Abstract
Purpose
To evaluate the effect of half-dose verteporfin photodynamic therapy (hPDT) on the physiology of the macula determined by focal macular electroretinograms (FMERGs) in eyes with chronic central serous chorioretinopathy (CSC).
Methods
Fourteen eyes of 13 patients with chronic CSC were treated with hPDT. The best-corrected visual acuity (BCVA) was measured, and optical coherence tomography (OCT) and FMERGs were performed at the baseline, and at 4 days, 1, 3, 6, and 12 months after the hPDT.
Results
The subreitnal fluid was resolved in 12 of the 14 eyes after the hPDT. The amplitude of the a-wave at 12 months was significantly increased by 1.28 times over that at the baseline. The amplitude of the b-wave was also increased but not significantly (P = 0.055). The implicit time of the a-wave was significantly reduced at 6 months, and that of the b-wave at 3 months. The amplitudes of the oscillatory potentials did not change significantly during the 12-month follow-up period.
Conclusions
hPDT led to an improvement in the FMERGs for at least 12 months without a transient depression of the FMERGs in eyes with chronic CSC. hPDT can be used safely to treat eyes with CSC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central serous chorioretinopathy (CSC) is a relatively common vision-threatening disease characterized by serous retinal detachments at the posterior pole with retinal pigment epithelial detachments (PEDs). Although the exact mechanism of the development of CSC is still not definitively determined, the current explanation is that CSC develops from an initial hyperpermeability of the choroidal vessels and not related to alterations in the retinal pigment epithelium (RPE). The hyperpermeability of the choroidal vessels leads to the formation of the PED, which causes a breakdown of the barrier function of the RPE. The breakdown then leads to leakage of fluid through the RPE into the subretinal space and a detachment of the neurosensory retina [1,2,3]. The natural course of acute CSC is self-limiting, and it resolves in 3–4 months either with or without changes in the RPE and photoreceptors as assessed by optical coherent tomography (OCT). However, some of the patients with CSC have a recurrence of the subretinal fluid and PED, and this condition is then defined as chronic CSC. Ultimately chronic CSC leads to RPE atrophy, formation of subretinal fibrin, disturbances in the photoreceptors, and a reduction of visual acuity [4,5,6,7]. Thus, to prevent permanent dysfunction of the macula, chronic CSC needs to be properly treated.
Laser photocoagulation is generally used to treat CSC with obvious focal leakage observed on fluorescein angiography (FA). However, CSC with subfoveal or parafoveal leakage points should not be treated with laser photocoagulation because of the complication of scotoma. In addition, iatrogenic choroidal neovascularization after laser photocoagulation for CSC is reported [8]. Recently, photodynamic therapy (PDT) with verteporfin has been used to treat chronic CSC, and it is reported that PDT can lead to a resolution of the subretinal fluid and improvement of the visual acuity [9, 10]. However, there are complications accompanying PDT such as secondary choroidal neovascularizaion, severe choroidal ischemia, and RPE atrophy [9,10,11].
To reduce the risk of PDT-induced complications, safety enhanced PDT, such as half-dose verteporfin PDT (hPDT), is reported to be effective with low incidences of complications during a one-year follow-up period [12,13,14]. However, the direct effect of hPDT on the physiology of the macula has not been determined.
Focal macular electroretinograms (FMERG) are used to assess the physiology of the macular area [15, 16]. A study from our laboratory reports that the functional impairments of the macula immediately after standard-dose PDT for age-related macular degeneration were correlated with the hypoperfusion of the choroidal vessels [17]. Therefore, we believe that assessments of the macular physiology after hPDT would provide information on the physiological status of the macula.
The purpose of this study was to evaluate the effect of hPDT on the physiology of the macular area in eyes with chronic CSC. To accomplish this, we retrospectively analyzed the records of FMERGs performed before and up to one year after the hPDT in eyes with chronic CSC.
Patients and methods
All the patients were informed on the procedures and the possible complications, and all signed an informed consent form before the hPDT. They also agreed to the recording of visual acuity, OCT, and FMERGs during the follow-up examinations. The treatment protocol conformed to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board Committee of Nagoya University Hospital.
We reviewed the medical records of 14 eyes of 13 patients who had been diagnosed with chronic CSC and received hPDT at the Nagoya University Hospital from July 2009 to May of 2010. The diagnosis of chronic CSC was based on the findings of FA, indocyanine green angiography (ICGA; Heidelberg Retina Angiography; Heidelberg Engineering GmbH, Heidelberg, Germany), and spectral-domain OCT (SD-OCT; Spectralis HRA+OCT; Heidelberg Engineering GmbH). CSC was diagnosed when a serous retinal detachment was present.
The inclusion criteria were: (1) presence of subretinal fluid involving the fovea for at least 3 months, (2) active leakage from the RPE on FA, and (3) hyperpermeablity of the choroidal vessels on ICGA within the macula where FMERG was performed. Some of the eyes in this study presented with recurrent CSC. Thus, the duration of symptoms may represent the entire period including recurrences. Eyes with other chorioretinal disorders that can produce subretinal exudations, e.g., choroidal neovascularization, were excluded. Patients who had already received laser photocoagulation, PDT, or intravitreal anti-vascular endothelial growth factor injections were also excluded.
The patients had comprehensive clinical examinations including FMERGs before the hPDT at the baseline, and at 4 days, and at 1, 3, 6, and 12 months after the hPDT.
Photodynamic therapy
hPDT treatment was performed as described in detail [17, 18]. A half-dose of verteporfin (3 mg/m2, Visudyne; Novartis AG, Basel, Switzerland) was injected intravenously over a 10-min. period. Fifteen min. after the beginning of the infusion, a 689-nm diode laser was used to deliver 600 mW/cm2 for at least 83 s. to produce a total energy dose of 50 J/cm2. The hPDT spot size was selected to cover the diameter of the hyperpermeable choroidal lesion in the images recorded in the mid-phase of ICGA. All patients had only one hPDT treatment even if a recurrence or resistance occurred during the follow-up period.
Best-corrected visual acuity (BCVA)
Measurements of the BCVA were made with the early treatment of diabetic retinopathy study (ETDRS) charts at 4 m at each visit.
Measurements of optical coherence tomographic (OCT) images
SD-OCT was performed at each visit, and the central macular thickness was measured in the SD-OCT images recorded with the 12-radial scan protocols. The enhanced depth-imaging technique was used to obtain clear images of the choroid. The subfoveal choroidal thickness was measured as the distance from outer border of the RPE to the inner sclera in the vertical and horizontal OCT images, and the values were averaged.
Focal macular electroretinograms (FMERGs)
Focal macular electroretinograms (FMERGs; ER-80; Kowa, Nagoya, Japan) were recorded before the hPDT, and on day 4, and months 1, 3, 6, and 12 after the hPDT as described in detail [19]. Briefly, a Burian-Allen bipolar contact lens electrode (Hansen Ophthalmic Development Laboratories, Iowa City, IA, USA) was used to pick-up the FMERGs. The stimulus spot size on the macula was 15° in diameter, and the background light covered nearly the entire visual field. The source of the background light was embedded in the camera (CF-60DSi; Canon, Tokyo, Japan). The luminance of the stimulus was 30 cd/m2, and that of the background was 1.5 cd/m2.
The responses were digitally band pass filtered from 5 to 500 Hz. Five hundred responses were averaged at a stimulation rate of 5 Hz (Neuropack S1 MEB-9400; Nihon Kohden, Tokyo, Japan).
The a-wave amplitude was measured from the baseline to the trough of the first negative wave, and the b-wave amplitudes from the first trough to the peak of following positive wave. The implicit times of the a-waves and b-waves were also measured to the peak of each wave. For the OPs amplitudes, the amplitude of each OP was measured from the trough to the peak (O1–O3), and the sum was used for the statistical analyses.
Statistical analyses
All statistical analyses were performed with SPSS ver. 23 (IBM-SPSS, Chicago, IL. USA). Shapiro-Wilk tests were used to determine whether the data were normally distributed. The mean ± standard error of the means of the data are presented. Repeated ANOVA followed by Dunnett’s multiple comparison tests were used for the normally distributed data, and the Friedman test was used for the non-normal distributed data. The differences were considered significant when P < 0.05.
Results
Fourteen eyes of 13 patients with chronic CSC underwent hPDT. The characteristics of the patients are presented in Table 1. One patient had chronic CSC in both eyes (patient’s number, 13). The mean age at the time of the hPDT was 52.2 ± 2.6 years with a range of 34 to 62 years. The average duration of the symptoms was 54.9 ± 24.5 months with a range of 3–360 months. The average spot size was 3578 ± 336 μm.
BCVA and OCT measurements
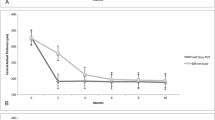
The mean BCVA before the hPDT was 74.1 ± 3.17 letters measured with ETDRS chart. The BCVA at 1 month after the hPDT was 77.8 ± 2.3 letters, which was significantly better than the pretreatment number (Tables 1, 2). At 12 months after the hPDT, the patients had an improvement in their BCVA by 10.1 ± 0.9 letters. None of the patients had a reduction in the BCVA or reported a central scotoma after the hPDT.
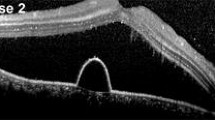
Optical coherence tomography images were examined to determine whether a SRD was present and to measure the thickness of central fovea and subfoveal choroid at each time point. The examinations showed that the subretinal fluid had resolved in 10 of the 14 eyes at 1 month after the hPDT, but a recurrence developed in one of these eyes (Fig. 1). In 3 other eyes, the subretinal fluid resolved in 3–6 months, and the one other eye had retention of the subretinal fluid over the 12-month follow-up period.
The foveal thickness was decreased significantly at month 1 compared to that of the pretreatment thickness (from 349.9 ± 28.5 μm to 184.1 ± 12.7 μm). Much of the decrease was due to the resolution of the subretinal fluid (Table 2). At 12 months after the hPDT, the foveal thickness was 191.9 ± 14.1 μm. None of the patients had retinal edema during the 12-month follow-up period.
The subfoveal choroidal thickness also decreased significantly from 421.4 ± 32.7 μm at the baseline to 346.8 ± 27.7 μm at month 1 after the hPDT (Table 2). The subfoveal choroidal thickness was reduced to about 20.3% of the baseline thickness at 12 months. Only one eye had a large PED at the subfovea at the baseline.
Changes in FMERGs
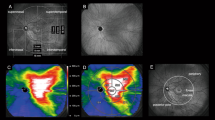
FMERGs were recorded to determine the physiology of the retina in a 15° area around fovea (Fig. 2a). The a-wave, b-wave, and oscillatory potentials (OPs) were analyzed (Fig. 2b). Representative OCT images showed that the subretinal fluid still remained on day 4 after the hPDT. The different components of the FMERGs had not improved on day 4 (Fig. 2c, d, Patient 13 L, Patient 2). The amplitude of the a- and b-waves of the FMERGs of patient 13 L had increased at 12 months and of patient 2 at 1 month. The one eye with a PED at the subfovea had a reduction of the a-wave amplitude by 56.3% and the b-waves amplitude by 7.9% of the baseline amplitude at day 4 compared to the baseline amplitudes; a-wave from 1.03 to 0.45 μV; b-wave, from 1.89 to 1.74 μV (Fig. 2e, Patient 11). However, the implicit times of the a-wave and b-wave were not prolonged in this case and this patient did not have a central scotoma or any reduction of the BCVA on day 4. At 12 months after the hPDT, the amplitudes and implicit times of a-wave and b-wave in all three cases had improved (Fig. 2c–e). The mean amplitude of the a-wave was 0.95 ± 0.12 μV and the b-wave was 2.02 ± 0.30 μV before the hPDT (Table 2). The amplitude of the a-wave improved significantly to 1.28 ± 0.09 times the baseline value at 12 months after the hPDT. The amplitude of the b-wave was also increased at 12 months but the increase was not significant (P = 0.055). The mean implicit time of the a-wave was significantly reduced from 24.8 ± 0.29 ms at the baseline to 23.8 ± 0.35 ms at 6 months and 23.9 ± 0.30 ms at 12 months. Similarly, the mean implicit times of the b-wave was reduced significantly from 45.81 ± 0.70 ms at the baseline to 44.08 ± 0.47 ms at 3 months, to 43.34 ± 0.51 ms at 6 months, and to 42.89 ± 0.43 ms at 12 months. The amplitudes and implicit times of the OPs did not change significantly.
Optical coherence tomographic (OCT) images and focal macular electroretinograms (FMERGs) of eyes with chronic CSC that responded favorably to hPDT. a The size of the stimulus to elicit the FMERGs was 15° (white circle). b FMERGs recorded from a normal eye. The upper line shows the a-wave and b-wave, and the lower line shows the oscillatory potentials (OPs). The sum of O1, O2, and O3 was used for the analyses. c–e Representative cases of chronic CSC treated with hPDT. The OCT images (left column) and FMERGs (right column) of each patient before treatment (Pre), day 4, month 1, and month 12 after the hPDT. The patient numbers are in accordance with those in Table 1. L Left eye
Discussion
The mechanism underlying the effectiveness of PDT is believed to be its ability to occlude pathological choroidal neovascular vessels as well as the normal choroidal vessels and choriocapillaris [20, 21]. Thus, the mechanism of the effect of hPDT for chronic CSC is assumed to provoke transient choriocapillaris hypoperfusion and choroidal vascular remodeling. Although the targeting of the hyperpermeable choroidal vasculature by PDT in eyes with CSC led to an immediate resolution of the subretinal fluid, several complications related to the PDT including choroidal ischemia and acute inflammation are reported [9, 10]. The results of earlier studies show that acute inflammatory reactions began almost immediately after PDT, and the subretinal fluid accumulation peaked in several days [22]. In addition, our group reported earlier that the amplitudes of the a- and b-waves of the FMERGs were reduced one week after PDT for eyes with age-related macular degeneration. The reduction was correlated with the reduction of choroidal circulation, and both the amplitude of the FMERGs and choroidal circulation improved to the pre-PDT level in 1–3 months [17].
In this study, we found that the hPDT did not depress the different components of the FMERGs especially in the acute phases on day 4 and 1 month after the hPDT. In addition, none of the patients reported a transient scotoma. Thus, hPDT appears to be a better treatment for eyes with chronic CSC.
Several studies on the use of hPDT in eyes with CSC that report that some patients developed a transient scotoma after hPDT. Lai, et al. report that two of 20 patients developed a transient scotoma within the first 2 days after hPDT. They also report that eyes with PED in CSC patients had a transient reduction in the multifocal ERGs at 4 days after hPDT, whereas eyes without a PED did not have the transient reduction after the hPDT [12]. In our study, one eye with a PED and serous retinal detachment at the subfovea before the hPDT had a transient reduction in the amplitudes of the a- and b-waves. Even though the amplitudes of the a- and b-waves improved to the baseline at 1 month, the amplitudes of both waves had not improved completely at 12 months after the hPDT. In addition, the implicit times of the a- and b-waves were also reduced after the hPDT. These findings suggest that in CSC with a subfoveal PED there might be differences in pharmacokinetics. Therefore, we need to remember that retinal function after hPDT for eyes with chronic CSC associated with a subfoveal PED may not completely improve.
Our results show that there were significant improvements in the different components of the FMERGs at one year after the hPDT suggesting improvements in macular function. We found that the implicit times of the a- and b- waves were reduced in a relatively short time after the hPDT accompanying the resolution of the subretinal fluid, which suggests the improvement of retinal functions. In other words, the physiology of the outer and inner retina can improve after both the remaining RPE and photoreceptors regain their cellular connections. However the amplitudes of the a- and b-waves required a longer time to improve compared to the implicit times. In addition, the amplitudes of the OPs did not improve significantly throughout the 12-month follow-up period. However, we followed the patients for only 12 months and the number of patients in this study was small. Thus, it might take a longer time to improve the amplitude of OPs. We had suggested earlier that the functional disturbances related to CSC may involve the inner retina as well as the photoreceptors [23]. We suggest that the disturbances of the inner and outer retina by long existing subretinal fluid are severe, and some neuronal cells that were severely damaged may have become permanently damaged. Thus, it may take a longer time for the macula to recover with an improvement of the amplitudes of the a- and b-waves and the OPs.
There are limitations in this study. The number of patients was small and a control group was not examined. The background of the patients, particularly the disease duration varied widely because some recurrences occurred without subjective symptoms.
In conclusion, we found that hPDT for chronic CSC improves the BCVA and FMERGs without severe dysfunction of macula in the early postoperative period. In addition, the macular function observed by FMERGs improved after reattachment of neurosensory retina. These results provide clinical evidence that hPDT can be good treatment option for chronic CSC.
References
Donald J, Gass M. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63:587–615.
Guyer DR, Yannuzzi LA, Slakter JS. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–62.
Piccolino FC, Borgia L, Zinicola E, Zingirian M. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye. 1995;9:324–32.
von Rückmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol. 2002;133:780–6.
Loo RH, Scott IU, Flynn HW, Gass JD, Murray TG, Lewis ML, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22:19–24.
Iida T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999;19:508–12.
Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103:2070–9.
Nomura Y, Obata R, Yanagi Y. Intravitreal bevacizumab for iatrogenic choroidal neovascularization due to laser photocoagulation in central serous chorioretinopathy. Jpn J Ophthalmol. 2012;56:245–9.
Chan WM, Lam DSC, Lai TYY, Tam BSM. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003;87:1453–8.
Piccolino FC, Eandi CM, Ventre L, de la Longrais RRC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003;23:752–63.
Lee PY, Kim KS, Lee WK. Severe choroidal ischemia following photodynamic therapy for pigment epithelial detachment and chronic central serous chorioretinopathy. Jpn J Ophthalmol. 2009;53:53–6.
Lai TYY, Chan WMM, Li H, Lai RY, Liu DT, Lam DS. Safety enhanced photodynamic therapy with half dose verteporfin for chronic central serous chorioretinopathy: a short term pilot study. Br J Ophthalmol. 2006;90:869–74.
Chan WMM, Lai TYY, Lai RY, Tang EW, Liu DT, Lam DS. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina. 2008;28:85–93.
Fujita K, Imamura Y, Shinoda K, Matsumoto CS, Mizutani Y, Hashizume K, et al. One-year outcomes with half-dose verteporfin photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology. 2015;122:555–61.
Miyake Y, Awaya S. Stimulus deprivation amblyopia. Simultaneous recording of local macular electroretinogram and visual evoked response. Arch Ophthalmol. 1984;102:998–1003.
Miyake Y, Shiroyama N, Ota I. Oscillatory potentials in electroretinograms of the human macular region. Invest Ophthalmol Vis Sci. 1988;29:1631–5.
Ishikawa K, Kondo M, Ito Y, Kikuchi M. Correlation between focal macular electroretinograms and angiographic findings after photodynamic therapy. Invest Ophthalmol Vis Sci. 2007;48:2254–9.
Uetani R, Ito Y, Oiwa K, Ishikawa K, Terasaki H. Half-dose vs one-third-dose photodynamic therapy for chronic central serous chorioretinopathy. Eye. 2012;26:640–9.
Hibi N, Ueno S, Ito Y, Piao C-HH, Kondo M, Terasaki H. Relationship between retinal layer thickness and focal macular electroretinogram components after epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2013;54:7207–14.
Schlötzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002;240:748–57.
Michels S, Schmidt-Erfurth U. Sequence of early vascular events after photodynamic therapy. Invest Ophthalmol Vis Sci. 2003;44:2147–54.
Costa RAA, Farah ME, Cardillo JAA, Calucci D, Williams GA. Immediate indocyanine green angiography and optical coherence tomography evaluation after photodynamic therapy for subfoveal choroidal neovascularization. Retina. 2003;23:159–65.
Miyake Y, Shiroyama N, Ota I, Horiguchi M. Local macular electroretinographic responses in idiopathic central serous chorioretinopathy. Am J Ophthalmol. 1988;106:50.
Acknowledgements
We thank Professor Duco Hamasaki of the Bascom Palmer Eye Institute for editing the final version of the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research B (20390448; 23390401; 15H04994, HT) from the Japan Society for the Promotion of Science.
Conflicts of interest
K. Oiwa, None; K. Kataoka, Lecture fees (Bayer Health Care, Novartis Pharma, Santen Pharmaceutical); R. Maruko, Lecture fees (Bayer Health Care, Novartis Pharma, Santen Pharmaceutical); S. Ueno, Grants (Santen Pharmaceutical), Lecture fees (Novartis Pharma, Pfizer Japan); Y. Ito, Lecture fees (Aichi Ophthalmologists Association, Bayer Health Care, Canon Life Care Solutions, Carl Zeiss Meditec, Kowa Pharmaceutical, Novartis Pharma, Okazaki City Medical Association, Pfizer Japan, Santen Pharmaceutical); H. Terasaki, Grants (Alcon Japan, HOYA, Kowa Pharmaceutical, NIDEK, Novartis Pharma, Otsuka Pharmaceutical, Pfizer Japan, Santen Pharmaceutical, Senju Pharmaceutical, Wakamoto), Consultant fees (Bayer Health Care, Ono Pharmaceutical), Lecture fees (Aichi Ophthalmologists Association, Alcon Japan, Astellas Pharma, Bayer Health Care, Carl Zeiss Meditec, Chiba Ophthalmologist Association, Fukushima Ophthalmologist Association, Japan Medical Association, Kowa Pharmaceutical, NIDEK, Nitten Pharmaceutical, Novartis Pharma, Otsuka Pharmaceutical, Pfizer Japan, Santen Pharmaceutical, Sanwa Kagaku Kenkyusho, Senju Pharmaceutical, Takeda Pharmaceutical, Wakamoto), Travel expenses (Bayer Health Care, Chiba Ophthalmologist Association, Fukushima Ophthalmologist Association, Japan Medical Association), Rohto Award Selection committee (Rohto Pharmaceutical), Writing assistance (Nitten Pharmaceutical).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Oiwa, K., Kataoka, K., Maruko, R. et al. Half-dose photodynamic therapy for chronic central serous chorioretinopathy evaluated by focal macular electroretinograms. Jpn J Ophthalmol 61, 260–266 (2017). https://doi.org/10.1007/s10384-017-0498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-017-0498-9