan A, Ang B, Bettencourt R, et al. Association between quantity of liver fat and cardiovascular risk in patients with nonalcoholic fatty liver disease independent of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2015;13:1513-520.e1.
Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25:6053–62.
Tada T, Iijima H, Kobayashi N, et al. Usefulness of attenuation imaging with an ultrasound scanner for the evaluation of hepatic steatosis. Ultrasound Med Biol. 2019;45:2679–87.
Ferraioli G, Maiocchi L, Savietto G, et al. Performance of the attenuation imaging technology in the detection of liver steatosis. J Ultrasound Med. 2021;40:1325–32.
Sugimoto K, Moriyasu F, Oshiro H, et al. The role of multiparametric US of the liver for the evaluation of nonalcoholic steatohepatitis. Radiology. 2020;296:532–40.
Bae JS, Lee DH, Lee JY, et al. Assessment of hepatic steatosis by using attenuation imaging: a quantitative, easy-to-perform ultrasound technique. Eur Radiol. 2019;29:6499–507.
Kleiner DE, Brunt EM, Van Natta M, et al. (2005) Nonalcoholic steatohepatitis clinical research network design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–21.
Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9.
Lee DH, Cho EJ, Bae JS, et al. Accuracy of two-dimensional shear wave elastography and attenuation imaging for evaluation of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2021;19:797-805.e7.
Jeon SK, Lee JM, Joo I, et al. Prospective evaluation of hepatic steatosis using ultrasound attenuation imaging in patients with chronic liver disease with magnetic resonance imaging proton density fat fraction as the reference Standard. Ultrasound Med Biol. 2019;45:1407–16.
Yoo J, Lee JM, Joo I, et al. Reproducibility of ultrasound attenuation imaging for the noninvasive evaluation of hepatic steatosis. Ultrasonography. 2020;39:121–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflicts of interest.
Ethical statement
All the procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. This study was approved by the Ethical Review Board of Tokyo Medical University.
Informed consent
Informed consent was waived because this was a retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sugimoto, K., Abe, M., Oshiro, H. et al. The most appropriate region-of-interest position for attenuation coefficient measurement in the evaluation of liver steatosis. J Med Ultrasonics 48, 615–621 (2021). https://doi.org/10.1007/s10396-021-01124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-021-01124-z