Summary
Background
The diffusion of the use of robotic surgical platforms, such as the da Vinci Xi Surgical System® (Intuitive Surgical, Sunnyvale, CA, USA), has been advocated by several authors to overcome the limitations of laparoscopy in hepatobiliary surgery.
Methods
We reported our experience of robot-assisted fenestration of giant hepatic cysts in posterosuperior segments with the use of indocyanine green fluorescence imaging. We described step by step our surgical technique including the operative room set-up, port placement and robotic instruments.
Results
We enrolled 11 patients: nine females and two males with a mean age of 65 years (range 52–80 yrs). All procedures were undertaken successfully without intraoperative or postoperative complications. The mean surgical operating time was 125 min. The mean blood loss was 30 ml. The median postoperative stay was two days (range, 1 to 3 days).
Conclusions
The most significant advantage of the robotic approach was the ability to access hepatic cysts close to the diaphragm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Simple, non-parasitic cysts represent the most common benign hepatic lesions, affecting up to 5% of the general population [1]. Several treatments have been proposed for symptomatic hepatic cysts. Laparoscopic fenestration is currently the gold standard due to its minimally invasive nature, short-term benefits and fast recovery times [2, 3]. However, long-term recurrence rates range from 14.9 to 25%, affecting most frequently the right posterior segments (S7, S8), due to their proximity to the diaphragm, which in some cases hinders adequate deroofing of the cystic wall [4].
Robotic surgical platforms, such as the da Vinci Xi Surgical System® (Intuitive Surgical, Sunnyvale, CA, USA), may overcome the limitations of laparoscopy by providing advances such as 3D vision, wristed instruments with seven degrees of freedom, and improved ergonomics. Despite the potential benefits of this technique, only few cases of robotic-assisted fenestration of hepatic cysts have been reported in the literature [5, 6]. We hereby report our surgical experience.
Materials and methods
We described step by step our surgical technique including the operative room set-up, port placement and robotic instruments. We also reported our experience of robot-assisted fenestration of giant hepatic cysts with the use of indocyanine green fluorescence (ICG) imaging.
Preoperative study
Indications for surgery included the presence of symptoms correlated with radiological findings by means of computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) to exclude the presence of neoplastic nodules inside the cysts. All patients underwent serology tests for Echinococcus and Entamoeba. ICG was administered one hour before surgery at a dose of 0.2 mg/kg of body weight.
Operative room set-up
The patients were placed in supine position with legs apart and both arms adducted. The operating table was placed in reverse Trendelenburg position with a right tilt (20°). The robotic cart was located at the patient’s left side and the docking set-up was in the upper abdomen. The viewing tower was positioned at the patient’s right shoulder, while the ultrasound machine was located at the patient’s feet. A laparoscopic assistant surgeon was standing between the legs. The scrub nurse was at the right side of the assistant (Fig. 1).
Port placement
We routinely induced pneumoperitoneum at 12 mm Hg through a Veress needle inserted at the Palmer’s point. Trocars were placed as follows: the optical port (8 mm) was placed on the right mid-clavicular line along the transverse umbilical line; the other three operative trocars (8 mm) were placed in a straight line, two on the right and one on the left side. The trocars on the right were used for the surgeon’s right hand (monopolar curved scissor or Vessel Sealer®) and for the surgeon’s third hand (Cadier forceps). The left unit was used for the surgeon’s left hand (fenestrated bipolar forceps). One additional assistant trocar (12 mm or 5 mm) was generally placed in the lower abdomen between two robotic trocars (Fig. 2). The assistant port was frequently used for retraction, aspiration and extraction.
Operative trocar’s position. Four robotic trocars (blue dots) were placed along the transverse umbilical line: n. 1: in the right flank for the fenestrated bipolar forceps (surgeon left hand). n. 2: in the right middle clavicular line (MCL) for the endoscope (optic trocar). n. 3: in the midline for the monopolar curved scissors and vessel sealer (surgeon right hand). n. 4: in the left flank for the Cadiere forceps (surgeon third hand). Two laparoscopic assistant 10 mm trocars (white dots) were placed in the hypogastrium and used to introduce the US probe and the suction/irrigation system
Surgical technique
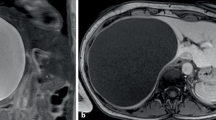
Surgical technique was standardized for all cases. Cystic margins were delineated using a robotic ultrasound probe (Hitachi Aloka Medical Ltd) and ICG fluorescence. EndoWrist technology (Vessel Sealer®) was used for cystic wall fenestration; the cystic dome was excised as close as possible to the hepatic parenchyma to reduce the risk of bleeding (Fig. 3). ICG was used to identify intramural compressed bile ducts (Fig. 4a, b c). A drainage was routinely placed at the end of the procedure and removed on postoperative day 3 in all cases.
Robot-assisted fenestration of a giant hepatic cysts in poster superior segments. a, b Hepatic cyst of the right posterior-superior segment tightly linked to the diaphragmatic muscle. c Complete deroofing of the superior cystic wall. d ICG staining of hepatic parenchyma which results completely exposed after the removal of cystic wall
Postoperative follow-up
Patients underwent follow-up by means of a surgical visit and an abdominal ultrasound at one, six and twelve months after surgery.
Results
Between January 2022 and February 2023 we enrolled 11 consecutive patients undergoing robotic fenestrations of giant hepatic cysts of posterosuperior segments (S7, S8, S4a). Our study includes nine females and two males, with a mean age of 65 years (range 52–80 yrs). Patient’s demographic and clinical characteristics are shown in Table 1. ICG was adopted in all cases and allowed identification of cystic margins and avoidance of bile duct injury. All procedures were conducted successfully, without intraoperative or postoperative complications. Mean operating time was 125 min, median blood loss was 30 ml and median post-operative stay was two days (range 1–3 days) (Table 2). Pathologic examination revealed a benign liver cyst in all the resected specimens. No patients developed symptoms or radiological signs of recurrence at six months.
Discussion
Many authors have reported on the routine use of indocyanine green fluorescence (ICG) in hepatobiliary surgery [7]. Near infrared fluorescent cholangiography (NIRF-C) represents a powerful real-time diagnostic tool for the detection of biliary anatomy during laparoscopic cholecystectomy (LC). Substantial evidence has proved the importance of routine use in elective LC to decrease bile duct injury and conversion to open surgery. NIRF‑C could also be used for the detection of bile leakage after hepatic resection [8,9,10].
Recently, several authors have advocated the use of ICG fluorescence to avoid bile duct injury during laparoscopic liver cyst fenestration [11,12,13].
In our experience, ICG was injected intravenously one hour before surgery, because the maximum concentration of ICG dye in bile has been observed within two hours from administration. ICG fluorescence was retained in the liver parenchyma and in the biliary tract, but not in the cystic wall, allowing a real-time assessment of surgical margins between the liver parenchyma and the cystic wall. Therefore, ICG proved to be useful to delineate the cystic margins and to reveal intramural compressed bile ducts within the cystic wall, aiding in the avoidance of accidental bile duct injury, which represents the main complication of hepatic cyst fenestration.
In literature, there are only a few cases of robot-assisted fenestration of hepatic cysts [14,15,16]. Reported advantages of the robotic approach are the three-dimensional view and the possibility to use an instrument with a sealing capability of vessels up to 7 mm in diameter (Vessel Sealer®) [14,15,16]. Tsirlis T et al. [15] reported the largest case series, enrolling seventeen patients in a single center prospective study. In this case series, the majority of the cysts (5/6) were located in the posterosuperior or central segments (VII, VIII, and IVa). In our small case series, there were no cases of conversion and intraoperative blood loss was minimal. None of the eleven patients developed any complications. During the follow-up period of 12 months, all patients presented without any type (symptomatic or radiological) of recurrence. Despite the absence of complications and recurrence in our case series, there is no actual evidence in the literature that robotic fenestration provides a lower recurrence rate compared to the laparoscopic approach. The robotic approach can decrease the learning curve and increase the application of minimally invasive surgery. In all cases, surgery was performed by a young hepatobiliary (HPB) surgeon; we believe that robotic fenestration of hepatic cysts may constitute an ideal tool in the training of young HPB surgeons.
References
Kaltenbach TE, Engler P, Kratzer W, et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol (ny). 2016;41(1):25–32. https://doi.org/10.1007/s00261-015-0605-7.
Chen A, Cai C, Fu Q, Wang X. Safety and efficacy of laparoscopic hepatectomy versus open hepatectomy for giant hepatic cysts: a systematic review and meta-analysis. Transl Cancer Res. 2022;11(5):1230–44. https://doi.org/10.21037/tcr-22-910.
Chandok N. Polycystic liver disease: a clinical review. Ann Hepatol. 2012 Nov-Dec;11(6):819–26.
Drenth JP. ChrispijnM, Nagorney DM, Kamath PS, Torres VE. Medical and surgical treatment options for polycystic liver disease. Hepatology. 2010;52(6):2223–30. https://doi.org/10.1002/hep.24036.
Ardito F, Bianco G, Vellone M, et al. Long-term outcome after laparoscopic fenestration of simple liver cysts. Surg Endosc. 2013;27:4670–4.
Kisiel A, Vass DG, Navarro A, et al. Surg Laparosc Endosc Percutan. tech. 2017;27(4):e80–e2. https://doi.org/10.1097/SLE.0000000000000441.
Potharazu AV, Gangemi A. Indocyanine green (ICG) fluorescence in robotic hepatobiliary surgery: A systematic review. Int J Med Robot. 2023;19(1):e2485. https://doi.org/10.1002/rcs.2485.
Dip F, LoMenzo E, Sarotto L, et al. Randomized Trial of Near-infrared Incisionless Fluorescent Cholangiography. Ann Surg. 2019;270(6):992–9. https://doi.org/10.1097/SLA.0000000000003178.
Ladd AD, Zarate Rodriguez J, Lewis D, Warren C, Duarte S, Loftus TJ, Nassour I, Soma D, Hughes SJ, Hammill CW, Zarrinpar A. Low vs Standard-Dose Indocyanine Green in the Identification of Biliary Anatomy Using Near-Infrared Fluorescence Imaging: A Multicenter Randomized Controlled Trial. J Am Coll Surg. 2023 Apr 1;236(4):711–717. https://doi.org/10.1097/XCS.0000000000000553.
Sakaguchi T, Suzuki A, Unno N, et al. Bile leak test by indocyanine green fluorescence images after hepatectomy. Am J Surg. 2010;200(1):e19–23. https://doi.org/10.1016/j.amjsurg.2009.10.015.
Kitajima T, Fujimoto Y, Hatano E, et al. Intraoperative fluorescent cholangiography using indocyanine green for laparoscopic fenestration of nonparasitic huge liver cysts. Asian J Endosc Surg. 2015;8(1):71–4. https://doi.org/10.1111/ases.12137.
Hanaki T, Yagyu T, Uchinaka E, et al. Avoidance of bile duct injury during laparoscopic liver cyst fenestration using indocyanine green: A case report. Clin Case Rep. 2020 Jun 10;8(8):1419–1424. https://doi.org/10.1002/ccr3.2840.
Shimagaki T, Itoh S, Toshida K, et al. Prevention of bile duct injury using indocyanine green fluorescence in laparoscopic liver cyst fenestration for giant liver cyst: a case report. J Surg Case Rep. 2022 Oct 19;2022(10):rjac479. https://doi.org/10.1093/jscr/rjac479.
Nota CL, Molenaar IQ, Borel Rinkes IH, Hagendoorn J. Robot-assisted Laparoscopic Fenestration of Giant Hepatic Cysts. Surg Laparosc Endosc Percutan Tech. 2015;25(5):e163–5. https://doi.org/10.1097/SLE.0000000000000193.
Tsirlis T, Thakkar R, Sen G, et al. Robotic fenestration of massive liver cysts using EndoWrist technology. Int J Med Robot. 2019;15(4):e1994. https://doi.org/10.1002/rcs.1994.
Madha ES, Mateo RB, Hawksworth JS. Identification of biliary duct branches with indocyanine green during robot-assisted laparoscopic hepatic cyst fenestration. ANZ J Surg. 2022;92(11):3061–2. https://doi.org/10.1111/ans.17527.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this articl
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G. Piccolo, M. Barabino, F. Lecchi, R. Masserano and P.P. Bianchi declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Video of a robot-assisted fenestration of a giant hepatic cysts in poster superior segments
Rights and permissions
About this article
Cite this article
Piccolo, G., Barabino, M., Lecchi, F. et al. Robot-assisted fenestration of giant hepatic cysts in posterosuperior segments. Eur Surg (2024). https://doi.org/10.1007/s10353-024-00834-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10353-024-00834-1