Summary
The treatment elements used for pancreatic ductal adenocarcinoma (PDAC) include surgical resection, systemic cytotoxic agents, and targeted drugs. For second- and third-line therapies in PDAC, approximately 15% of patients have actionable mutations although only 2.5% receive matched targeted treatment but with a significant improvement in survival of around 16 months. For the majority of PDAC patients the current most effective strategy is surgical resection of the primary tumor and systemic combination chemotherapy. The chemotherapy regimens and the order of delivery relative to the resection reference point have been based to a large extent on randomized trials using a newly developed empirical staging (Em) system. Although the reductionist TNM based AJCC and UICC systems work well for pathology staging, they are less accurate and less manageable for treatment decision-making. This Em system defines locally resectable (EmR), borderline resectable (EmBR), and unresectable (EmUR) stages, plus the emerging entity of oligometastatic disease (EmOm). For EmR patients, 6 months of adjuvant chemotherapy achieves 5‑year survival rates of 30–50%. In EmBR short-course (2 months) neoadjuvant plus 6‑month adjuvant chemotherapy increases 12-month survival rates to around 77%, compared to 40% for upfront surgery, despite resection rates of 64–85% and 75%, respectively. Longer-course (4 months) neoadjuvant chemotherapy has also been shown to achieve an 18-month overall survival of 67%. In EmUR, induction therapy (3–6 months) may result in resections rates of 20–60% with significantly improved survival rates compared to no resection. For all stages including the polymetastatic (EmPm) setting, patients with good performance status receive combination chemotherapies based on either oxaliplatin (FOLFIRINOX or NALIRIFOX) or gemcitabine (GEM-CAP, or Gem-NabP). Molecular subtypes (Moffitt, Collisson, Bailey, and Cheng-Sen-Yue) are shown to be associated with treatment responses. Transcriptomic signatures have also been developed as classifiers for determining either oxaliplatin- or gemcitabine-based therapies (PurIST, Tiriac, GemPred+, and ESPAC) and are being evaluated in various studies. Most notably the ESPAC transcriptomic signature is being used as the treatment classifier in the experimental arms of the randomized ESPAC6 adjuvant trial in EmR patients and the ESPAC7 induction therapy trial in EmUR patients. Genomic and transcriptomic profiling at baseline and over time is an integral part of ESPAC6/7 to deepen our understanding of tumor plasticity during the course of therapy, identifying the intrinsic (persister cell) and acquired (genetic) tumor plasticity evolving over time and in reaction to different therapies in order to enable a scientific approach to overcoming clonal-resistance clades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal cancers with a 5-year relative survival of 11% [1]. The late stage of diagnosis, rapid progression, and resistance to systemic therapies are the most important factors that are attributed to the low survival rate. Nevertheless, the past two decades have witnessed momentous strides in combating this relentless disease. The advent of improved surgical techniques combined with multimodal systemic chemotherapy and the introduction of empirical staging systems, centered on the assessment of resectability, have emerged as pivotal tools in helping to improve survival [2,3,4,5,6]. By contrast, few patients benefit from targeted therapies and for only a limited survival period [7]. Primary surgical resection in localized PDAC is now achieved in 15–20% of patients as well as secondary resection after neoadjuvant or induction chemotherapy in many of the 30–35% of patients with locally advanced disease [3, 7]. The integration of adjuvant multiagent chemotherapy after primary resection has significantly improved prognosis from 10% or less with surgery ± chemoradiotherapy to 30–50% with combination regimens, depending on patient selection [8,9,10,11,12,13]. The pivotal proof-of-concept study was the first European Study Group for Pancreatic Cancer (ESPAC) trial using adjuvant 5‑fluorouracil (5-FU) monotherapy and the 5‑FU enhancer folinic acid (FA; [8, 9]). The ESPAC3 showed that while there was less toxicity with adjuvant gemcitabine, it did not improve the overall survival of 5‑FU/FA [11]. Combination regimens have increased survival even longer. The evolution in adjuvant chemotherapy regimens has been developed to more potent combination therapies such as gemcitabine plus capecitabine (GEMCAP) with a 5-year survival rate of 30% in unselected patients including those aged over 80 years, and in younger patients with more stringent criteria a 5-year survival rate of 50% has been achieved using modified FA, 5‑FU, irinotecan, and oxaliplatin (mFOLFIRINOX) albeit with greater toxicity [12, 13].
Despite significant strides in achieving higher resectability and improving survival outcomes through advanced surgical techniques and adjuvant chemotherapy, the prospect of long-term survival remains elusive for most patients with PDAC. While empirically driven systemic cytotoxic drugs and reductionist-driven targeted therapies show promise in patients with advanced-stage and metastatic PDAC, they have yet to yield major breakthroughs in extending survival [3,4,5,6,7]. More recently neoadjuvant therapies have garnered immense interest in the PDAC landscape [3]. The goal is to extend the survival of patients with resectable tumors and to increase resection rates and survival rates in those with borderline-resectable tumors, even exploring possibilities in selected patients with locally advanced tumors and/or oligometastatic disease [3].
With the development of sequencing technology, several molecular classifications of PDAC tumors based on transcriptomic or whole-genome sequencing have been identified in the past 12 years aiming to better understand treatment responses and hence treatment selection, which has been shown to be associated with treatment responses (Fig. 1; [14,15,16,17]). While molecular subtypes enable a broad phenotypic classification, they are too broad at present to permit more direct treatment allocation, and several transcriptomic signatures have been developed, serving as classifiers to distinguish between oxaliplatin- or gemcitabine-based therapies, notably PurIST, Tiriac, GemPred+, and ESPAC ([18,19,20]; NCT05314998). These signatures are currently under evaluation in various research studies and differ significantly in their mode of development: The PurIST signature derived from various types of tumor samples from 321 patients and has 16 genes defining FOLFIRINOX; the Tiriac signature has 138 genes defining gemcitabine and 98 genes defining oxaliplatin derived from 60 organoids; and the GemPred+ has 420 genes derived from 38 primary cell cultures defining gemcitabine treatment [18,19,20]. The ESPAC signature is unique in using RNASeq data from standardized fresh frozen PDAC tumor samples that have undergone epithelial cell enrichment, defining gemcitabine-based and oxaliplatin-based therapies [NCT05314998].
The traditional view of basing the treatment of pancreatic cancer is to use a reductionist pathological staging systems such as the Union for International Cancer Control (UICC) American Joint Committee on Cancer (AJCC) TNM systems, avoiding surgery in patients in whom an R0 resection cannot be achieved, to consider the tumor as a fixed phenotype, and to give all patients what seems to be the most effective systemic chemotherapy—presently mFOLFIRINOX [21].
Empirical staging: assessment of resectability
The UICC and AJCC TNM staging systems are commonly used to classify the pathological disease stages of PDAC based on tumor, nodes, and metastasis criteria. While this system is effective for pathological and radiological assessments, it has limitations in accurately determining maximum tumor diameter, lymph node involvement, and small metastases [22, 23].
More importantly, PDACs are genetically and biologically heterogeneous, and even using the simplest dichotomized molecular classification subtypes (basal-like and classic-like) there are considerable differences across all of the TNM stages, as well as PDAC neighborhood subtypes and responses to therapy [14,15,16,17, 24]. The TNM pathology systems used in PDAC have been based on general (assumed a priori and reductionist) oncological principals. There is a shortfall, however, in this reductionist approach, as in clinical practice the TNM does not completely match decision-making (resection being the treatment nodal point), nor the biological nature of PDAC at the diagnostic start point and the subsequent biological behavior in response to time (tumor evolution and acquired mutational resistance) and interventions including surgery and chemotherapy (enrichment of intrinsic persister cell resistance). Thus, an empirically derived staging (Em), based on practical (a posterior) experience, is an alternative approach, with an unbiased discovery scope [3].
Surgical resectability is determined practically using empirical staging criteria established by various organizations (Table 1), including the Americas Hepato-Pancreato-Biliary Association, the Society of Surgical Oncology, the Society for Surgery of the Alimentary Tract (AHPBA/SSO/SSAT), The University of Texas MD Anderson Cancer Center, the Alliance for Clinical Trials in Oncology, and the National Comprehensive Cancer Network (NCCN; Fig. 2a–c; [21, 23, 25, 26]). Each of these criteria sets characterizes the anatomy of the primary tumor and its relationships with crucial blood vessels such as the superior mesenteric vein, portal vein, superior mesenteric artery (SMA), common hepatic artery, its first-order branches, and the celiac trunk (Table 1). They can be applied to stage tumors located in the head, body, or tail of the pancreas. However, there are differences in how radiologists, surgeons, and multidisciplinary teams interpret and utilize each system for staging tumors. To ensure consistency and accuracy, it is essential to clearly report the staging criteria used and rigorously apply them as a condition for patient enrollment in clinical trials. Most of trials such as ESPAC‑5 and Alliance A021101, A021501, and A021806 have stricter enrollment criteria ([26,27,28]; NCT04340141). They mandate prospective centralized specialized review of the staging images of each patient, ensuring that all enrolled patients meet the stringent staging requirements.
Empirical staging shown on preoperative computed tomography (CT) scans (a–c); resection photographs demonstrating the triangle procedure for EmUR without hepatic portal vein (HPV) resection (d) or with HPV and superior mesenteric vein (SMV) resection and an interposition graft (e); and preservation of the celiac axis (CA), left gastric artery and common hepatic artery (CHA), using the divestment technique, all of which were encased as shown by the preoperative CT scan in the inset (f)
To include other important prognostic factors, such as the inherent biological characteristics of the tumor, the physiological condition of the patient, or radiographically occult disease that might not be detectable with routine imaging protocols, the MD Anderson system takes a more comprehensive approach by incorporating additional factors such as serum carbohydrate antigen 19‑9 (CA19-9) levels and performance status, in addition to tumor anatomy [29, 30]. This system has been integrated into international consensus criteria to facilitate more nuanced prognosis assessments. Biological staging of potentially resectable PDACs focuses on a subset of tumors that are anatomically potentially resectable (EmBR, EmUR, EmOm) but may exhibit clinical features suggestive of occult distant polymetastatic disease (EmPm). These worrying features may include a serum CA19‑9 level > 500 kU/L or the presence of regional lymph node metastases diagnosed through biopsy sampling and/or positron emission tomography–computed tomography (PET-CT). Considerable further refinement of this concept is required as studies from France and Japan have shown that the biological aspect of these criteria has limited clinical impact [31, 32]. Therefore, the investigation into biological staging and its relationship to tumor plasticity and acquired and inherent resistance continues to be a critical focus of research [3].
Surgery and cytotoxic therapies in empirically staged tumors
Over the past 50 years numerous clinical trials have been conducted in pancreatic cancer. The majority of these studies have been phase I/II trials, evaluating the potential survival benefits of newer agents and various treatment approaches, including chemoradiation. Unfortunately, many of these trials have not yielded successful outcomes. Currently, clinicaltrials.gov lists over 3000 registered PDAC trials, with 1099 in phase I, 1441 in phase II, and 306 in phase III. Among them, 254 trials are neoadjuvant, of which 23 are in phase III. The EU Clinical Trials Register shows 487 PDAC trials, with 87 in phase III, including 10 neoadjuvant trials.
Given the vast amount of information available, the focus should be on well-conducted phase II/III randomized trials that include appropriate control arms. Combination chemotherapy remains the mainstay of systemic treatment for PDAC with no role for chemoradiation regarding survival, at least in the adjuvant setting. Interestingly, only a relatively small number of agents and combinations have demonstrated sufficient efficacy to gain regulatory approval or become standard-of-care options [3]. An overview of adjuvant, neoadjuvant, and induction chemotherapy based on empirical staging is illustrated in Fig. 3.
Empirical resectable PDAC
The standard of care for EmR PDAC remains upfront resection followed by 6 months of adjuvant chemotherapy but there is no survival advantage to using chemoradiotherapy, resulting only in additional toxicity (Table 2, 2.1). A ceiling in optimal survival has now been reached with combination chemotherapies and is unlikely to be improved without radically new effective therapies, and/or more intelligent utilization of existing cytotoxic drugs based on the actual biology of the tumor at the point of treatment. When comparing the overall survival outcomes of adjuvant trials it is very important to consider the different selection eligibility criteria as well as the toxicity of each regimen [50]. The APACT study (which failed its primary endpoint and hence adjuvant GEM-NabP is not approved by the FDA) only included patients with an Eastern Cooperative Oncology Group performance status of ≤ 1 and a serum 19‑9 level of < 100 kU/L and PRODIGE24 was restricted to patients ≤ 79 years, with a WHO performance score of 0/1, no significant cardiovascular disease, and a serum CA19‑9 level of < 180 kU/L, whereas ESPAC4 had none of these restrictions [12, 23, 44]. These discrepancies are reflected in the notable differences in overall survival for the same gemcitabine control arms used in these studies with overall median (95% CI) survival rates of 37.7 months (range: 31.1–40.5) for APACT, 35.0 months (range: 28.7–43.9) for PRODIGE24, and 25.5 months (range: 22.7–27.9) for ESPAC4 [12, 23, 44]. Thus, the APACT trial had the most favorable selection criteria leading to longer overall survival irrespective of the type of intervention. It is also noteworthy that both ESPAC4 and PRODIGE24 had an absolute increase in 5‑year overall survival in the experimental arms by 12% but only 7% in the APACT trial compared to the control gemcitabine arms [12, 23, 44].
Although a considerable number of patients with pancreatic cancer initially have surgically resectable disease, a significant portion of them eventually experience distant recurrences [51, 52]. The promising outcomes seen with adjuvant chemotherapy have led to the investigation of the same drug regimens in the neoadjuvant and perioperative settings for EmR disease. Strong arguments for the use of neoadjuvant therapy have been put forward, but the randomized evidence clearly shows a lack of survival advantage in this setting (Table 2, 2.2; [3, 53,54,55]).
The combination of resectable (EmR) and borderline resectable (EmBR) populations in several of the clinical trials created some confusion, at least at first, in understanding the outcome data. Some early trials, like those led by Golcher et al. and Casadei et al., had to be closed prematurely due to slow patient accrual during a period when neoadjuvant therapy was less accepted [56, 57]. The Prep-02/JSAP-05 trial claimed an improvement in median overall survival for the neoadjuvant group (neoadjuvant gemcitabine and S1 followed by resection then adjuvant S1) at 36.7 months compared to 26.6 months in the upfront resection group (adjuvant S1; [59]). This was a problematic study, however, (only ever published as an abstract), as in the phase III JASPAC-01 trial, patients who had received adjuvant S1 had a median overall survival of 46.5 months, surpassing the adjuvant gemcitabine group with an overall survival of 25.5 months [43, 59]. The trial, however, did not lead to a change in practice (in Japan) as recommended by the Clinical Practice Guidelines for Pancreatic Cancer 2019 of the Japan Pancreas Society [66].
The NORPACT‑1 is the latest multicenter trial to report the results of short-course neoadjuvant FOLFIRINOX versus upfront surgery for EmR pancreatic head cancer, which again does not support the use of neoadjuvant therapy for EmR [65]. In this study 140 patients were randomly assigned to neoadjuvant chemotherapy, (n = 77) or upfront surgery (n = 63) with a median (95% CI) survival that was actually less for the neoadjuvant group (25.1 [17.2–34.9] months) than for upfront surgery (38.5 months [27.6–not reached]) although not statistically significant (p = 0.096; [65]).
Empirical borderline-resectable PDAC
The PREOPANC1 and ESPAC5 trials now provide strong evidence for the use of neoadjuvant chemotherapies for EmBR PDAC while the Alliance A021501 trial suggests that stereotactic body radiotherapy added to neoadjuvant chemotherapy adds to toxicity and may also depress survival (Table 3; [27, 28, 60, 61]). In the PREOPANC1 trial, patients with EmBR had a significant survival impact with neoadjuvant therapy (chemoradiation plus gemcitabine and then adjuvant gemcitabine compared to upfront surgery and adjuvant gemcitabine; [60, 61]). The role of chemoradiation in this context remains unproven especially since survival rates were poor in both groups and the control arm employed mono-gemcitabine, which is no longer the standard of care. In the Alliance A021501 phase II study for EmBR, patients were randomized to receive neoadjuvant mFOLFIRINOX with or without 33–40 Gy hypofractionated radiation therapy, and both groups received adjuvant 5‑FU and oxaliplatin (mFOLFOX6) after resection. The median overall survival was 29.8 months for chemotherapy alone versus 17.1 months for chemotherapy plus radiotherapy [28]. Similarly, the ESPAC5 study for EmBR PDAC found that neoadjuvant chemotherapy with randomization to either FOLFIRINOX or GEMCAP resulted in superior survival compared to upfront surgery, while neoadjuvant 50.4-Gy capecitabine-based chemoradiotherapy did not show the same benefit [27]. The ESPAC5 trial is the only randomized trial that has a head-to-head comparison between FOLFIRINOX and GEMCAP, which demonstrated similar survival outcomes but with less toxicity for GEMCAP.
In the PREOPANC1 trial, the resection rates for EmBR were 52% for the neoadjuvant group and 64% for the upfront surgery group, while the R0 resection rates were 79% and 13%, respectively [60]. In the ESPAC5 trial, the resection rates in the three neoadjuvant arms were 65% after FOLFIRINOX, 85% after GEMCAP, and 80% after chemoradiotherapy, with a resection rate of 75% for upfront surgery. The R0 resection rates after neoadjuvant therapy were 12% with FOLFIRINOX, 15% with GEMCAP, and 30% with chemoradiotherapy, compared to 11% with upfront surgery [27]. In the Alliance A021501 trial, the resection rates were 35% with neoadjuvant chemotherapy followed by radiotherapy and 49% with neoadjuvant chemotherapy only (without radiotherapy; [28]). The R0 resection rates were 74% after neoadjuvant chemotherapy followed by radiotherapy and 88% after neoadjuvant chemotherapy only [28]. Thus, for EmBR, neoadjuvant chemotherapy favors overall survival but resection rates and R‑status do not predict survival, while the role of neoadjuvant radiation therapy is not proven.
Empirical locally advanced, unresectable PDAC
Induction chemotherapy
According to NCCN guidelines, selected patients without systemic metastases are recommended to receive 4–6 months of induction combination chemotherapy, followed by chemoradiation or stereotactic body radiation therapy. Surgical resection should be considered if feasible after induction therapy, with adjuvant chemotherapy as clinically indicated [21]. Among the various induction therapy regimens, FOLFIRINOX is the preferred choice for patients with favorable performance status, while gemcitabine with nab-paclitaxel (GEM-NabP) is an alternative (Table 4). The CONKO-007 included 495 patients who received 3 versus 6 months of either FOLFIRINOX or gemcitabine, with or without chemoradiation, followed by surgical exploration (if technically resectable; [70]). The primary endpoint of overall survival was later amended to R0 resection due to delayed patient accrual. The study showed no significant difference in overall survival between the groups, indicating that the addition of radiation during induction therapy did not provide a survival benefit. Importantly, however, this prospective study showed that a resection rate of 23.2% was achieved in EmUR PDAC after induction chemotherapy.
The NEOLAP trial treated EmUR patients with two cycles of induction GEM-NabP, and those without progression were randomized to receive four cycles of FOLFIRINOX or two additional cycles of GEM-NabP [71]. The study found no statistically significant difference in resection rates (the primary endpoint) between the two groups, with both showing overall median survival rates of around 20 months, but importantly again demonstrated in this prospective study an overall reaction rate of 31% [71]. A decade ago, none of these patients would have even been considered for surgical resection.
Surgical exploration after induction chemotherapy
Determining resectability is based on the expertise of the center and surgeon’s experience. Surgical exploration should be undertaken if resection and vascular reconstruction seem feasible, as conventional cross-sectional imaging may not accurately reflect response to induction therapy or the likelihood of resection [2, 3, 73]. A decrease in serum CA19‑9 levels after induction therapy is associated with an increase in successful resections [74,75,76,77]. Different surgical techniques, such as “artery first,” “triangle resection,” and “arterial divestment,” should be considered to optimize resection rates [78,79,80,81,82]. A clean dissection of lymphatic and neural tissue structures in the anatomical triangle bordered by the SMA, celiac axis, and portal vein is crucial for a successful operation (Fig. 2d,e; [81]). The periarterial divestment technique aims to achieve radical tumor clearance without the need for arterial dissection (Fig. 2f; [78, 79, 82, 83]). In a retrospective analysis of EmUR patients who had R0 portal venous resections, the median overall survival was 24 months with a 5-year overall survival of 20% [84].
Empirical oligometastatic disease PDAC
Patients with limited metastases can be considered for resection of the primary tumor and simultaneous metastasectomy achieving median overall survival of 12.3–14.5 months in selected patients [3, 84, 85]. In the largest recent series, in patients with a good pathological response at metastatic sites (ypM0) a median overall survival of 25.5 months was achieved, which increased to 29.0 months with further adjuvant chemotherapy [86]. Patients with lung metastases generally seem to survive longer than those with liver metastases, and moreover pulmonary resection can result in a median survival of 29.2 months from the time of diagnosis of disease recurrence compared with 19.6 months in those with unresected lung metastases [87, 88].
Adjuvant therapies after neoadjuvant treatment
It remains unclear whether patients who have undergone pancreatectomy for localized PDAC after neoadjuvant treatment still benefit from adjuvant chemotherapy. In a retrospective study, adjuvant chemotherapy was found to offer no survival benefit in this setting [89]. By contrast, adjuvant chemotherapy has been found to be associated with improved survival after adjustment for treatment and tumor characteristics in multivariable analyses in a comprehensive analysis of the National Cancer Database [90].
In a multicenter, retrospective study, patients with localized PDAC (EmR, EmBR und EmUR) who underwent pancreatic surgery after at least two cycles of neoadjuvant FOLFIRINOX chemotherapy were retrospectively analyzed. In patients with node-positive disease, adjuvant chemotherapy after neoadjuvant FOLFIRINOX treatment and resection was associated with significantly improved median overall survival of 26 vs. 13 months, respectively [91]. However, adjuvant chemotherapy did not show a significant survival benefit in patients with node-negative disease with a median overall survival of 38 vs. 54 months, respectively [91]. A National Cancer Database study showed that the associated survival was significantly improved in patients who has received adjuvant chemotherapy after neoadjuvant treatment and resection compared with neoadjuvant treatment alone [92]. Furthermore, the survival benefit of additional adjuvant chemotherapy remained significant for those with node-negative disease, a lymph node ratio of < 0.15, low-grade tumor histology, and negative resection margin status [92]. A separate National Cancer Database analysis revealed that additional adjuvant chemotherapy was significantly associated with substantially better median overall survival of 26.6 vs. 21.2 months, with a varied benefit by age, tumor stage, and tumor differentiation [93]. These findings suggest that patients with localized PDAC may benefit from additional adjuvant chemotherapy to achieve prolonged survival after multiagent neoadjuvant or induction chemotherapy and surgical resection. Patients receiving FOLFIRINOX are more prone to greater cumulative toxicity than with other regimens; thus, for example, GEMCAP followed by, for instance, gemcitabine monotherapy could be given for many more cycles, but what is really needed right now is more evidence from well-designed randomized trials.
Targeted therapies
The development of cytotoxic therapies for different stages of pancreatic cancer has primarily relied on empirical approaches. Reductionist attempts to develop treatments by targeting key pathogenic gene alterations have seen limited success, with most targets having a prevalence ranging from 0.1% to 5%, and the survival advantage provided by these agents being only a few months, despite the frequency of altered mutational pathways [94, 95]. The Know Your Tumor Registry represents the largest pancreatic cancer targeted therapy program. Out of 1856 referred patients, 282 (15.2%) had actionable mutations, but only 46 (2.5%) received matched therapy [7]. Survival since diagnosis varied among patients, with a median overall survival of 1.3 years for those with no actionable alteration, 1.5 years for patients with actionable mutations who received unmatched therapy, and 2.6 years for those who had matched therapy [7]. Maintenance therapy with the poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitor olaparib improved progression-free survival from 3.8 to 7.4 months in patients with metastatic pancreatic cancer and germline mutations in the BRCA1/2 genes, provided they had not progressed after at least 4 months of platinum-based first-line chemotherapy [96]. The potential fraction of PDAC patients with druggable driver alterations may significantly increase if novel KRAS inhibitors, targeting the G12D mutation present in about 40% of PDAC patients, prove successful in clinical trials [97].
Drug-resistant persisters in PDAC
In the evolution of PDAC, acquired genomic mutations accumulate in pancreatic exocrine cells leading to increasing dysplastic pancreatic intraepithelial neoplasia, PanIN1 and PanIN2, then carcinoma in situ PanIN3 before progressing to invasion [98]. Low-grade PanIN1A consists of a flat to papillary ductal epithelium with abundant supranuclear mucin and loss of polarity while high-grade PanIN3 is characterized by cytonuclear atypia, dysplasia, or carcinoma in situ with a more complex papillary architecture, nuclear hyperchromatism, and pleomorphism. Telomere shortening and oncogenic KRAS activation are initiating events followed by hypermethylation and/or intragenic mutations and/or loss of heterozygosity of CDKN2A/p16, and mutational activation and or/loss of TP53, as well as homozygous loss or intragenic mutation and second allele loss of DPC4/SMAD4 [99].
Loss of TP53 enables a deterministic pattern of genome evolution in PDAC, suggesting that TP53 mutations are associated with heterogeneous gains in KRAS, MYC, and GATA6, which can further drive metastasis and/or influence PDAC subtypes [100]. The development of PDAC toward greater genomic diversity offers proof that subclonal heterogeneity probably contributes to the unsatisfactory reactions to therapy in PDAC. Within this framework, the simultaneous development of distinct subclones in the identical tumor and at varying stages along a set pathway might clarify the prevalent incidence of varied therapy responses in PDAC. Although these findings propose a traditional model of step-by-step genomic evolution in therapy resistance, mounting proof proposes that non-genetic priming or adjustment significantly adds to therapy resistance [101].
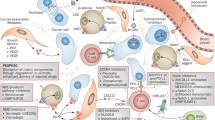
Emerging findings from our research laboratory indicate that intrinsic drug-tolerant cells, often referred to as “persisters,” have the capacity to arise from a preexisting subset of cancerous cells subsequent to neoadjuvant chemotherapy (Fig. 4; [95, 101,102,103]). These persister-like cells adapt to the impact of chemotherapy by enhancing the expression of genes such as CYP3A5 and other co-expressed drug-metabolizing genes. These genes are responsible for metabolizing irinotecan, a constituent of FOLFIRINOX, into forms that lack therapeutic activity. Cancer persister cells can enter a state of dormancy or quiescence, rendering them impervious to the effects of chemotherapy and/or chemoradiotherapy. Consequently, while chemotherapy might effectively eliminate the majority of cancer cells, it could falter in eradicating a minor subset of persister cells, which manage to endure and subsequently repopulate the tumor. Within this conceptual framework, the persister cell state mirrors the notion of minimal residual disease, potentially leading to relapse if treatment discontinues. Accordingly, the identification and comprehensive understanding of persister cells could potentially pave the way for innovative strategies to combat drug-resistant forms of cancer [103].
Resistance mechanisms developing during the evolution of pancreatic cancer may be broadly classified into (1) intrinsic mechanism due to enrichment of persister cells with specific resistance features and (2) acquired resistance over time due to new genetic mutations and structural chromosomal changes
Molecular classifications and personalized therapy of PDAC
Pancreatic ductal adenocarcinomas exhibit genetic and biological heterogeneity. The simplest molecular classification includes basal-like and classic-like subtypes, which vary across different disease stages and respond differently to various treatments (Fig. 1; [14,15,16,17, 95, 104]). Classic-like subtypes define well-differentiated tumors that tend to be associated with better outcomes and expression of the key pancreatic-specific transcription factors GATA6, HNF1A, and PDX. Basal-like subtypes are less differentiated tumors with mesenchymal characteristics, including upregulated expression of ∆NP63 and TGFβ-signaling and tend to be associated with poor prognosis [104]. While current subtyping schemas can identify prognostic subgroups among patients with resectable tumors, they are less effective in those with advanced-stage disease.
Different molecular subtypes also respond differently to chemotherapies [14,15,16,17, 95, 104]. Basal-like PDACs show poor response to chemotherapy in locally advanced or metastatic PDAC [14, 15]. The utility of the classic and basal-like subtypes for predicting survival and response to two major first-line schemes—mFOLFIRINOX and gemcitabine nab-paclitaxel—in advanced PDAC was assessed in the COMPASS study (NCT02750657; [105]). There was a nearly 4‑month longer overall survival for patients with classic-like than for those with a basal-like phenotype [105]. Additionally, patients with high expression of the classic-like marker GATA6 had 2 months longer survival than those with GATA6 low expression. Patients with the classic-like subtype who had mFOLFIRINOX had longer survival than those with the basal-like subtype [105]. Collectively, these findings suggest that GATA6-low and basal-like subtype may be associated with poor response to mFOLFIRINOX.
Intratumor heterogeneity is linked to stromal heterogeneity, leading to various tumor microenvironment programs, including “reactive,” “intermediate,” and “deserted” cellular and transcriptomic subtypes [24]. A deserted-like microenvironment is found in untreated tumors and has been associated with poor treatment response in patients. Drug-tolerant persister cells, arising from different tumor cell lineages during first-line and/or second-line therapy, contribute to disease relapse. Both FOLFIRINOX and chemoradiation therapy have been reported to induce phenotype switching from classic-like to basal-like subtypes, resulting in greater chemoresistance and reduced survival [95, 103,104,105]. What may be of more importance is the acquisition of hybrid molecular phenotypes with both classic- and basal-like characteristics and persister cell type features. Understanding and addressing post-therapy cellular plasticity and persister cell enrichment will require well-designed clinical trials with multidimensional omics analysis approaches.
New trials
Several studies are exploring the use of transcriptomic profiling in the clinical trial setting. The PANCREAS study (PurIST Classification-Guided Adaptive Neoadjuvant Chemotherapy by RNA Expression Profiling of EUS Aspiration Samples) aims to enroll 41 patients, determining the PurIST molecular subtypes in tumor samples obtained by endoscopic ultrasound fine-needle aspiration (EUS/FNA) to establish the pancreatic cancer subtype (NCT04683315). Therapy is directed on the basis of molecular subtype (classic vs. basal). Patients with the classic subtype will receive mFOLFIRINOX and patients with the basal subtype will receive neoadjuvant Gem-NabP. The primary outcome measure is the number of patients who receive PurIST classification-directed therapy and have a treatment response following 12 weeks of therapy—but the stage of disease is not specified.
PASS-01 (Pancreatic Adenocarcinoma Signature Stratification for Treatment) is a randomized phase II trial that will enroll 150 patients with PDAC of any stage to receive either mFOLFIRINOX or Gem-NabP (NCT04469556). The primary outcome measure is progression-free survival, and it is the first randomized trial to have a head-to-head comparison of these two regimens. Important secondary outcome measures include overall survival associated with treatment-specific signatures, and GATA6 concordance between organoid transcriptomic profiles and patient transcriptomic profiles.
The ESPAC6 trial (NCT05314998) will randomize resectable (EmR) patients 1:1 to an experimental arm in which patients will receive either adjuvant mFOLFIRINOX or GEMCAP based on the ESPAC transcriptomic treatment-specific signature or to the control arm in which all patients will receive adjuvant mFOLFIRINOX. The primary endpoint is disease-free survival, with multiple secondary endpoints in transcriptomic and genomic analyses. The ESPAC7 is similarly designed for patients with EmUR locally advanced PDAC tumors, with the primary endpoint in this case being the rate of resection.
Several standard clinical trials are comparing neoadjuvant and adjuvant alternatives in EmR and EmBR settings (Table 5). The Phase III Alliance 21806 trial is evaluating perioperative mFOLFIRINOX (eight cycles neoadjuvant and four cycles adjuvant) compared to adjuvant mFOLFIRINOX (12 cycles). The PREOPANC-3 trial in The Netherlands is evaluating the same treatment regimens. PREOPANC‑2 is also evaluating neoadjuvant mFOLFIRINOX (eight cycles) compared to neoadjuvant gemcitabine-based chemoradiotherapy, with both groups receiving four cycles of adjuvant gemcitabine in the borderline and resectable PDAC populations.
Further evidence on the role of surgical resection in patients with EmOm is expected from several ongoing studies. The non-randomized single-arm, phase II HOLIPANC study will enroll 150 patients with hepatic oligometastatic PDAC to receive induction therapy with liposomal irinotecan, oxaliplatin, and 5‑FU/FA (NAPOX) followed by surgical exploration and synchronous resection of both primary and metastatic lesions if feasible.
The METAPANC study will randomize 272 patients with EmOm to receive either FOLFIRINOX until disease progression or surgery after at least eight cycles of FOLFIRINOX.
Conclusion
The personalized treatment in localized pancreatic cancer is now firmly established based on the empirical system of staging. For locally resectable (EmR), this requires primary resection followed by adjuvant combination chemotherapy, utilizing either mFOLFIRINOX for patients aged 79 or younger with good performance status and no significant cardiovascular issues, or GEMCAP in other cases. For borderline resectable (EmBR), a neoadjuvant chemotherapy regimen followed by adjuvant therapy, particularly FOLFIRINOX or GEMCAP, is favored. For individuals initially diagnosed with unresectable (EmUR) disease, a 3–6-month induction course of combination chemotherapy, preferably mFOLFIRINOX, is recommended. A similar approach may be taken in selected patients with EmOm. The total number of cycles and dosing when both pre- and postoperative chemotherapy is administered needs to be evaluated in future trials. Chemoradiotherapy in the adjuvant setting is not supported, while its role in the neoadjuvant and induction settings is questioned by a number of studies (Lap-07, Alliance A021501, and ESPAC5). Surrogate markers in outcome evaluation in neoadjuvant and induction strategies are not supported by the evidence, requiring disease-free and overall survival as the primary endpoints. Real progress is now dependent on integrating the empirical staging systems with a deeper understanding of tumor plasticity according to tumor evolution with resistance mechanism based on acquired mutations and the selection of cell persister populations with intrinsic resistance mechanisms. Several key studies that are ongoing are designed to do precisely this, notably PASS-01, ESPAC6, and ESPAC7.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48
Strobel O, Neoptolemos J, Jager D, Buchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11–26.
Springfeld C, Ferrone CR, Katz MHG, Philip PA, Hong TS, Hackert T, et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. 2023;20(5):318–37.
Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–48.
Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022.
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73–85.
Pishvaian MJ, Wang H, He AR, Hwang JJ, Smaglo BG, Kim SS, et al. A Phase I/II Study of Veliparib (ABT-888) in Combination with 5‑Fluorouracil and Oxaliplatin in Patients with Metastatic Pancreatic Cancer. Clin Cancer Res. 2020;26(19):5092–101.
Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–85.
Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–10.
Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77.
Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073–81.
Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011–24.
Conroy T, Hammel P, Hebbar M, Abdelghani BM, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406.
Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–3.
Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–78.
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52.
Chan-Seng-Yue M, Kim JC, Wilson GW, Ng K, Figueroa EF, O’Kane GM, et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat Genet. 2020;52(2):231–40.
Rashid NU, Peng XL, Jin C, Moffitt RA, Volmar KE, Belt BA, et al. Purity independent subtyping of tumors (PurIST), a clinically robust, single-sample classifier for tumor subtyping in pancreatic cancer. Clin Cancer Res. 2020;26(1):82–92.
Tiriac H, Belleau P, Engle DD, Plenker D, Deschenes A, Somerville TDD, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8(9):1112–29.
Nicolle R, Gayet O, Duconseil P, Vanbrugghe C, Roques J, Bigonnet M, et al. A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma. Ann Oncol. 2021;32(2):250–60.
NCCN Guidelines V1.2022, Pancreatic Adenocarcinoma. 2022. https://www.nccn.org/guidelines/category_1. Accessed 21 August 2023.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. https://doi.org/10.3322/caac.21388.
Callery MP, Chang KJ, Fishman EK, Talamonti MS, Traverso WL, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–33.
Grunwald BT, Devisme A, Andrieux G, Vyas F, Aliar K, McCloskey CW, et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell. 2021;184(22):5577–5592.e18.
Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–46.
Katz MH, Shi Q, Ahmad SA, Herman JM, Marsh Rde W, Collisson E, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151(8):e161137.
Ghaneh P, Palmer D, Cicconi S, Jackson R, Halloran CM, Rawcliffe C, et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(2):157–68.
Katz MHG, Shi Q, Meyers J, Herman JM, Chuong M, Wolpin BM, et al. Efficacy of preoperative mFOLFIRINOX vs mFOLFIRINOX plus hypofractionated radiotherapy for borderline resectable adenocarcinoma of the pancreas: The A021501 phase 2 randomized clinical trial. JAMA Oncol. 2022;8(9):1263–70. https://doi.org/10.1001/jamaoncol.2022.2319.
Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833–46. discussion 46–8.
Isaji S, Mizuno S, Windsor JA, Bassi C, Fernandez-Del CC, Hackert T, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2–11.
Hayasaki A, Isaji S, Kishiwada M, Fujii T, Iizawa Y, Kato H, et al. Survival analysis in patients with pancreatic ductal adenocarcinoma undergoing chemoradiotherapy followed by surgery according to the international consensus on the 2017 definition of borderline Resectable cancer. Cancers. 2018;10(3):65.
Medrano J, Garnier J, Ewald J, Marchese U, Gilabert M, Launay S, et al. Patient outcome according to the 2017 international consensus on the definition of borderline resectable pancreatic ductal adenocarcinoma. Pancreatology. 2020;20(2):223–8.
Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899–903.
Bakkevold KE, Arnesjo B, Dahl O, Kambestad B. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater—results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A(5):698–703.
Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685–95.
Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5‑fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230(6):776–82. discussion 82–4.
Smeenk HG, Incrocci L, Kazemier G, van Dekken H, Tran KT, Jeekel J, van Eijck CH. Adjuvant 5‑FU-based chemoradiotherapy for patients undergoing R‑1/R‑2 resections for pancreatic cancer. Dig Surg. 2005;22(5):321–8.
Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–81.
Regine WF, Winter KA, Abrams R, Safran H, Hoffman JP, Konski A, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5‑year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18(5):1319–26.
Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299(9):1019–26.
Ueno H, Kosuge T, Matsuyama Y, Yamamoto J, Nakao A, Egawa S, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101(6):908–15.
Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T, et al. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol. 2012;30(33):4077–83.
Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S‑1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–57.
Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol. 2017;35(29):3330–7.
Sinn M, Liersch T, Riess H, Gellert K, Stubs P, Waldschmidt D, et al. CONKO-006: A randomised double-blinded phase IIb-study of additive therapy with gemcitabine + sorafenib/placebo in patients with R1 resection of pancreatic cancer—Final results. Eur J Cancer. 2020;138:172–81.
Abrams RA, Winter KA, Safran H, Goodman KA, Regine WF, Berger AC, et al. Results of the NRG oncology/RTOG 0848 adjuvant chemotherapy question-erlotinib+gemcitabine for resected cancer of the pancreatic head: a phase II randomized clinical trial. Am J Clin Oncol. 2020;43(3):173–9.
Conroy T, Castan F, Lopez A, Turpin A, Abdelghani BM, Wei AC, et al. Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 2022;8(11):1571–8.
Tempero M, O’Reilly E, Van Cutsem E, Berlin J, Philip P, Goldstein D, et al. LBA-1 Phase 3 APACT trial of adjuvant nab-paclitaxel plus gemcitabine (nab‑P + Gem) vs gemcitabine (Gem) alone in patients with resected pancreatic cancer (PC): Updated 5‑year overall survival. Ann Oncol. 2021;32:S226.
Tempero MA, Reni M, Riess H, Pelzer U, O’Reilly EM, Winter JM, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37(15_suppl):4000.
Springfeld C, Büchler MW, Neoptolemos JP. Interpreting primary end points in randomized trials of pancreatic cancer. J Clin Oncol. 2023;41(25):4182–3. https://doi.org/10.1200/JCO.23.00779.
Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–45.
Klaiber U, Hackert T, Neoptolemos JP. Adjuvant treatment for pancreatic cancer. Transl Gastroenterol Hepatol. 2019;4:27.
Neoptolemos JP, Springfeld C, Hackert T. A review of pancreatic cancer. JAMA. 2021;326(23):2436.
Springfeld C, Neoptolemos JP. The role of neoadjuvant therapy for resectable pancreatic cancer remains uncertain. Nat Rev Clin Oncol. 2022;19(5):285–6.
Buchler MW, Neoptolemos JP. The NEONAX study. Ann Oncol. 2023;34(4):442–3.
Golcher H, Brunner TB, Witzigmann H, Marti L, Bechstein WO, Bruns C, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191(1):7–16.
Casadei R, Di Marco M, Ricci C, Santini D, Serra C, Calculli L, et al. Neoadjuvant chemoradiotherapy and surgery versus surgery alone in resectable pancreatic cancer: a single-center prospective, randomized, controlled trial which failed to achieve accrual targets. J Gastrointest Surg. 2015;19(10):1802–12.
Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2–3 trial. Lancet Gastroenterol Hepatol. 2018;3(6):413–23.
Unno M, Motoi F, Matsuyama Y, Satoi S, Matsumoto I, Aosasa S, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S‑1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). JCO. 2019;37(4_suppl):189.
Versteijne E, van Dam JL, Suker M, Janssen QP, Groothuis K, Akkermans-Vogelaar JM, et al. Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022;40(11):1220–30.
Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763–73.
Sohal DPS, Duong M, Ahmad SA, Gandhi NS, Beg MS, Wang-Gillam A, et al. Efficacy of perioperative chemotherapy for resectable pancreatic adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2021;7(3):421–7.
Seufferlein T, Uhl W, Kornmann M, Algul H, Friess H, Konig A, et al. Perioperative or only adjuvant gemcitabine plus nab-paclitaxel for resectable pancreatic cancer (NEONAX)—a randomized phase II trial of the AIO pancreatic cancer group. Ann Oncol. 2023;34(1):91–100.
Schwarz L, Bachet J‑B, Meurisse A, Bouché O, Assenat E, Piessen G, et al. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy: A multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). J Clin Oncol. 2022;40(16_suppl):4134.
Labori KJ, Bratlie SO, Biörserud C, Björnsson B, Bringeland EN, et al. Short-course neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer: A multicenter randomized phase-II trial (NORPACT-1). J Clin Oncol. 2023;41:LBA4005–LBA4005.
Okusaka T, Nakamura M, Yoshida M, Kitano M, Uesaka K, Ito Y, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan pancreas society: a synopsis. Pancreas. 2020;49(3):326–35.
Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 2018;268(2):215–22.
Yamaguchi J, Yokoyama Y, Fujii T, Yamada S, Takami H, Kawashima H, et al. Results of a phase II study on the use of neoadjuvant chemotherapy (FOLFIRINOX or GEM/nab-PTX) for borderline-resectable pancreatic cancer (NUPAT-01). Ann Surg. 2022;275(6):1043–9.
Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–53.
Fietkau R, Ghadimi M, Grützmann R, Wittel UA, Jacobasch L, Uhl W, et al. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresectable locally advanced pancreatic cancer: First results of the CONKO-007 trial. J Clin Oncol. 2022;40(16_suppl):4008.
Kunzmann V, Siveke JT, Algul H, Goekkurt E, Siegler G, Martens U, et al. Nab-paclitaxel plus gemcitabine versus nab-paclitaxel plus gemcitabine followed by FOLFIRINOX induction chemotherapy in locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): a multicentre, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(2):128–38.
Ducreux MP, Desgrippes R, Rinaldi Y, Fiore F, Guimbaud R, Follana P, et al. 1296MO PRODIGE 29-UCGI 26(NEOPAN): A phase III randomised trial comparing chemotherapy with folfirinox or gemcitabine in locally advanced pancreatic carcinoma (LAPC). Ann Oncol. 2022;33:S1136.
Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261(1):12–7.
Heger U, Sun H, Hinz U, Klaiber U, Tanaka M, Liu B, et al. Induction chemotherapy in pancreatic cancer: CA 19‑9 may predict resectability and survival. HPB. 2020;22(2):224–32.
Springfeld C, Jager D, Buchler MW, Strobel O, Hackert T, Palmer DH, Neoptolemos JP. Chemotherapy for pancreatic cancer. Presse Med. 2019;48(3 Pt 2):e159–e74.
Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5(3):285–94.
Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C, et al. CA19‑9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol. 2013;20(7):2188–96.
Cai B, Lu Z, Neoptolemos JP, Diener MK, Li M, Yin L, et al. Sub-adventitial divestment technique for resecting artery-involved pancreatic cancer: a retrospective cohort study. Langenbecks Arch Surg. 2021;406(3):691–701.
Diener MK, Mihaljevic AL, Strobel O, Loos M, Schmidt T, Schneider M, et al. Periarterial divestment in pancreatic cancer surgery. Surgery. 2021;169(5):1019–25.
Klompmaker S, Boggi U, Hackert T, Salvia R, Weiss M, Yamaue H, et al. Distal pancreatectomy with celiac axis resection (DP-CAR) for pancreatic cancer. How I do it. J Gastrointest Surg. 2018;22(10):1804–10.
Klotz R, Hackert T, Heger P, Probst P, Hinz U, Loos M, et al. The TRIANGLE operation for pancreatic head and body cancers: early postoperative outcomes. HPB. 2022;24(3):332–41.
Schneider M, Hackert T, Strobel O, Buchler MW. Technical advances in surgery for pancreatic cancer. Br J Surg. 2021;108(7):777–85.
Habib JR, Kinny-Koster B, van Oosten F, Javed AA, Cameron JL, Lafaro KJ, et al. Periadventitial dissection of the superior mesenteric artery for locally advanced pancreatic cancer: Surgical planning with the “halo sign” and “string sign”. Surgery. 2021;169(5):1026–31.
Hackert T, Niesen W, Hinz U, Tjaden C, Strobel O, Ulrich A, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43(2):358–63.
Tachezy M, Gebauer F, Janot M, Uhl W, Zerbi A, Montorsi M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160(1):136–44.
Hank T, Klaiber U, Hinz U, Schütte D, Leonhardt CS, Bergmann F, et al. Oncological outcome of conversion surgery after preoperative chemotherapy for metastatic pancreatic cancer. Ann Surg. 2022;277(5):e1089–98. https://doi.org/10.1097/SLA.0000000000005481.
Jones RP, Psarelli EE, Jackson R, Ghaneh P, Halloran CM, Palmer DH, et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC‑4 randomized adjuvant chemotherapy trial. JAMA Surg. 2019;154(11):1038–48.
Homma Y, Endo I, Matsuyama R, Sho M, Mizuno S, Seyama Y, et al. Outcomes of lung metastasis from pancreatic cancer: a nationwide multicenter analysis. J Hepato Biliary Pancreat. 2022;29(5):552–61.
Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021;273(2):341–9.
Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, et al. Neoadjuvant therapy followed by resection versus Upfront resection for resectable pancreatic cancer: a propensity score matched analysis. J Clin Oncol. 2017;35(5):515–22.
van Roessel S, van Veldhuisen E, Klompmaker S, Janssen QP, Hilal AM, Alseidi A, et al. Evaluation of adjuvant chemotherapy in patients with Resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020;6(11):1733–40.
Olecki EJ, Stahl KA, Torres MB, Peng JS, Dixon M, Shen C, Gusani NJ. Adjuvant chemotherapy after neoadjuvant chemotherapy for pancreatic cancer is associated with improved survival for patients with low-risk pathology. Ann Surg Oncol. 2021;28(6):3111–22.
Sugawara T, Rodriguez Franco S, Sherman S, Kirsch MJ, Colborn K, Ishida J, et al. Association of Adjuvant chemotherapy in patients with Resected pancreatic adenocarcinoma after multiagent neoadjuvant chemotherapy. JAMA Oncol. 2023;9(3):316–23.
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–6.
Bailey P, Zhou X, An J, Peccerella T, Hu K, Springfeld C, et al. Refining the treatment of pancreatic cancer from big data to improved individual survival. Function. 2023;4(3):zqad11.
Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–27.
Huang L, Guo Z, Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021;6(1):386.
Perez-Mancera PA, Guerra C, Barbacid M, Tuveson DA. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142(5):1079–92.
Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142(4):730–733.e9.
Baslan T, Morris JP, Zhao Z, Reyes J, Ho YJ, Tsanov KM, et al. Ordered and deterministic cancer genome evolution after p53 loss. Nature. 2022;608(7924):795–802.
Shen S, Vagner S, Robert C. Persistent cancer cells: the deadly survivors. Cell. 2020;183(4):860–74.
Cabanos HF, Hata AN. Emerging insights into targeted therapy-tolerant persister cells in cancer. Cancers. 2021;13(11):2666.
Zhou X, An J, Kurilov R, Brors B, Hu K, Peccerella T, et al. Persister cell phenotypes contribute to poor patient outcomes after neoadjuvant chemotherapy in PDAC. Nat Cancer. 2023. https://doi.org/10.1038/s43018-023-00628-6.
Xu Z, Hu K, Bailey P, Springfeld C, Roth S, Kurilov R, et al. Clinical impact of molecular subtyping of pancreatic cancer. Front Cell Dev Biol. 2021;9:743908.
O’Kane GM, Grunwald BT, Jang GH, Masoomian M, Picardo S, Grant RC, et al. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin Cancer Res. 2020;26(18):4901–10.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.P. Neoptolemos, K. Hu, P. Bailey, C. Springfeld, B. Cai, Y. Miao, C. Michalski, C. Carvalho, T. Hackert and M.W. Büchler declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neoptolemos, J.P., Hu, K., Bailey, P. et al. Personalized treatment in localized pancreatic cancer. Eur Surg 56, 93–109 (2024). https://doi.org/10.1007/s10353-023-00814-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-023-00814-x