Summary
Background
Since implementation of the laparoscopic approach for removal of the gallbladder in the early 90s, a shift in numbers from open cholecystectomy (OC) to laparoscopic cholecystectomy (LC) has been observed. Despite initial reservations, LC has become the most favoured technique in the past 3 decades. Consecutive technical improvements support this trend. Considering this development, the question regarding the relevance of OC nowadays inevitably arises.
Methods
This study represents a literature review performed in PubMed.
Results
Laparoscopic cholecystectomy has evolved to be the leading technique for cholecystectomy. This technique has become well established in elective as well as in acute indications. However, concomitant to increasing numbers of performed LC, the proportion of performed OC is declining, leading to a decreasing expertise and a lack in training in OC nowadays. However, in complex situations, when the operation strategy has to be changed to maintain patient safety, conversion to OC is the most common strategy.
Conclusion
Owing to past and current developments, open cholecystectomy can hardly be assumed to be common surgical knowledge anymore. Hence, OC as bail-out strategy in cases of intraoperative difficulties must be critically evaluated. From this point of view, alternative patient management and treatment concepts should be considered to maintain patient safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Surgical removal of the gallbladder has a history of nearly 140 years—a period allowing for technical progression with most advances in the past 30 years, when minimally invasive surgery became implemented in clinical routine. This article addresses current developments and limitations regarding cholecystectomy, reflected against a historical background.
Introduction
In 1882 Carl Langenbuch was the first to perform a cholecystectomy. The implemented technique represented the standard surgery for indications like gallstones, polyps, acute and chronic cholecystitis, as well as for carcinoma of the gallbladder, for more than 100 years.
In 1985 Erich Muehe performed the first laparoscopic cholecystectomy in Boeblingen, Germany. At this time, he and his technique didn’t receive much attention. Phillipe Mouret of Lyon and Francois Dubois of Paris, France, adapted this approach in 1987 and 1988, respectively, and popularized it in the surgical society [1].
Despite initial scepticism and rejection of this new technique, surgeons began to recognize the advantages of the laparoscopic approach and it became widely implemented in the early 90s. In 1993 a National Institute of Health consensus officially approved this technique for all patients with symptomatic and asymptomatic gallstones [2].
Operative skills and perioperative management have been developing ever since. Nowadays, laparoscopic cholecystectomy seems to be feasible not only in uncomplicated cholelithiasis, but also challenges cholecystectomy in complex patient situations. Although the Society of American Gastrointestinal and Endoscopic Surgeons claimed acute cholecystitis to be a relative contraindication for laparoscopic cholecystectomy in 1993 [3], the laparoscopic technique has evolved into the most favoured method for this indication these days.
A 2015 published meta-analysis compared 677 patients treated with laparoscopic cholecystectomy (LC) and 697 patients treated with open cholecystectomy (OC) for acute cholecystitis regarding postoperative morbidity. The results revealed a benefit for patients with LC in terms of a reduction of the overall morbidity rate by nearly half (OR = 0.46) compared to OC. In detail: postoperative wound infection rate (OR = 0.54), postoperative pneumonia (OR = 0.51) and also postoperative mortality rates (OR = 0.2) were reduced. The conversion rate ranged from 9.5–35.5% (mean 20.87%). Coccolini et al. found no significantly higher incidence of biliary leakage or intraoperative blood loss. They also did not observe prolonged operative times and the postoperative hospital stay in the LC group was reduced. Taken together, these results plead for a laparoscopic attempt in acute cholecystitis [4].
The Tokyo Guidelines from 2018, in their third revision, classify acute cholecystitis into three severity grades with different appraisals of laparoscopic feasibility. Compared to the 2007 revision, the 2018 version also recommends LC in grade III cases, with the severest inflammatory alterations in the cystohepatic triangle, if certain conditions are met [5].
Because of frequently present comorbidities, LC in elderly patients had been avoided so far. Stress of the capno-peritoneum or possible extended operation times have been mentioned among other reasons for why OC was favoured.
A 2013 published meta-analysis including 2 randomized and 11 observational studies with 101,559 patients compared the outcome of patients age 65 and older regarding postoperative morbidity, cardiac and respiratory complications, and mortality, and displayed a beneficial trend for laparoscopic surgery compared to open surgery (OR 0.44, 0.55, 0.55 and 0.24, respectively). Morbidity data from the 2 RCTs including symptomatic cholecystolithiasis and acute cholecystitis clearly indicate a trend in favour of the laparoscopic approach, although statistical significance could not be reached [6].
Gad et al. showed that LC is practicable even in cirrhosis according to Child–Pugh stage, with conversion rates of 0–17% in centres experienced in perioperative management of such patients [7]. Also, growing expertise in laparoscopic common bile duct explorations can be registered. There have been reports that even management of type II Mirizzi syndrome seems practicable laparoscopically [8].
This demonstrates that the limits of feasibility of the laparoscopic technique have not yet been set.
Regarding all these advances, the supreme goal is to improve patient safety and morbidity.
Safety
Compared to open surgery the laparoscopically operating surgeon is challenged by the lack of intraoperative tactile perception. Another challenge can be found in the reduction of the stereoscopic vision to a two-dimensional image and, consequently, a susceptibility to optical illusions and misinterpretation of depth.
Way et al. analysed 252 laparoscopic bile duct injuries and claimed that 97% of errors that had led to bile duct injury were caused by visual perceptual illusion and not due to faults in technical skills, knowledge or judgment [9]. This study was conducted in the pre-3D imaging era, whereas nowadays, 3D laparoscopy is available in many centres. To evaluate the possible benefit of this technical evolution, Schwab et al. randomized 112 elective patients between conventional and 3D laparoscopic cholecystectomy. They could show that the 3D view reduced operative time in complex cases and also the number of gallbladder perforations [10]. A Korean group studied possible differences between 3D LC and 2D LC in acute cholecystitis and found no significant differences between the groups in terms of conversion rate or blood loss, but did in terms of operation time and postoperative stay (54.88 ± 28.68 vs. 86.31 ± 35.07 min and 5.71 ± 4.75 vs. 6.99 ± 6.79 days, respectively) [11]. A publication addressing the question of whether 3D LC improves the rate of bile duct injuries by reducing visual misperception remains outstanding.
Among the technical complications, common bile duct injuries (BDI) are those most serious [12]. Despite growing experience in laparoscopy, improvements in operative skills and technological advances in optical and surgical devices, bile duct injuries occur nearly twice as frequently in patients with laparoscopic cholecystectomy than in those with open surgery [13]. Also, the pattern of bile duct injuries seems to be different to that in open surgery. Complex injuries (e.g., central injuries, thermal injuries or complete transections) represent one third of bile duct complications. Combined vasculobiliary injuries occur in nearly one out of four bile duct injuries [14].
Prevention of bile duct injuries
To prevent bile duct injuries or combined vasculobiliary injury (CVBI), Strasberg et al. proposed a concept under the term of “critical view of safety” (CVS), which should be achieved when performing the “infundibular” attempt of LC. This approach includes the meticulous dissection of all fatty and fibrous tissue in the triangle of Calot, visualisation of the posterior liver bed by separating the lowest part of the gallbladder from the cystic plate and identification of only two tubular structures entering the gallbladder [15]. Dissection above the Rouviére’s sulcus and a short time-out and re-evaluation of anatomical structures before transecting were additionally claimed by the Delphi consensus to reduce BDI [16]. If these requirements cannot be fulfilled, a bail-out strategy should be considered to minimize the risk of BDI or CVBI (Fig 1).
Bail-out strategies
One bail-out strategy is represented by changing to the “fundus first” technique, where the separation of the gallbladder begins at the fundus. By following the cystic wall, the cystic duct will be identified as the remaining structure leaving the gallbladder (Fig 1c).
The most frequently used strategy to overcome a critical situation or to prevent major complication in the case of intraoperative difficulties in LC is conversion to open surgery. The haptic feedback and the possibility of applying local pressure to control bleedings makes this strategy suitable in any difficult cases (Fig 1d).
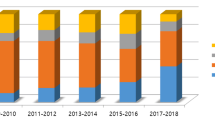
In fact, in the early years of LC, a low threshold for conversion to improve patient safety and prevent severe complications was demanded. A problem of this approach is that with increasing skills of surgeons in LC, training in open cholecystectomy decreases concomitantly. This is revealed in a questionnaire survey, where only 17.5% of expert surgeons in Taiwan, Japan and South Korea declared that conversion made the surgery easier [17]. This evaluation mirrors a common development among surgeons, reflecting a general decrease in training and skills to perform OC, despite an overall increase of performed cholecystectomies in the US. In the period from 2010 to 2018, the numbers of performed LC increased by 39% from 81 to 117 per graduating general surgery resident [18]. On the other hand, OCs per surgical residency have declined from 70.4 to 3.6 over the past 30 years according to the observation of a single centre in Texas [19].
Subtotal cholecystectomy is another bail-out strategy. In open surgery, this concept is well implemented. In laparoscopic surgery, this strategy is also an appropriate option. Therefore, the gallbladder is opened at the most proximal secure site, ideally at the level of Hartmann’s pouch, and gallstones are removed. The remaining biliary structures are left without approaching to the cystohepatic triangle. The gallbladder remnant is subsequently closed or left open, depending on remaining bile secretion via the cystic duct. If dissection in the liver bed turns out to be unsafe, resection of merely the anterior wall seems to be a reasonable option (Fig 1e). Drainage of the situs is mandatory in any case.
A Canadian study compared postoperative outcomes of 105 LC patients and 46 with laparoscopic subtotal cholecystectomy (LSC) in severe cholecystitis. They counted four bile duct injuries in the LC group compared to none in the LSC group. Bile leaks had an RR of 3.4 combined with a higher rate of ERCP (Endoscopic retrograde cholangiopancreaticography) (RR 3.2) and stent implantation (RR 4.6) in the LSC group. Long-term bile fistulas were not observed. Overall postoperative morbidity did not differ between the LC and the LSC groups, supporting this strategy as an approbate bail-out option [20].
Discussion
Considering the recent developments in biliary surgery and extrapolating these trends, recommendations for surgical strategies, especially in exceptional situations where the operating surgeon has to change course to ensure patient safety, have to be re-evaluated constantly.
Regarding obvious changes in expertise, the question arises of whether open cholecystectomy or conversion to open surgery is still a safe bail-out procedure in complex cases or in the event of intraoperative complications.
Currently, there is a sufficient proportion of surgeons within the surgical staff who grew up being trained in open surgery, but in 10 years, most general surgeons will lack routine and skills in open cholecystectomy. So how should we manage in the future? Is it necessary to restrict this operation to a few high-volume centres and not consider cholecystectomy as “general surgical knowledge”, as we have done so up until now? Do we have to change treatment strategies, for example, initially starting conservative therapy with antibiotics in acute cholecystitis and transferring patients to centres with multimodal interdisciplinary expertise? Should we convert or indicate open surgery more liberally to regain routine? Or should we place more emphasis on laparoscopic bail-out strategies?
New developments or variations of LC like single-incision laparoscopic cholecystectomy (SILC), solo single-incision laparoscopic cholecystectomy (S-SILC), laparoscopic surgery through natural orifices (NOTES) or trans-cylindrical (gas-free) small-incision cholecystectomy, 3D vision in laparoscopic cholecystectomy, robotic cholecystectomy or the use of fluorescent ICG to visualize critical structures have found their ways into clinical implementation. All technical and methodological advances should support the surgeon in preventing complications. Further objectives are the reduction of postoperative morbidity by reducing the operative trauma or the improvement of cosmetic results.
The answer to the question of whether open cholecystectomy is a reasonable option for complicated cholecystectomies cannot be answered without considering actual trends and developments of practical and technical nature. Due to changes in training and expertise, the question has to be asked against the background of current rather than historical perspectives, keeping in mind for the future the one crucial fact no technical improvement can overcome: surgical quality comes only with practice.
Abbreviations
- BDI:
-
Common bile duct injury
- CVBI:
-
Combined vasculobiliary injury
- ERCP:
-
Endoscopic retrograde cholangiopancreaticography
- ICG:
-
Indocyanine green
- LC:
-
Laparoscopic cholecystectomy
- LSC:
-
Laparoscopic subtotal cholecystectomy
- OC:
-
Open cholecystectomy
- OR:
-
Odds ratio
- RR:
-
Relative risk
References
Reynolds W Jr.. The first laparoscopic cholecystectomy. JSLS. 2001;5(1):89–94.
Gollan JL, Bulkley GB, Diehl AM, et al. Gallstones and laparoscopic cholecystectomy. JAMA. 1993;269(8):1018–24.
Society of American Gastrointestinal Endoscopic Surgeons (SAGES). The role of laparoscopic cholecystectomy (L.C.). Guidelines for clinical application. Surg Endosc. 1993;7(4):369–70.
Coccolini F, et al. Open versus laparoscopic cholecystectomy in acute cholecystitis. Systematic review and meta-analysis. Int J Surg. 2015;18:196–204.
Wakabayashi G, et al. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):73–86.
Antoniou SA, et al. Meta-analysis of laparoscopic vs open cholecystectomy in elderly patients. World J Gastroenterol. 2014;20(46):17626–34.
Gad EH, et al. Laparoscopic cholecystectomy in patients with liver cirrhosis: 8 years experience in a tertiary center. A retrospective cohort study. Ann Med Surg (Lond). 2020;51:1–10.
Senra F, et al. Laparoscopic management of type II Mirizzi syndrome. Surg Endosc. 2020; https://doi.org/10.1007/s00464-019-07316-6.
Way LW, et al. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237(4):460–9.
Schwab KE, et al. 3D laparoscopy does not reduce operative duration or errors in day-case laparoscopic cholecystectomy: a randomised controlled trial. Surg Endosc. 2019; https://doi.org/10.1007/s00464-019-06961-1.
Yun JJ, et al. A retrospective single-center study comparing clinical outcomes of 3‑dimensional and 2‑dimensional laparoscopic cholecystectomy in acute cholecystitis. Ann Hepatobiliary Pancreat Surg. 2019;23(4):339–43.
Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy. Factors that influence the results of treatment. Arch Surg. 1995;130(10):1123–8. discussion 1129.
Rystedt J, Lindell G, Montgomery A. Bile duct injuries associated with 55,134 cholecystectomies: treatment and outcome from a national perspective. World J Surg. 2016;40(1):73–80.
Avgerinos C, et al. One thousand laparoscopic cholecystectomies in a single surgical unit using the “critical view of safety” technique. J Gastrointest Surg. 2009;13(3):498–503.
Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180(1):101–25.
Iwashita Y, et al. What are the appropriate indicators of surgical difficulty during laparoscopic cholecystectomy? Results from a Japan-Korea-Taiwan multinational survey. J Hepatobiliary Pancreat Sci. 2016;23(9):533–47.
Hibi T, et al. The “right” way is not always popular: comparison of surgeons’ perceptions during laparoscopic cholecystectomy for acute cholecystitis among experts from Japan, Korea and Taiwan. J Hepatobiliary Pancreat Sci. 2017;24(1):24–32.
Warner RL, et al. A review of general surgery resident experience in common bile duct exploration in the ERCP era. Am J Surg. 2020; https://doi.org/10.1016/j.amjsurg.2020.02.032.
Sirinek KR, Willis R, Schwesinger WH. Who will be able to perform open biliary surgery in 2025? J Am Coll Surg. 2016;223(1):110–5.
Purzner RH, et al. Safe laparoscopic subtotal cholecystectomy in the face of severe inflammation in the cystohepatic triangle: a retrospective review and proposed management strategy for the difficult gallbladder. Can J Surg. 2019;62(6):402–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Ammann and F. Längle declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ammann, M., Längle, F. The complicated gallbladder—is old-school treatment an alternative?. Eur Surg 53, 114–118 (2021). https://doi.org/10.1007/s10353-020-00653-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-020-00653-0