Abstract
Prior to 1985, the open waters of Lake Malawi were free from schistosome transmission. Over the past decades, however, the prevalence of urinary schistosomiasis has increased dramatically in the southern part of the lake. We found the prevalence of human schistosomiasis in school-aged children to be negatively correlated with the density of molluscivorous fishes. Specifically, the increased infection rate in southern Lake Malawi between 1978 and 1991 is coincident with the reduction in numbers of snail-eating fishes. During 2003, we determined the relative abundance of molluscivorous fishes and snail density at 18 sites throughout the lake and schistosome infection in school-aged children living in selected lakeshore communities of Lake Malawi. At the 18 sites sampled in 2003, we found that snail abundance decreased with an increase in abundance of snail-eating fishes. Furthermore, the 2003 samples showed that the abundance of snail-eating fishes increased and there was a reduction in schistosomiasis in school-aged children in Chembe Village. We believe that we will not observe a return to the 1978 infection rates until these fishes continue to increase and inhabit shallower waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is a parasitic disease of major public health importance in many countries in Africa, Asia, and South America, with an estimated 200 million people infected worldwide (World Health Organization, 2002). The disease is caused by trematodes of the genus Schistosoma that require specific freshwater snail species to complete their life cycles. People contract schistosomiasis when they come in contact with water containing the infective larval stage (cercariae) of the trematode. Five species of schistosomes are recognized as important metazoan parasites of humans. Schistosoma mansoni Sambon, S. haematobium (Bilharz), and S. japonicum Katsurada are widespread, with S. intercalatum Fisher and Schistosoma mekongi Voge, Bruckner, and Bruce having a more restricted distribution (Rollinson and Simpson, 1987). In Malawi, S. haematobium causes urinary schistosomiasis and its known intermediate snail hosts are Bulinus globosus and B. nyassanus.
Prior to 1985, the open waters of Lake Malawi were free from schistosome transmission (Stauffer et al., 1997). Over the past decades, the prevalence of urinary schistosomiasis has increased dramatically in the southern part of the lake and now affects tourism as authorities such as the Centers for Disease Control issue warnings about the risk of contracting schistosomiasis in Lake Malawi (Centers for Disease Control, 2005). In Malawi, gynecological lesions appeared in some patients with schistosomiasis; and although these lesions were unlikely to cause death directly, they added to overall morbidity (Wright et al., 1982) and could theoretically facilitate transmission of acquired immunodeficiency syndrome (AIDS) (Feldmeier et al., 1994; Harms and Feldmeier, 2002).
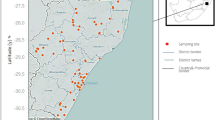
The increase in the prevalence of schistosomiasis coincided with overharvesting of snail-feeding cichlids in Lake Malawi, which resulted in a dramatic decrease in the abundance of snail-feeding cichlid species (Stauffer et al., 1997). Studies have shown that the facultative molluscivore and popular food fish Trematocranus placodon is effective at controlling snails that serve as intermediate hosts for schistosomes in fishponds (Chiotha et al., 1991a, 1991b). Here, we present data to show the relationship among abundance of molluscivorous fishes, intermediate snail-host density, and schistosome infection in school-aged children living in lakeshore communities of Lake Malawi (Fig. 1).
Methods
Fish density was estimated along transects extending up to 200 m from shore at depths of 1.5, 3.0, 4.6, 6.0, 7.6, and 9.1 m at 18 stations in Lake Malawi (Fig. 1) during January, April, and October 2003. A 50 m measuring tape was laid down at each depth parallel to the shoreline, and all molluscivorous fishes seen within a 2 m zone on either side of the tape were videoed by a diver swimming along the tape in one direction; the same procedure was immediately followed swimming in the opposite direction. The video was reviewed later in the laboratory; the number of T. placodon was determined for each pass along the tape, and the average was used as the number of fish per 200 m2 (50 × 4 m). A similar technique was used to estimate fish density at station 6 in 1978 and along the Nankumba Peninsula (stations 4–8) in 1980, but the fish were counted instead of videoed.
Snail density was determined monthly from February to December 2003 along the same transects and depths as described above. Three 1 × 1 m quadrants at each transect depth were carefully sampled by SCUBA divers using plastic buckets to remove the top 2–3 cm of sediment. This sediment sample was transferred to one plastic bag for each 1 × 1 m area. Samples were brought to the shore and carefully sorted for snails on location. The sediment was sieved through a wire mesh of 1.5 mm and the retained material systematically searched for live snails. The density of B. nyassanus is reported as number per meter squared. B. nyassanus were checked for cercarial shedding, while other snails were counted and preserved in 80% ethanol. To check for cercarial shedding, snails were transferred individually to small plastic beakers (12.5 ml) with 6 ml of water. Snails were exposed to light outside (not direct sunlight) for 2–3 hours before containers were visually inspected for the presence of cercariae using a dissecting microscope. Snails collected during the morning were checked for cercarial shedding on the same day; if snails could not be checked before 1400 hours, they were transferred to water and checked the following morning.
Urine samples were collected from school-aged children in 2003 between 1000 and 1400 during April around Nankumba Peninsula (stations 1–11, Fig. 1) and during October at Chizumulu (Kabuthu School, station 15) and Likoma (Mpima and Chilonga schools, stations 13 and 14) islands. Examination of urine samples followed the filtration technique of Plouvier et al. (1975). S. haematobium egg counts were expressed as the number per 10 ml. All infected children were offered treatment after the examination.
Snail and fish counts were logarithmically transformed (i.e., log10[x+1]) for the calculations, and then average values were transformed back to the original scale. For convenience, these back-transformed values are referred to as geometric means. We were able to compare data collected in this study with historical information collected at one of the stations in southern Lake Malawi (station 6, Fig. 1) in 1978 and at all the Cape Maclear stations (stations 4–8, Fig. 1) in 1980.
Results
The number of T. placodon per 200 m2 was much lower in 1991 than in 1978 (Stauffer et al., 1997) (Fig. 2). Stauffer et al. (1997) used the same technique for estimating fish density as reported herein, except they counted the fish observed instead of videoing them. Fishing was restricted somewhat by people living at the Fisheries Research Station (station 6) in 1998, and these efforts may be linked to the increased densities of T. placodon observed in 2003. Nevertheless, peak densities of T. placodon at stations 4–8 in 2003 were shifted to deeper waters when compared to observations made in 1980 (Fig. 3). Increased prevalence of the disease was coincident with a decrease in density of molluscivorous fish (Fig. 2). No B. globosus were found in the open waters of the lake. Abundance of B. nyassanus was relatively consistent between 3.0 and 7.6 m, with a decrease observed at 9.1 m (Fig. 3). B. nyassanus occurred at 16 of the 18 sites sampled in 2003, and abundance decreased with an increase in abundance of T. placodon (Fig. 4). Infection rates of school-aged children around the Nankumba Peninsula varied between 10.6% and 57.4% (Fig. 1a) and at Chizumulu and Likoma islands between 27.1% and 30.1% (Fig. 1b).
Discussion
Fishes from Lake Malawi comprise approximately 70% of all the animal protein consumed by Malawians; therefore, fishing pressure on inshore fishes is intense. Fish are particularly vulnerable to the artesian fishing gear when they are spawning or in shallow areas. We attribute the observed decrease in density of T. placodon (Figs. 2 and 3) to overfishing and the increased use of fine-meshed beach seines. During the 1970s and early 1980s, most fishing activity took place in offshore waters. As these fishing stocks became depleted, fishermen moved inshore with illegal fine-meshed nets. As these fishing pressures built, people started using beach seines and the fish in water less than 7 m were removed. The peak abundance of T. placodon then shifted to deeper waters, where they were not susceptible to beach seines (Fig. 3).
When Stauffer et al. (1997) showed that the decrease in the number of snail-feeding fishes was coincident with the increase in schistosomiasis in school-aged children, we postulated that B. globosus, the then only known intermediate host of S. haematobium, had invaded the predator-free areas of Lake Malawi. Subsequently, Madsen et al. (2001) discovered that B. nyassanus was also an intermediate host of human urinary schistosomes. Our data show that B. nyassanus is the only known intermediate host of human schistosomes that occurs in the open waters of the lake, including areas along sand beaches. Throughout the lake, we observed that as the density of T. placodon increases, the abundance of B. nyassanus decreases (Fig. 4). Human urinary schistosomes, however, are transmitted via other water sources (e.g., streams, ponds, swamps) in Malawi, where B. globosus resides; thus, any reduction of intermediate host snails (i.e., B. nyassanus) facilitated by an increase in molluscivorous fishes in the shallow waters of Lake Malawi will not completely eliminate the disease.
We hypothesize that before 1980 densities of B. nyassanus in the waters surrounding Chembe Village (stations 4–8) were so low that either it was not significantly involved in cercariae production or it was immune to infection by indigenous human schistosomes. The school-aged children residing in Chembe Village have the highest prevalence of urinary shistosome infection recorded in Malawi (>87% in 1991, Fig. 2) and currently have the highest we have measured (57.4%, Fig. 1a). Although B. nyassanus was collected at all sites including those at Chizumulu and Likoma islands, the only sites where we collected B. nyassanus infected with S. haematobium were at Chembe Village (stations 4–8). Therefore, we conclude that infection at the islands occurs only from inland water sources. The historical (1978) infection rate among school-aged children from Chembe Village (36%, Fig. 2), when B. nyassanus was not known to be a host, is comparable to 2003 infection rates of children residing on Chizumulu (27%) and Likoma (30.1%) islands (Fig. 1b). Therefore, we postulate that the current high human prevalence at Chembe Village is linked to B. nyassanus being an intermediate host in this portion of Lake Malawi and that a background infection rate of school-aged children from schistosomes released only from B. globosus is around 30%.
Because of transmission of S. haematobium from water sources other than the open waters of Lake Malawi, the reduction of intermediate host snails (i.e., B. nyassanus) in the shallow waters of Lake Malawi will not completely eliminate the disease. Data do not exist on the abundance of B. nyassanus in Lake Malawi prior to 1980; however, increased human density of Chembe Village since 1980 may have led to ecological changes that could have contributed to an increase in densities of B. nyassanus to levels high enough to actively transmit S. haematobium. Conversely, perhaps B. nyassanus was not an intermediate host of Lake Malawi’s indigenous strain of S. haematobium. It is conceivable that tourists from other parts of Africa who visited Chembe Village in increased numbers in the 1980s may have introduced a different strain of S. haematobium that was capable of using B. nyassanus as an intermediate host.
Certainly, chemotherapy campaigns at Chembe Village contributed to a decrease in infection levels of school-aged children from those observed in 1998 (Fig. 2); however, the fact that reinfection to the current high levels occurred rapidly is coincident with our theory that transmission at Chembe Village occurs in both inland waters and the open waters of Lake Malawi via B. globosus and B. nyassanus, respectively. Therefore, we believe an increase in the density of molluscivorous fishes will reduce cercariae production in the open waters of the lake. We further hypothesize that the elimination of cercariae production in the open waters of the lake will initiate a return to the low infection rates reported in 1978. The indigenous snail-eating fishes in Lake Malawi, as do crayfish in Kenya (Mkoji et al., 1999), can and did function as a biological control of intermediate hosts of urinary schistosomes.
References
Centers for Disease Control (2005) Health information for travelers to East Africa. Available: www.cdc.gov/travel/eafrica.htm [accessed May 12, 2005]
Chiotha SS, McKaye KR, Stauffer JR Jr. (1991a) Prey handling in Trematocranus placodon, a snail-eating cichlid fish from Malawi. Ichthyological Explorations in Freshwaters 2:203–208
Chiotha SS, McKaye KR, Stauffer JR Jr. (1991b) Use of indigenous fishes to control schistosome snail vectors in Malawi, Africa. Biological Control 1991:316–319
Feldmeier H, Krantz I, Poggensee G (1994) Female genital schistosomiasis as a risk factor for the transmission of HIV. International Journal of STD and AIDS 5:368–372
Harms G, Feldmeier H (2002) HIV infection and tropical parasitic diseases: deleterious interactions in both directions? Tropical Medicine and International Health 7:479–488
Madsen H, Bloch P, Kristensen TK, Furu P (2001) Bulinus nyassanus is intermediate host for Schistosoma haematobium in Lake Malawi. Annals of Tropical Medicine and Hygiene 95:353–360
Mkoji GM, Hofkin BV, Kuris AM, Stewart-Oaten A, Mungal BN, Kihara JH, et al. (1999) Impact of the crayfish Procambarus clarkia on Schistosoma haematobium transmission in Kenya. American Journal of Tropical Medicine and Hygiene 61:751–759
Plouvier S, Leroy JC, Colette J (1975) A propos d’une technique simple de filtration des urines dans le diagnostic de la bilharziose urinaire en enquete de masse. Medecine Tropicale 35:229–230
Rollinson D, Simpson AFG (1987) The Biology of Schistosomes: From Genes to Latrines, New York: Academic Press
Stauffer JR Jr, Arnegard ME, Cetron M, Sullivan JJ, Chtsulo LA, Turner GF, et al. (1997) Controlling vectors and hosts of parasitic diseases using fishes. Bioscience 47:41–49
World Health Organization (2002) Prevention and Control of Schistosomiasis and Soil Transmitted Helminthiasis: Report of a WHO Expert Committee, Technical Report Series 912, Geneva: World Health Organization
Wright ED, Chimphangwi J, Hutt MSR (1982) Schistosomiasis of the female genital tract: a histopathological study of 176 cases from Malawi. Transactions of the Royal Society of Tropical Medicine and Hygiene 76:822–829
Acknowledgments
We thank the government of Malawi for giving us permission to work in Lake Malawi and the University of Malawi for providing us with the proper permits to collect fishes and snails. Funding was provided by the NSF/NIH joint program in ecology of infectious diseases (DEB-0224958) and the World Wide Fund for Nature, Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stauffer, J.R., Madsen, H., McKaye, K. et al. Schistosomiasis in Lake Malawi: Relationship of Fish and Intermediate Host Density to Prevalence of Human Infection. EcoHealth 3, 22–27 (2006). https://doi.org/10.1007/s10393-005-0007-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-005-0007-3