Summary
Background and purpose
The control of intra-abdominal pressure (IAP) values clinically has gained considerable importance, as they affect organ functions and organ damage. Hence, the discussions on secure IAP values to be used in laparoscopic interventions still continue. In this study, the effects of low IAP values simultaneously on intrathoracic, intra-abdominal, and extra-abdominal organ damage are presented.
Methods
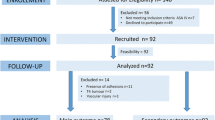
This study was conducted on 40 male Sprague Dawley rats in total, with an average weight of 300 ± 20 g. A saline infusion of 10 ml/kg/h was administered to all the rats from the tail vein. Mechanical ventilator support was maintained for 1 h after the tracheotomy was opened. The rats in the study were separated into four equal groups. No pneumoperitoneum, 6-mmHg pneumoperitoneum, 9-mmHg pneumoperitoneum, and 12-mmHg pneumoperitoneum was applied to Group A (control), Group B, Group C, and Group D, respectively. A total of 30 min after completion of the procedure, lung, terminal ileum, and testicle tissues taken from the rats were examined histopathologically. The results obtained were evaluated, and a statistical package was used for statistical analysis.
Results
A statistically significant difference was not detected between groups in the evaluation of presence of organ damage with respect to intestinal damage (p > 0.05). However, statistically significant difference was detected between groups with respect to lung damage (χ2 = 16.684; p = 0.001 < 0.05) and testicle damage (χ2 = 15.508; p = 0.001 < 0.05). With respect to group variable, although a statistically significant difference was not determined between mean intestinal damage scores in the groups (p > 0.05), a statistically significant difference was determined between mean lung damage scores (Kruskal–Wallis H (KW) = 16.743; p = 0.001 < 0.05) and mean testicle damage scores (KW = 15.088; p = 0.002 < 0.05).
Conclusion
In line with the data obtained from the study, when organs in different compartments of the body are evaluated as a whole, we predict that secure IAP values are between 6 and 9 mmHg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood flow in all venter organs significantly decreases with the increase of intra-abdominal pressure (IAP). When IAP practically exceeds 10 mmHg, it is accepted that there are changes in the physiological parameters and organ functions [1, 2–4]. However, there are studies denoting that organ functions are affected at pressures below 10 mmHg [1, 5–7]. The control of IAP values clinically has gained considerable importance, as they affect organ functions and damage. Nowadays, laparoscopic interventions are used widely in the diagnosis and treatment of many diseases, and for this reason, organ damages secondary to elevated IAP increase the importance of the subject. Hence, the discussions on secure IAP values to be used in laparoscopic interventions still continue.
At present, laparoscopic interventions instead of conventional methods are used in the diagnosis and treatment of many diseases. Control of IAP values is considerably important because IAP rise observed both in laparoscopic interventions and in intensive care patients causes organ damage. In literature, there are various studies that show the effects of IAP increase on organ damage. However, data showing simultaneous damage in intrathoracic, intra-abdominal, and extra-abdominal organs with increase in IAP are limited. Besides, most of the studies in literature involve IAP values greater than 10 mmHg. Here, we present the hazardous effects of low IAP values on intrathoracic, intra-abdominal, and extra-abdominal organs simultaneously. In this study, IAP values of 6, 9, and 12 mmHg were applied for 1 h, and simultaneous changes were examined histopathologically in lung tissue as intrathoracic organ, in terminal ileum tissue as intra-abdominal organ, and in testicle tissue as extra-abdominal organ. Histopathological examination is used instead of ischemia markers in the evaluation of organ damage.
Methods
This study was performed in the Animal Testing Laboratory of the Karadeniz Technical University between 13 and 17 July 2013 with the consent obtained from the Animal Experiments Ethical Committee, Karadeniz Technical University. In this study, 40 male Spraque Dawley rats in total with an average weight 300 ± 20 g were used. All the rats were treated humanely during the study according to “Guide for the Care and Use of Laboratory Animals.” All interventions were performed under anesthesia. Ketamine hydrochloride (Ketalar® vial, 50 mg/ml, Eczacıbaşı. Istanbul, Turkey) 50 mg/kg intraperitoneal (IP) and xylazine hydrochloride (Rompun® vial, 23.32 mg/ml, Bayer. Istanbul, Turkey) 5 mg/kg IP were used in anesthesia induction. Vascular access was established under anesthesia through tail vein with 26G angiocut (BD Neoflon®, Helsingborg, Sweden). After shaving the neck region and anterior abdominal wall, operative site disinfection was performed with povidone iodine in operating room environment, and tracheostomy was performed with 16G cannula (Nova Cath IV canulla, Medipro, Turkey). A saline infusion of 10 ml/kg/h was performed in all rats under mechanical ventilator support (under pressure-controlled mode; peak inspiratory pressure: 12 cmH2O, positive end expiratory pressure: 8 cmH2O, fraction of inspired oxygen: 1.0, frequency: 50/min, tidal volume: 10 ml/kg, I/E ratio: 1/1 [Versamed Ivent 201, Kadima, Israel]) [8]. During the procedure, all rats were monitored and their peak and plateau airway pressures were maintained under 30 cmH2O. Continuity of anesthesia was maintained with intramuscular xylazine (10 mg/kg/h). Pneumoperitoneum was achieved in all rats other than the ones in the control group, at 6, 9, and 12 mmHg pressures with 18G angiocut (BD Neoflon®, Helsingborg, Sweden) for 1 h. The rats in the study were separated into four groups:
Group A (control, n = 10): mechanical ventilator support was applied and 10 ml/kg/h saline infusion was administered.
Group B (n = 10): pneumoperitoneum was formed at 6 mmHg under mechanical ventilator support with 10 ml/kg/h saline infusion.
Group C (n = 10): pneumoperitoneum was formed at 9 mmHg under mechanical ventilator support with 10 ml/kg/h saline infusion.
Group D (n = 10): pneumoperitoneum was formed at 12 mmHg under mechanical ventilator support with 10 ml/kg/h saline infusion.
Thoracotomy and laparotomy were performed under sterile conditions 30 min after completion of 1-h mechanical ventilator support in the control group and desufflation in the pneumoperitoneum group. After sampling of tissues from lung and terminal ileum approximately from the same areas, both testicle tissues were taken. The samples with which histopathological examination was to be performed were placed in 10 % formaldehyde separately.
Histopathological method
After the samples taken for histopathological examination were fixed in 10 % formaldehyde solution, 5-µm paraffin sections were prepared, stained with hematoxylin and eosin, and examined by the same pathologist with 10 × and 40 × magnification.
In lung histopathology, five randomly selected areas were classified under high magnification according to the criteria described earlier. Accordingly, it was evaluated semi-quantitatively as Grade 0: normal morphology, Grade 1: mild intra-alveolar edema and inflammatory cell infiltration, Grade 2: moderate alveolar edema and inflammatory cell infiltration, Grade 3: severe alveolar edema and inflammatory cell infiltration with focal hemorrhage, and Grade 4: pervasive inflammatory cell infiltration and distortion in alveolar structure [9].
Modified Chiu point scoring system, presented in Table 1, was used in the histopathology of terminal ileum [1, 10].
For testicles, a similar four-graded classification scale that was defined by Cosentino et al. [11] was used to measure histopathological damage. Grade 1 denotes normal testicular structure with orderly arrangement of germinal cells; Grade 2, a less orderly, noncohesive germinal cells and closely packed seminiferous tubules; Grade 3, disordered, sloughed germinal cells with shrunken, pycnotic nuclei and less distinct seminiferous tubule borders; and Grade 4, seminiferous tubules that are closely packed with coagulative necrosis of the germinal cells [12].
Statistical method
A statistical program was used for statistical analysis to evaluate findings obtained from the study. Kolmogorov–Smirnov distribution test along with descriptive statistical methods (frequency, percent, median, and interquartile range) was used for the evaluation of normally distribution data. Pearson chi-square test was used for the comparison of qualitative data. For intergroup comparisons of parameters that exhibited normal distribution, one-way analysis of variance test was used for the comparison of quantitative data when there were more than two groups. For intergroup comparisons of parameters that did not exhibit normal distribution, Kruskal–Wallis test was used for the comparison of quantitative data when there were more than two groups, and Mann–Whitney U test with Bonferroni correction was used for the detection of group causing difference. Bonferroni correction was determined as 0.05/6 = 0.008333. The results were evaluated at p < 0.05 and p < 0.01 significance level, 95 % confidence level, and p < 0.008333 advanced significance level.
Power analysis
Gpower program was used for power analysis. Effect size and power were calculated as 0.7071 and 0.96 according to the rate of intestinal damage and 0.6853 and 0.95 according to the rate of testicle damage, respectively.
Results
Rat number 8 in Group B and number 3 and 7 in Group D died during the study, so tissue samples of these rats were not included in the study. Statistically significant difference was not detected with regard to average weights of the groups (F = 0.003; p = 1.000 > 0.05).
Organ damage levels of rats in the groups are presented in Table 2.
In evaluating organ damage in groups without considering the degree of damage, all results other than normal histopathology were assessed as “with damage (+)” and normal histopathological results were assessed as “without damage (−)”. In the view of this knowledge, statistically significant difference was not detected between groups with regard to the presence of intestinal damage (χ2 = 2.890; p = 0.409 > 0.05). However, a statistically significant difference was detected with regard to the presence of lung damage (χ2 = 16.684; p = 0.001 < 0.05) and testicle damage (χ2 = 15.508; p = 0.001 < 0.05). The findings regarding organ damages of groups are presented in Table 3.
Statistically significant difference was not detected with regard to group variable between mean intestinal damage scores within groups (Kruskal–Wallis H (KW) = 2.812; p = 0.422 > 0.05). Statistically significant difference was determined with regard to group variable between mean lung damage scores within groups (KW = 16.743; p = 0.001 < 0.05). In the statistical analysis performed to determine which group was the source of difference, mean lung damage scores of Group C and Group D were found to be higher than mean lung damage score of Group A (Mann–Whitney U = 8.5, p = 0.001 < 0.05; and Mann–Whitney U = 8, p = 0.001 < 0.05). Statistically significant difference was determined with regard to group variable between mean testicle damage scores in groups (KW = 15.088; p = 0.002 < 0.05). In the statistical analysis performed to determine which group was the source of difference, mean testicle damage scores of Group C and Group D were found to be higher than mean testicle damage score of Group A (Mann–Whitney U = 20, p = 0.004 < 0.05; and Mann–Whitney U = 5, p = 0.000 <0.05). Mean organ damage scores of the groups are presented in Table 4.
Discussion
There are many studies in literature about the effects of IAP increase. However, in line with our research, we did not achieve adequate data showing effects on organ functions in different compartments at the same time. Considering that different body compartments are affected at the same time with the increase of IAP, the usage of these data in clinical practice is disputable. Hence, we present reliable IAP values in this study that may be used in clinical practice by examining organ damage in different compartments of the body at the same time.
When IAP practically exceeds 10 mmHg, it is accepted that there are changes in the physiological parameters and organ functions [1, 2–4]. However, there are studies denoting that organ functions are affected at pressures below 10 mmHg [1, 5–7]. In literature, there are many data related to IAP values equal to and higher than 10 mmHg, but data relating to pressure values lower than 10 mmHg are limited. In line with our research, sufficient data, especially related to testicle damage, were not found for IAP below 10 mmHg.
In a previous study, in the experimental sepsis model, a significant difference was not detected with regard to intestinal damage at an IAP value of 8 mmHg for 1 h [1]. In this study, a significant difference was not detected with regard to the presence of intestinal damage and mean damage scores for IAP values of 6, 9, and 12 mmHg for 1 h. According to these results, it was determined that 12-mmHg pneumoperitoneum administered for 1 h was safe with regard to intestinal damage. This result was compatible with the results of the study performed by Ozmen et al. [13].
In the study performed by Karapolat et al. [14], lung tissue damage was reported in laparoscopic surgery performed with 15 mmHg for 30 min. Although mild alveolar edema was detected in 55.5 % of the rats administered 6 mmHg IAP in our study, a significant difference was not detected with regard to mean damage score. This result shows that 6 mmHg IAP can be a critical value that starts lung damage. Severe alveolar edema was detected in both 9- and 12-mmHg IAP-applied groups. Hence, it was concluded that significant lung damage with regard to mean damage score starts at an IAP value of 9 mmHg. A significant difference with regard to presence of lung damage and mean damage score was not detected between 9- and 12-mmHg IAP-applied groups.
In one of our previous experimental studies, it was reported that testicular damage occurred at an IAP of 10 mmHg [12]. However, in one of our later studies published recently, when late results (after 6 weeks) of the same experimental model was examined, it was detected that testicular damage occurred at 10-mmHg IAP, and that this situation could lead to sub-/infertility [15]. In our study, significant testicular damage was detected at 9- and 12-mmHg IAP-applied groups. However, there was no significant difference with regard to the presence of testicular damage and mean testicular damage score. The results of our study include histopathological data related to tissue damage obtained in early period. In consequence, we consider that late-period data are required to find out whether this damage is irreversible and causes infertility. In the study performed by Rifaioglu et al. [16], it was reported that testicular damage occurred at 15-mmHg IAP. In the study performed by Istanbulloglu et al. [17], an increase in testicular germ cell apoptosis level at 20-mmHg IAP was reported. Both the results of our study and the results of other studies in literature support the opinion that the testicle tissue is very sensitive to IAP increase. In contrast to other studies in literature, our study results include data regarding IAP values lower than 10 mmHg. So, we consider that the results of our study can contribute to determine safe IAP values that can be used in the clinical practice.
Schäfer and Krähenbühl [18] suggested that the IAP should be kept between 8 and 12 mmHg during laparoscopy to minimize alterations in blood flow and consequent oxidative stress-related adverse effects [19]. However, there are studies in the literature reporting that organ functions are negatively affected at IAP values lower than 8 mmHg [6, 7]. This situation exposes the necessity of studying lower IAP values and/or the development of protective agent or techniques reducing these negative effects. Glantzounis et al. [20] has emphasized that to prevent the adverse effects of increased oxidative stress caused by laparoscopic procedures in critically ill patients, there is a need for new research on the potential protective value of antioxidant therapy or alternative methods using a non-gas-using technique [19]. Some studies in literature also support this opinion [14, 19, 21, 22].
This study was conducted in rats, so exact clinical application is not practically possible. However, there are studies reporting the effects of IAP on physiologic parameters [6, 7] in pressures lower than the recommended IAP values of 8–12 mmHg [18]. On the basis of this knowledge, discussions on clinically safe IAP values are continuing. For this reason, in this study, we aimed to introduce the effects of standard IAP values used in clinical practice and lower IAP values on rats. Data obtained from the study revealed safe IAP values between 6 and 9 mmHg. Results of this study denote the need of clinical studies evaluating lower pressure values than the standard IAP used in clinical practice and may be a basis for future studies.
The results obtained in our study reveal the necessity for keeping IAP levels low both in laparoscopic operations and patients followed in intensive care. Significant damage was not observed in testicle and lung tissue at 6-mmHg IAP, but at 9 mmHg. So, a precise safe IAP value could not be detected. Based on the results of this study, it can be said that safe IAP value is between 6 and 9 mmHg. However, we consider that studies performed at pressure values between 6 and 9 mmHg are required to obtain a precise pressure value. Besides, time was limited to 1 h because we focused on low pressure values. Thus, we think that time-oriented studies are needed to reveal the changes that may be observed in a longer time.
In conclusion, IAP increase affects the functions of all organs. Practically, IAP should be kept between 8 and 12 mmHg both in laparoscopic operations and in intensive care patients. However, studies in literature that show organ functions are affected at IAP values lower than the proposed values present the necessity for lowering these values and/or development of protective agent or alternative methods that will eliminate these effects. An important limitation of the studies in literature is that they do not include simultaneous information regarding the organ functions in different body compartments. We consider that the results obtained in this study can contribute to determine safe IAP values to be preferred in clinical applications, as organ damages in different body compartments were simultaneously examined. According to the data obtained in our study, when organs in different body compartments are assessed as a whole, we predict that IAP values between 6 and 9 mmHg are safe. However, we believe that this study should be supported by new experimental and clinical studies to be performed at pressure values between 6 and 9 mmHg.
Conflict of interest
The authors have no conflict of interest and no funding exists.
Abbreviations
- IAP:
-
Intra-abdominal pressure
References
Kesici U, Kesici S, Polat E, et al. Effect of intra-abdominal pressure increase on intestinal ischemia and bacterial translocation in experimental sepsis model. Saudi Med J. 2011;32(8):813–17.
Saggi B, Sugerman H, Ivatury R, Bloomfield G. Abdominal compartment syndrome. J Trauma. 1998;45:597–609.
Kenneth WS, Richard JL. Abdominal packing for surgically uncontrollable hemorrhage. Ann Surg. 1992;215:467–75.
Ivatury RR, Diebel L, Porter JM, Simon RJ. Intra-abdominal hypertension and the abdominal compartment syndrome. Surg Clin North Am. 1997;77:783–800.
Malbrain ML. Intra-abdominal pressure in the intensive care unit: clinical tool or toy? In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. Berlin: Springer-Verlag; 2001.
Dessol S, Rubattu G, Capobianco G, Caredda S, Cherchi PL. Utility of bipolar electrocautery scissors for abdominal hysterectomy. Am J Obstet Gynecol. 2000;183:396–9.
Kologlu M, Sayek I, Kologlu LB, Onat D. Effect of persistently elevated intra-abdominal pressure on healing of colonic anastomoses. Am J Surg. 1999;178:293–7.
Akyol A, Ulusoy H, Imamoglu H, et al. Does propofol or caffeic acid phenethyl ester prevent lung injury after hindlimb ischaemia-reperfusion in ventilated rats? Injury. 2006;37:380–7.
Tekinbaş C., Ulusoy H, Yulug E, Alver A, Muharrem Erol M, Yenilmez E, Geze Ş, Topbaş M. One-lung ventilation: for how long? J Thorac Cardiovasc Surg. 2007;134 405–10.
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic and metabolic reappraisal. Arch Surg. 1970;101:478–83.
Cosentino MJ, Nishida M, Robinowitz R, et al. Histopathology of prepubertal rat testes subjected to various durations of spermatic cord torsion. J Androl. 1986;7:23–31.
Imamoğlu M, Cay A, Unsal MA, et al. The effects of increased intra-abdominal pressure on testicular blood flow, oxidative stress markers, and morphology. J Pediatr Surg. 2006;41:1118–24.
Ozmen MM, Aslar Kessaf A, Besler HT, Cinel I. Does splanchnic ischemia occur during laparoscopic cholecystectomy? Surg Endosc. 2002;16:468–71.
Karapolat S, Gezer S, Yildirim U, Dumlu T, Karapolat B, Ozaydin I, Yasar M, Iskender A, Kandis H, Saritas A. Prevention of pulmonary complications of pneumoperitoneum in rats. J Cardiothorac Surg. 2011;6:14.
Imamoglu M, Sapan L, Tekelioglu Y, Sarihan H. Long-term effects of elevated intra-abdominal pressure on testes an experimental model of laparoscopy. Urol J. 2013;10(3):953–9.
Rifaioglu MM, Davarci M, Nacar A, Alp H, Celik M, Sefil NK, Inci M. Caffeic acid phenethyl ester (CAPE) protects against acute urogenital injury following pneumoperitoneum in the rat. Ren Fail. 2014;36:98–103.
Istanbulluoglu MO, Piskin M, Zor M, Celik A, Ozgok A, Ates M, Ustun H, Ozgok Y. The acute effects of increased intra-abdominal pressure on testicular tissue: an experimental study in pigs. Urology. 2011;77(2):510.e12–6. doi:10.1016/j.urology.2010.06.009.
Schäfer M, Krähenbühl L. Effects of laparoscopy on intra-abdominal blood flow. Surgery. 2001;129:385.
Cay A, Imamoğlu M, Unsal MA, Aydin S, Alver A, Akyol A, Sarihan H. Does anti-oxidant prophylaxis with melatonin prevent adverse outcomes related to increased oxidative stress caused by laparoscopy in experimental rat model? J Surg Res. 2006;135(1):2–8.
Glantzounis GK, Tselepis AD, Tambaki AP, et al. Laparoscopic surgery-induced changes in oxidative stress markers in human plasma. Surg Endosc. 2001;15:1315.
Yilmaz S, Polat C, Kahraman A, et al. The comparison of the oxidative stress effects of different gases and intra-abdominal pressures in an experimental rat model. J Laparoendosc Adv Surg Tech A. 2004;14:165.
Demirbas M, Güler C, Samli M, et al. The effect of verapamil on the prevention of ischemia/reperfusion injury in the experimental retroperitoneoscopic donor nephrectomy model. Surg Endosc. 2004;18:1272.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aydin, H., Kesici, S., Kesici, U. et al. Effects of different intra-abdominal pressure values on different organs: what should be the ideal pressure?. Eur Surg 46, 203–208 (2014). https://doi.org/10.1007/s10353-014-0271-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-014-0271-y