Abstract
Small reefal bioconstructions that developed in lagoonal settings are widespread in a few horizons of the Late Jurassic (Oxfordian) succession of the Korallenoolith Formation, exposed southwest of Hannover, Northwest Germany. Especially the florigemma-Bank Member, “sandwiched” between oolite shoal deposits, exposes a high variety of build-ups, ranging from coral thrombolite patch reefs, to biostromes and to coral meadows. The reefs show a distribution with gradual facies variations along an outcrop belt that extends about 30 km from the Wesergebirge in the NW to the Osterwald Mts in the SE.
The patch reefs from the Deister Mts locality at the “Speckhals” are developed as coral-chaetetid-solenoporid-microbialite reefs and represent a reef type that was hitherto unknown so far north of its Tethyan counterparts. They are mainly built up by coral thickets that are preserved in situ up to 1.5 m in height and a few metres in diameter. They contain up to 20 coral species of different morphotypes but are chiefly composed of phaceloid Stylosmilia corallina and Goniocora socialis subordinately. The tightly branched Stylosmilia colonies are stabilized by their anastomosing growth. The coral branches are coated with microbial crusts and micro-encrusters reinforcing the coral framework. Encrusters and other biota within the thicket show a typical community replacement sequence: Lithocodium aggregatum, Koskinobullina socialis and Iberopora bodeuri are pioneer organisms, whereas the occurrence of non-rigid sponges represents the terminal growth stage. The latter are preserved in situ and seem to be characteristic so far poorly known constituents of the Late Jurassic cryptobiont reef dweller community. The distance and overall arrangement of branches seems to be the crucial factor for the manifestation of a (cryptic) habitat promoting such community replacement sequences. Widely spaced branches often lack any encrusting and/or other reef dwelling organisms, whereas tightly branched corals, as is St. corallina, stimulate such biota. Hence, such reefs are well suited for research on coelobites and community sequences of encrusting and cavity dwelling organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coral reef development reaches a climax in the Late Jurassic (Kiessling et al. 1999; Leinfelder et al. 2002), and a wide variety of different coral reef types were established in different environmental settings. This can be attributed to a widened “reef window” during that time (Leinfelder and Nose 1999; Leinfelder et al. 2002). Owing to intensified examination of these reefs, the knowledge about their faunal composition, structure, palaeoecology, and sedimentology increased significantly during the last decades (see Leinfelder 2001, and references therein; Schmid et al. 2001). Due to a more equilibrated greenhouse type climate, Late Jurassic reefs are distributed in high latitudinal settings – up to about 40° north – as well as in the low latitude reef belt of the Tethyan realm (e.g., Leinfelder et al. 1993, 1994, 1996; Leinfelder 2001). In contrast to the reefs from the Tethys, those from the high latitudes of the North-Tethyan shelf areas (sensu Leinfelder et al. 2002), for example from southern Great Britain and northwestern Germany, are only insufficiently known (Insalaco et al. 1997; Insalaco 1999; Leinfelder et al. 2002: 504). Therefore, a comparison between them and the Tethyan reefs was almost impossible. Insalaco (1999) described Middle Oxfordian coral reefs from southern Great Britain. He recorded only a few eurytopic and r-selected corals that dominate the reef building assemblages even in highly different local environments and ecological conditions. These assemblages frequently display characters of pioneering communities among which typical Tethyan elements, especially micro-encrusters (Leinfelder et al. 1993; Schmid 1996) and microbialite, widely to completely lack. These observations agree with the environmental instability model developed by Fürsich and Sykes (1977) for the subboreal faunal province in the Upper Jurassic.
The Northwest German coral reefs of the Korallenoolith Formation that developed contemporaneously at similar palaeolatitudes in the European archipelago (Fürsich and Sykes 1977) were expected to show largely similar features. This was questioned by the observations of Helm and Schülke (1998, 1999), Helm et al. (2001, 2003b: concerning reef corals, 2003c: concerning calcareous algae), Reuter et al. (2001), and Helm (2005) who were able to demonstrate the close palaeobiogeographical affinity of many Northwest German reefs to the Tethyan ones.
The reefs presented in this paper are patch reefs that mainly consist of branched corals (Stylosmilia corallina and Goniocora socialis). Similar coral thickets show a worldwide distribution and are developed as patch reefs, biostromes, or coral meadows, the latter mostly consisting of only one coral species. Such reefal bioconstructions are largely restricted to lagoonal environments in very shallow water and less turbulent conditions. Although the overall fossil record of such build-ups is rather good (Roniewicz and Roniewicz 1971; Eliášová 1981; Beauvais 1985; Werner 1986; Geister and Lathuilière 1991; Flügel et al. 1993; Fookes 1995; Geister 1995; Nose 1995; Bertling and Insalaco 1998; Helm and Schülke 1998; Laternser 2001; Leinfelder et al. 2002; Olivier et al. 2003, 2004, 2006), Late Jurassic coral bioconstructions mainly formed by branched corals are rarely examined in detail and poorly known so far. The Northwest German reefs can be taken as type examples for the growth of such build-ups and the associated epifaunal communities (see Vytopil and Willis 2001). The descriptions also add further detail to reefal development in the Northwest German Late Jurassic.
Late Jurassic coral reef development in NW Germany
During the early Late Jurassic, calcareous clay – to sandstones (Heersum Formation) were deposited in the Lower Saxony Basin, which are overlain by the platform carbonates of the Korallenoolith formation extending from the Middle Oxfordian to the earliest Early Kimmeridgian (Gramann et al. 1997; Helm et al. 2001). Corals and coral bioconstructions are largely restricted to five horizons within the Korallenoolith Formation (Helm et al. 2003b, and references therein) that is mainly formed by oolites.
The Untere Korallenbank Member and the florigemma-Bank/Hainholz Member are the most important reef-bearing intervals. The corals of the Untere Korallenbank Member form biostromes that have a lateral extent of several kilometres and reach up to 10 m in height. The low-diversified coral fauna is dominated by ubiquitous platy forms like Thamnasteria concinna, Isastrea, and turbinate solitary corals (Montlivaltia). Micro-encrusters and microbial crusts largely lack, whereas encrusting oysters, serpulids, and bryozoans as well as other reef dwellers (molluscs, brachiopods) occur frequently (Bertling 1989, 1993, 1994, 1997a,b; Schülke et al. 1998).
The florigemma-Bank Member is the most widespread coral-bearing horizon intercalated in a sequence of oolite shoal deposits. The term “florigemma-Bank” refers to the occurrence of echinoid spins of Paracidaris florigemma (Phillips) in this Member. However, remains of P. florigemma are of no stratigraphic value, since spins of this echinoid species are also present in the footwall deposits and the overlying strata. The biostratigraphical assignment of the florigemma-Bank Member (middle Oxfordian, variocostatus subzone; for discussion see Helm et al. 2003c) is based on rare and poorly preserved ammonoid specimens that were recovered during the last decades from deposits below and above the Member.
Although reef-like bioconstructions are widespread in the florigemma-Bank Member, they were largely unconsidered in the regional literature save a few short notes (Klüpfel 1931; Lambelet 1968; Bertling 1989, 1993; Mönnig and Bertling 1995). The facies mapping of the florigemma-Bank Member in its distribution area resulted in a characteristic facies and reef type distribution patterns (Helm et al. 2003b, and references therein). The spatial variations of reef types appear to be mainly controlled by both local scale tectonic movements in the course of the North Sea rift development and eustatic sea-level fluctuations (Ziegler 1990; Jacquin et al. 1998; Schülke et al. 2004).
In the Süntel Mts, extending over an area of approximately 50 km2, there occur small patch reefs up to a few metres in diameter and less than 4 m in height. The patch reefs are embedded in a micritic lime-mud inter-reef facies. Most of these reefs are formed by coral-thicket dominated by ramose Thamnasteria dendroidea Lamouroux (Helm and Schülke 2000a; Helm 2005). These coral thickets are strengthened by microbialite that exhibits frequent (micro-)encrusters. At a few localities, in particular overlying the patch reefs, large solenoporids occur in considerable amounts (Helm and Schülke 1998; Helm 2005).
East of the Süntel Mts, in the Kleiner Deister area, Th. dendroidea forms meadows of mostly thin branched and open-spaced colonies with usually upward directed branches (sparse pillarstone sensu Insalaco 1998). Microbial crusts and micro-encrusters largely lack. The coral meadows are interpreted as sediment-stressed because they were continuously covered with carbonate deposits during growth and did not elevate above the sediment surface (constratal growth fabric; Insalaco 1998).
North of the Kleiner Deister area, in the Deister Mts, the florigemma-Bank is excellently exposed and forms outcrop strings of about 5 km in lateral extent. According to Helm and Schülke (2000b), a threefold lateral facies variation can be observed: (1) biostrome facies, (2) coral-bearing marl facies, and (3) patch reef facies. The presence of chaetetids (Helm and Schülke 2000b; Helm et al. 2001) is a unique feature of the florigemma-Bank in the Deister Mts since they were so far only known from Tethyan type reefs. The biostrome facies reaches up to 3.5 m in thickness and is formed by a coral-microbialite community that has a lateral extent of several km2. Almost all possible coral morphotypes (platy, hemisphaerical, pseudocolumnar, ramose, phaceloid, dendroid, solitary) occur in this particular biostrome that is overlain by a horizon rich in large solenoporaceans. In the coral-bearing marl facies scattered corals and rare chaetetids are imbedded in fossiliferous lime-mud (e.g., Hirschkopf section, see Schülke and Helm 2000). The patch reef facies in the Deister Mts is characterized by coral thickets that are described in detail in the following chapters.
The largest occurrences of coral reefs are situated in the Hainholz Member (Helm et al. 2003a; Oberer Korallenoolith after Hoyer 1965), the presumed stratigraphical equivalent of the florigemma-Bank Member in the Osterwald Mts. They consist of patch reefs up to 12 m in height which are embedded in reefal debris (Reuter et al. 2001; Helm et al. 2003a).
Investigated sections
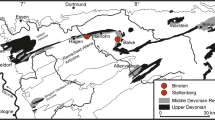
Two abandoned quarries that expose patch reefs in the Deister Mts, north of the town of Springe and about 25 km in the southwest of Hannover, Lower Saxony (Fig. 1) are investigated in detail. Further data about the regional distribution of this facies results from mapping (screening of hand samples) in the adjacent areas.
The first quarry (section no. 20 following Hoyer 1965; r 3538790 h 5789990) is situated near the Speckhals (Fig. 1; Speckhals quarry in the following). It has a lateral WE-extent of approximately 100 m and exposes the sedimentary succession up to 9 m in thickness of the gently N-dipping Korallenoolith Formation. The eastern part of the quarry is largely covered by rubble and dense vegetation, whereas the strata in the southern, western, and northwestern parts are well exposed, though their surfaces are frequently covered with algae and lichen. Due to the comparatively long lasting exposure of the rock surfaces, the massive patch reef units are less weathered when compared to the surrounding peri-reefal deposits (see Fig. 2A).
Abandoned Speckhals quarry, Deister Mts, NW Germany; corals of the florigemma-Bank Member. A Abandoned Speckhals quarry I, southern quarry wall (height of quarry wall is about 8 m, black arrow indicates hammer for scale). The section starts with bedded oolite that is overlain by reefal deposits of the florigemma-Bank Member: a coral thrombolite patch reef (thicket) is exposed in the centre of the photograph. Note lateral facies transition into indistinctly bedded micritic peri-reefal limestone. Bedded oolite follows up-section. B Goniocora socialis, a coral species similar to St. corallina; the interstices between the branches are filled up with fossiliferous micritic limestone. Arrow points to extratentacular budding. Thin-section Sph-M-1.1 m. C Submassive colony of St. corallina. Note calcareous cement layer connecting single branches. Thin-section Sph-IX-0.35 m(3). D Colony of Stylosmilia corallina in longitudinal section. Note fused fasciculate branches. T, in situ tetractinellid demosponge; S, serpulids; B, bryozoans; sp, shelter pores. Bivalve boreholes are circled with a black line. Thin-section Sph-IX-0.35 m(1)
The second quarry (section no. 21 following Hoyer 1965, r 3538960 h 5790300) is situated about 400 m NNE of locality 1 on the northern slope of the Speckhals (Fig. 1). Only the base and the middle part of the florigemma-Bank Member are poorly exposed in its easternmost part.
Methods
At locality 1 (Fig. 1), 11 sections of up to 3.5 m in thickness and containing the florigemma-Bank Member were analysed. Samples were taken at intervals of 10–20 cm. Due to favourable outcrop conditions, we focused on the western part of the quarry. The position of the sections is given in Fig. 1, sections C, E, F, I, J, and K are shown detailed in Fig. 3b. Owing to the bad exposure conditions of the succession at locality 2, only one section has been investigated (Fig. 3a). Bulk sampling and standard preparation techniques led to a total number of 290 polished slabs and 110 thin-sections. In addition, large samples of reefal limestone were broken down and prepared using hydrogen peroxide. These samples were examined for borings and resolution moulds (comp. Fürsich et al. 1994a; Bertling and Insalaco 1998), e.g., of corals (Fig. 4D). Several of these external moulds revealed detailed micromorphology of skeletal structures and were casted with latex (Fig. 2B, E–G). The facies examinations in both field analyses and microscopic studies resulted in a three-dimensional reconstruction of the Stylosmilia/Goniocora patch reef community (Fig. 5).
The figured thin-sections, polished slabs, moulds, and latex casts are reposited in the collection of the Institut für Geologie und Paläontologie, University of Hannover under catalogue no. GPH 2002 III 1-ff.
Facies description
Pre-florigemma-Bank Member deposits
Bedded and well-winnowed oolite (grainstone), which thickness amounts to at least 4 m in quarry 1 underlies the deposits of the florigemma-Bank. It is formed by ooids, grapestones/lumps, and some oolite-clasts. Bioclasts are mainly represented by echinoderm and Terquemella fragments. Remains of bryozoans, molluscs, and foraminifers (in particular lenticuliniids, textulariids, and Everticyclammina sp.) occur in minor amounts. Larger bioclasts and ooids are frequently encrusted by foraminifers. The oolite grades vertically into fossiliferous packstone to rudstone (Fig. 4A) followed by wackestone to floatstone (Fig. 6H) of the peri-reefal facies.
Corals from patch reef of the florigemma-Bank Member exposed in the Deister Mts. A Fossiliferous oolite from the base of the florigemma-Bank Member with a small coral colony (cf. Stylina) growing on an oyster shell fragment. Polished slab Sph-L. B Stylina tubulifera, Sample B (latex cast). C Specimen of the solitary caryophyllid coral Caryophyllia cf. suevica encrusted by serpulids; bottom: cross-section (central cut) through the corallite that exposes the spongeous columella; top: cross-section of the uppermost part of the corallite. Thin-sections Sph-VI-1.75 m(1) and Sph-VI-1.75 m(2). D and E Ramose branches of cf. Actinastraea ramulifera preserved as external moulds (D sample A1; E latex cast, sample A3). The margins of the septa are regularly ornamented with granules. F and G Calicular surface of cf. Dendrohelia coalescens showing small irregular-spaced corallites and granulated coenosteum surface. Sample Sph-IV-0.6 m (latex cast)
florigemma-Bank Member
The florigemma-Bank Member consists of reefal facies (Stylosmilia/Goniocora thickets and Stylina patch reefs) and peri-reefal facies (Figs. 2A and 7). Its thickness reaches 2–2.5 m. A summary of reef organisms and organisms of the peri-reefal facies is given in Figs. 8 and 9.
Reefal facies
Stylosmilia/Goniocora thickets
This is the most prominent reef type within the florigemma-Bank Member in the Speckhals area. The outline of the thickets that are well exposed in the western part of the Speckhals quarry 1 is typically ellipsoid in vertical section (Fig. 2A). The thickets reach up to a few metres in diameter and up to 2 m in height. They are either grouped together with only a few metres of peri-reefal deposits between each reef (Figs. 5 and 7), or they are loosely spaced.
The thickets are made up by a framework that is formed by the fasciculate Stylosmilia corallina (Figs. 2C and D) and Goniocora socialis (Fig. 2B) subordinately. The corals are generally preserved in life position and have only scarcely been toppled over. Large hemispherical colonies of Stylina tubulifera (Fig. 4B) are abundantly interspersed among the thickets. Other frequent corals are ramose colonies of cf. Actinastraea ramulifera (Fig. 4D and E). Plate-like colonies of Actinastraea pentagonalis, Isastrea, and microsolenid corals are less common. Rare reef dwelling coral species that do not contribute to the reefal framework are Actinaraea granulata, Thamnasteria concinna, Thecosmilia cf. trichotoma, Microphyllia spp., Dimorpharea sp., Cyathophora sp., Calamophyllia moreauana, cf. Caryophyllia suevica (Fig. 4C), Montlivaltia sp., and Rhipidogyra costata. According to the classification of Insalaco (1998), the reef growth fabric fits well with the dense pillarstone.
Individual colonies of the dominant phaceloid corals (St. corallina and G. socialis) are up to a few dm in diameter and resemble each other in thin polished slabs to a high degree with regard to the cylindrical corallite diameter (2.5–4 mm), the hexamer septal cycle with 24 septa, the weakly to strongly developed costae, and the styliform columella. Moreover, the septa of the first cycle of both species are often fused with the columella. However, there are significant differences concerning the branching mode and the arrangement of corallites (Lauxmann 1991). Unfortunately, these features are hardly recognizable in the field because the branches are covered with microbial crusts. Details are only visible in external moulds. In G. socialis, the budding is extratentacular (Fig. 2B), commonly in pairs of laterally opposing corallites. Over short distance the branches are bent up and grow subparallel to the predeceding corallite in a comparably wide distance. In St. corallina, the budding is also extratentacular, but the corallites are more or less closely packed within a colony, and commonly anastomotic. This branch arrangement results in submassive coralla (Turnšek 1975: pl. 2/4, 1997: 196, fig. A–F; Errenst 1990: 176; Reuter et al. 2001: fig. 7/4; Helm et al. 2003b: Fig. 11E). In addition, the costae of St. socialis are granulated, whereas those of G. socialis are rather rib-like.
Cf. Actinastraea ramulifera exhibits ramose colonies with widely spaced branches that reach ca. 5 mm in diameter; budding is rare (Fig. 4D and E). The calices are small and expose a columella. Twenty-four confluent to subconfluent septa are commonly developed, but smaller numbers of septa can also occur. Moulds of the branches show a fine granulation of the septa. The calices are frequently polygonal and appear to be cerioid. In other cases, even on the same branch, the calice boundaries are less sharp and the septa are thamnasterioid. The Northwest German material agrees well with the descriptions of Rosendahl (1985) and Lauxmann (1991), but the separation at species level remains uncertain due to the weakly developed walls towards adjacent corallites. The colonies of cf. Act. ramulifera have been observed mainly at the base of the thickets.
Within the Stylosmilia/Goniocora thickets, the interstices between coral branches are almost completely filled by microbialite, micro-encrusters, and cavity dwelling fauna. In the southwestern part of the Speckhals quarry (exposed along sections G and H), reticulate to dendroid microbialite (sensu Schmid 1996) overlies the thicket facies and usually wraps foliaceous microsolenid corals (poorly preserved and commonly leached colonies of cf. Actinarea granulata), Thecosmilia cf. trichotoma, and scarcely St. corallina/G. socialis.
Stylina patch reefs
In the Stylina patch reefs from the eastern part of the Speckhals quarry 1 (Figs. 3 and 7, section K), massive corals are more common than the above listed framebuilding branching corals. They predominantly consist of hemispherical to plate-like plocoid or cerioid colonies, varying around 10 cm in diameter. In order of decreasing frequency, the Stylina patch reefs contain the following corals: Stylina tubulifera (Fig. 4B), Heliocoenia variabilis, Actinastraea pentagonalis, Microphyllia spp., Isastrea sp., Stylina limbata, and Dendrohelia coalescens (Fig. 4F and G). The branched St. corallina, G. socialis, cf. Act. ramulifera, and Th. cf. trichotoma also occur abundantly but do not dominate the frame builders. Plate-like microsolenid corals are only of minor importance. The reef growth fabric (sensu Insalaco 1998) corresponds to domestone.
As in the Stylosmilia/Goniocora thickets, the framework is heavily encrusted by microbialite. Towards the top, the microbialite encrustation increases. The other encrusting fauna largely resembles that of the thicket facies.
Microbialite, associated reef organisms, and bioerosional organisms
Microbes are secondary reef builders, and microbialite significantly contributes to the framework. They commonly encrust the lateral surfaces of branched corals strengthening the framework or form up to a few cm thick coatings on the surfaces of massive corals. Microbial crusts at the sides of single coral colonies or thickets are developed as down-facing hemispheroids (Leinfelder and Schmid 2000) or mamillated microbialites (Olivier et al. 2003). This type has frequently been observed in Late Jurassic coral-thombolite reefs (Leinfelder 2001), in particular as wrappings of bushy corals (Nose 1995: 88; Olivier et al. 2003, 2004, 2006). The microbial crusts show mostly clotted to peloidal textures. According to the classification of Jurassic microbialite fabric offered by Schmid (1996), the following types have been observed: pure, clotted, and layered thrombolite as well as clotted and pure leiolite.
Encrusting serpulids are common in the studied reefs (Figs. 2D and 4C), but only “Glomerula” is distinguishable due to its round tubes. Apart from Terebella lapilloides that usually develops small tubes up to 1 mm in diameter, there occur specimens with larger tube diameters varying around 3 mm. However, variations in tube diameter and wall thickness are well known from Terebella in southern Germany (e.g., Brachert 1986). A few large arenaceous tubes agglutinating ooids, peloids, foraminifers, and spiculae have been observed, fixed on coral branches. They reflect a hitherto undescribed species of “terebelloid” polychaets. Additional material of this type is figured and referred to by Herrmann (1996: pl. 8/6, “agglutinated polychaetid worm gen. et sp. indet.”) from the Oxfordian of central Dobrogea (eastern Romania).
Sponges from patch reefs of the florigemma-Bank Member exposed in the Deister Mts. A Non-rigid tetractinellid sponge, a common cavity dweller of Stylosmilia/Goniocora colonies. The megascleres are preserved in situ and are embedded in clotted peloidal matrix. Thin-section Sph-IX-0.35 m(1). B and C Non-rigid tetractinellid sponge exhibiting in situ cementation of calthrop megascleres and microscleres (C: detail of B); thin-section Sph-IX-0.35 m(2). D and E Mesohyl area of non-rigid pachastrellid sponges showing dichotriaene dermal spicules. D: thin-section Sph-Br.-0.9 m(1), E: thin-section Sph-Br.-0.9 m(1). F Eudea cf. globata attached to a microbial crust. The sponge skeleton is covered with a rigid hemispherical cortex. Two openings with slightly elevated margins are visible. Thin-section Sph-Br.-0.9 m(2). G Ptychochaetetes polyporus growing on Thecosmilia sp. Thin-section Sph-IX-2.95 m. H Peri-reefal deposits with two nodules of Ptychochaetetes polyporus (P) and a branched colony of Thecosmilia sp. (branches encircled with black line) with varying stages of preservation (well-preserved corallites together with leached corallites). Polished slab Sph-VI-1.5 m
Facies architecture of the florigemma-Bank Member exposed in the western part of the Speckhals quarry 1 with location of sections A–I. The drawing is based on field mapping and shows the general distribution of patch reefs, their geometry, and most important framework building corals and microbialite as well as accompanying fauna/flora of the peri-reefal deposits; see Fig. 3B for legend
Fossil content of the florigemma-Bank Member except for coral species (shown in Fig. 8) with information on habitat (patch reefs versus peri-reefal deposits), semiquantitative abundance, and guild assignment
Among the foraminifers, nubeculariids and “Placopsilina” are dominantly present in the reefs. The latter is very similar to Haddonia sp. figured by Bucur et al. (1996) and commonly occurs enriched in clusters. The Lithocodium aggregatum/Troglotella incrustans-consortium (Schmid 1996; Schmid and Leinfelder 1996) is less frequent. Attached foraminifers with calcareous hyaline tests belonging to Bullopora (B. tuberculata and B. rostrata), Ramulina, Ramulinella, and Tentilenticulina latens also occur. They preferently inhabit micro-cavities within coral colonies (Fig. 11L). T. latens has only rarely been observed and seems to have a regionally restricted distribution, because it is so far only known from mid-Oxfordian coral reefs of the Coral Rag, England (Hitchings 1980; Insalaco 1999) and northwestern Germany (Helm 2005).
Sheet-like bryozoans of the “Berenicea”-group (Fig. 11K), which includes different genera (Walter 1969), are common. To a lesser degree, runner-like bryozoans (Stomatopora, “Proboscina”) are present. “Massive” colonies of bryozoans similar to Kolophos in thin-sections (Fig. 11J) occur only rarely. Boring bryozoans have been identified only by their boreholes (Talpina).
Concerning the brachiopods, the pedicle-attached Septaliphoria pinguis occurs abundantly in interstices of the framework. In addition, thecideidinids are very abundant (Fig. 11M). They are represented by Rioultina pustulosa, which has been described from a section southwest of the Speckhals area (Schülke and Helm 2000).
Sponges are very common in the patch reefs (Fig. 6A–H). Apart from chaetetids (Ptychochaetetes polyporus; Fig. 6G and H), the sponges are rather small and normally do not exceed 1 cm in size. They are represented by different taxonomic groups including sponges with rigid skeleton and non-rigid (soft-bodied) sponges. Owing to difficulties in the determination of sponge remains in thin-sections, the number of species that contribute to the patch reef fauna is uncertain but seems to be moderately high. Most frequent are non-rigid tetractinellid demosponges with in situ preserved tetractinellid megascleres and micro-triaens. Such aggregations of megascleres and micro-triaens are surrounded by bean-shaped rhaxes (Fig. 6A–C). Non-rigid pachastrellid sponges with dichotriaen megascleres in the mesohyl (Fig. 6D and E) and sterraster type microscleres in the dermal layer are rare and belong to the accessory fauna. All non-rigid sponges are restricted to the cavities left in the thickets after coating of coral branches with microbialites and encrusting organisms. They are preserved in situ and are imbedded within a mostly peloidal matrix that results from sponge automicrite formation (Delecat et al. 2001).
Such reef dwelling non-rigid sponges belong to the rather poorly known organisms (Leinfelder 2001: 266). Cryptic non-rigid sponges communities similar to the described material are known to occur elsewhere in the Lower Saxony Basin (Reitner 1994; Delecat et al. 2001: Lower Kimmeridgian oyster patch reefs characterized by prolific non-rigid sponge growth; Helm and Schülke 1998, 1999; Helm 2005: Oxfordian Thamnasteria dendroidea patch reefs exposed in the Süntel Mts).
Pharetronid Calcarea with basal skeleton are less frequent. The observed specimens comprise forms with stout and strongly linked fibres, but also specimens with fine reticulate skeleton structure (comp. Delecat et al. 2001: pl. 38/1). Specimens of Eudea cf. globata are rarely present. They are a few mm in diameter and globular in outer shape. Their surface is covered with a dermal layer (cortex) with apertures (Fig. 6F). Coralline sponges are rather rare and are represented by Neuropora. Additional sponges comprise clionids that belong to the destroyer guild.
Apart from small reef dwelling gastropods, numerous specimens of small patelloid gastropods (limpets) are recognized in the patch reefs (Fig. 11H). They belong to Emarginula sp. (comp. Jaintly et al. 2000). The surface ornamentation of cap-shaped shell appears grid-like due to the equally pronounced development of concentric and radial ribs. The patelloid gastropods occur in small reef cavities or dwell inside phaceloid coral colonies.
The examined patch reefs exhibit a wide variety of bioerosion features. They are extensively penetrated by bivalves that locally removed about 50% of the microbialite volume. The lithophagids are the most common of these taxa (Lithophaga subcylindrica and others; comp. Fürsich et al. 1994a). Also, a probably new pholadid species of the genus Jouannetia has frequently been identified. Borings of gastrochaenids are less frequent. They derive from two different species. A rare form is Gastrochaenopsis sp.. Its valves occur in elongated, flask-like borings (Gastrochaenolites dijugus). The valves of Carterochaena pulcherrima can also be observed in the same type of boreholes.
Bivalve borings penetrate microbialite as well as coral skeletons. The boreholes abundantly cut through both substrates (Fig. 2D). Lithophagid specimens were observed to primarily attack coral skeletons (nearly 80%), whereas about 75% of the Jouannetia sp. borings are found in microbial crusts. Gastrochaenid borings are present in corals as well as in microbial crusts, but their relatively small number does not allow a statistical analysis.
Apart from the juvenile boring stage of the foraminifer Troglotella incrustans (Fig. 11I), microborings are represented by small pits, straight or digitating tubes, and tubes forming networks. Occasionally, structureless leiolite is penetrated by boring fungi (sensu Reitner 1994) which form anastomosing and digitating tubes subsequently filled with sparite. The following forms of several larger borings have been observed: long tube-like borings between 0.1 and 2 mm in diameter (Trypanites ichnsp.), camerate and noncamerate borings of clionid sponges (Entobia ichnsp.; Fig. 11L), borings of cirripedians (Rogerella ichnsp.), spirally coiled, 0.2–0.4 mm wide borings (Spirichnus spiralis), phoronid borings (Talpina ichnsp.), and a single occurrence of a brachiopod boring (Podichnus ichnsp.).
Peri-reefal deposits
The poorly bedded limestone deposits that represent the peri-reefal facies have a micritic matrix and are highly fossiliferous (microfacies vary from wackestone/packestone to floatstone). These deposits abut sharply against the patch reefs whereas the transition from the underlying oolite is gradual: ooids become scarce and pelmicrites to fine calcarenites prevail upsection. However, the transitional horizon is also a stratigraphical equivalent to the onset of the reef development because it passes laterally into coral limestone that represents the base of the coral thickets (Fig. 3). Thus, ooid sedimentation continued during the beginning reef growth and ceased successively.
The deposits of the transition zone and those directly underlying the patch reefs are enriched with reef debris (rudstone/floatstone; Fig. 4A). The reef debris is generally well preserved and consists of broken massive or branched corals. Colonies of Thecosmilia cf. trichotoma are also present in the peri-reefal deposits (Fig. 6H). They are in life position and only rarely toppled. Other corals preferently growing in inter-reef areas are species of Microphyllia (M. brevivallis and M. thurmanni). They develop only small meandroid colonies.
Reef dwellers (bryozoans, foraminifers, thecideidinid brachiopods, gastropods) from the patch reefs of the florigemma-Bank Member and biota of the peri-reefal deposits. A and B Erect bryozoan colony with central tube, peri-reefal deposits: A cross section, thin-section Sph-VI-2.5 m; B oblique longitudinal section; thin-section Sph-VI-2.5 m. C Pelmicrite with abundant tests of Everticyclammina sp.; thin-section Sph-III-2.7 m(2). D Everticyclammina sp. in longitudinal section. The test consists of peloids and small foraminifers. Thin-section Sph-III-2.7 m(1). E Tetraxis type foraminifer (?Pseudomarssonella sp.); thin-section Sph-III-2.7 m(2). F Comaliamma gediki; thin-section Sph-III-2.7 m(2). G Reophax sp.; thin-section Sph-IV-1.9 m. H Patelloid gastropod Emarginula sp., “apex” is not preserved; sample Sph-E (latex cast). I Troglotella incrustans boring into a coral skeleton; thin-section Sph-IX-0.25 m. J Bryozoan (aff. Kolophos sp.) fixed on a St. corallina branch; thin-section Sph-IX-0.35 m(3). K Berenicean type bryozoan. Thin-section Sph-Br.-0.9 m(1). L cf. Actinastraea ramulifera branch penetrated by clionid sponge borings (Entobia ichnosp.). The cavity is inhabited by a hyaline foraminifer (Ramulina sp.). Note abundant needle-like processes of the outer wall of the worn tube-like test. Thin-section Sph-M-1.8 m. M Microbialite with two thecideidinid brachiopods (cf. Rioultina pustulosa) in cross section (above) and longitudinal section (below). Sample Sph-Br.-1.5 m
Pseudocolumnar bulbs (up to 20 cm in diameter) of Solenopora jurassica occur very frequently in the peri-reefal deposits and can be found both in growth position and toppled. Most of the bulbs expose characteristic annual growth patterns (Wright 1985), but frequently they are mutually intergrown with Bacinella irregularis. This is interpreted as “boring behaviour” of B. irregularis (Herrmann 1996; Kołodziej 1997; Dupraz and Strasser 1999; Ourribane et al. 2000). Larger components of the deposits, e.g., coral fragments, are frequently overgrown by the chaetetid sponge Ptychochaetetes polyporus that forms small nodules up to a few cm in diameter (Fig. 6G and H).
The associated benthic macrofauna appears generally well preserved. However, the preservation and, consequently, taxonomic identification suffers from the extensive lithification of the host sediments. A restricted number of gastropod, bivalve, and echinoderm species were observed occasionally (Fig. 9). Pedicle-attached brachiopods occur abundantly and are mainly represented by Septaliphoria pinguis.
Pelmicritic deposits of the inter-reef areas are partly enriched in rhaxes and Terquemella remains. Further remarkable remains are fragments of an erect bryozoan (Fig. 11A and B) that are probably detached from their patch reef habitat. Apart from rare ostracods, the microfauna also includes a diverse assemblage of benthic foraminifers of approximately 20 species (Figs. 8 and 11C–G). Most common are large tests of the lituolid foraminifer Everticyclammina sp. (Fig. 11C and D) that resemble those of Otaina magna (Ramalho). Also abundant are lenticulid species and small, coarse-agglutinated tests of Ammobaculites or Haplophragmium. Remains of the worm (?)tube Carpathiella triangulata and the enigmatic Aeoliasaccus occur only rarely.
Post-florigemma-Bank Member deposits
The deposits of the florigemma-Bank Member are overlain by a bedded and partly dolomitized oolite (grainstone). The sediments locally contain reef rubble (strongly abraded corals and nodules of Solenopora) at the base, which probably is material reworked from the underlying reefal and peri-reefal deposits. Bivalve shells are enriched in non-persistent intervening beds. In addition, oolite intraclasts, bored by bivalves (Gastrochaenolites ichnsp.), rarely occur.
The boundary between the florigemma-Bank deposits and the overlying strata is gradual by the successive increase in ooid content. Evidence of subaerial exposure (e.g., karst cavities) that is well known from the adjacent Süntel Mts (Helm 1998; Helm et al. 2002) has not been observed. However, coral skeletons from the top of the florigemma-Bank Member have been dissolved in places, and subsequently filled with sediment. This may indicate early freshwater diagenesis.
A calcarenite bed a few dm in thickness with fine quartz grains is intercalated in the oolite succession about 1 m above the top of the florigemma-Bank. This bed possibly corresponds to the siliciclastic Zwischenregion or Liegendquarzit Member (Klüpfel 1931) in the Süntel Mts.
Discussion
Environmental interpretation
The succession starts with oolites extensively showing HCS structures that were probably deposited as shoals or tidal bars under a storm-dominated turbulent high-energy environment below fair-weather wave base. The base of the florigemma-Bank Member possibly marks a major flooding surface. This deepening of considerable amplitude provided the accommodation space for the reef development. The initial patch reef growth started by coral colonization of large bioclasts that offered a sufficient hard substrate (Fig. 4A). Other favouring conditions were presumably reduced sedimentation rates during transgression and a stabilisation of the substrate (comp. Fürsich et al. 1994a; Insalaco 1999). The deposits of the florigemma-Bank Member reflect an open lagoonal depositional environment in relatively calm and shallow water above storm wave-base (<20 m). Storms account for episodically raised water energy that led to breakdown, fragmentation, and toppling of coral colonies. Organisms that preferred shallow water depths (L. aggregatum, B. irregularis, K. socialis, solenoporids, chaetetids) point to well illuminated and clear water (e.g., Leinfelder et al. 1993; Schmid and Leinfelder 1996), different from mesotrophic or eutrophic conditions during later reef growth (see below). The upper part of the florigemma-Bank Member reflects moderate shallowing. It is followed again by oolite shoal or tidal-bar deposits disadvantageous for reef growth. In general, this succession corresponds well to the one of the Süntel Mountains (Schülke et al. 2004; Helm 2005) but otherwise lacks the pronounced emersion surface known as the “Hauptdiskontinuitätsfläche”, that is developed as a minor facies shift probably marked by the quartz-bearing deposits above the reef horizon (Schülke et al. 2004).
Community replacement sequence in the Stylosmilia/Goniocora thickets
The succession of reef organisms that inhabit the thickets show a characteristic settling or replacement sequence, characterized by a successive colonization by encrusters and cavity dwellers and contemporaneous biological destruction. Such community replacement sequences associated to coral thickets or rubble piles were studied repeatedly during the last decades in both recent and fossil settings (Scoffin and Hendry 1984; Helm and Schülke 1998, and references therein; Perrin 2000; Perry 2001; Wood 2001; Shiraishi and Kano 2004). The formation of such successions is controlled by the progressive restriction of reef cavities (reduction of light exposure, water circulation, and oxygenation; Scoffin 1993) and follows a development from mainly photoautotrophic to heterotrophic feeding modes (Wilson 1998; Dupraz and Strasser 1999). Moreover, the fossilization inside protected cryptic habitats allows the examination of marine biota that are otherwise only poorly preserved or suffered from water turbulence (Brett 1988; Scoffin 1992).
The replacement stages from pioneering organisms to cryptobionta (Kobluk 1988) or coelobites (Rasmussen and Brett 1985) observed in this study are defined according to the dominant or characteristic organisms. We propose a subdivision into three stages comprising (1) a pioneer assemblage, (2) a micro-encruster/microbial crust assemblage, and (3) a terminal non-rigid sponge assemblage (Figs. 12 and 13).
The diagram shows Stylosmilia corallina that is extensively encrusted by microbialite intergrown with encrusting metazoans. The coenosarc of St. corallina extends down the sides of each branch. The community replacement sequence starts with photoautotrophic organisms. Remaining cavities supported the cryptic non-rigid sponge community
-
1.
Lithocodium/Koskinobullina stage (pioneer assemblage). The pioneer assemblage is defined by the scattered appearance of the Lithocodium-Troglotella foraminiferal consortium (cf. Schmid and Leinfelder 1996) and sheets of Koskinobullina socialis together with Iberopora bodeuri (Helm et al. 2003c) that directly cover the coral branches. Both decrease in abundance during the ensuing development of stage 2. The foraminiferal consortium has even been observed within the calices of phaceloid corals. Occasionally, the contact to the substrate is diffuse or micritic and the corallite walls below L. aggregatum encrustations seem to be etched or bored. Also clionid sponges, represented by the ichnogenus Entobia, are confined to this stage. They seem to exclusively penetrate corallites of branched corals before encrustation has taken place, because borings that cut both coral skeletons and microbialite have yet not been observed. In addition, boring bivalves may occur (documented by the existence of small borings), but their appearance depends on skeletal characteristics of the penetrated coral species (Edinger and Risk 1996; Perry and Bertling 2000; Laternser 2001). They flourish during the following stages when coatings around the branches are available for excavation.
-
2.
Micro-encrusters/microbial crust assemblage. In particular microbialites contribute to this stage. In addition, this stage comprises all organisms that grow contemporaneously to microbial crusts (Figs. 9, 12 and 13) or feed on microbialite, thus forming composite crusts. During advanced growth most organisms become successively rare, so that only T. lapilloides and nubeculariids continue to flourish even in the following stage 3. Both occur in close association with microbial crusts as they are incorporated within. Composite coatings in the branched coral colonies are developed as thin veils to thick crusts a few mm to a few cm in thickness, but they never fill the whole interstices between coral branches. In these small reef cavities small patelloid gastropods (Emarginula sp.) are occasionally present. The remaining space offers a habitat for cavity-dwelling, cryptobiontic fauna. This fauna flourishes in the following stage 3.
-
3.
Non-rigid sponge stage (terminal sponge assemblage). The encrusting sequence in the thickets is terminated with a non-rigid sponge community that postdates the encrustation described above. This community comprises small geodiid and pachastrellid demosponges (“container” sponges) that are confined to small cryptic habitats and preserved in situ. This preservation is possible because of the following conditions. First, the rigid reef framework protects all interstitial sediment and organisms from compaction (Scoffin 1992). Secondly, the intense microbial activity during and after decay of the sponge soft tissues leads to an early in situ lithification (Keupp et al. 1993; Reitner 1993; Delecat et al. 2001). Finally, the complete thickets are post mortem encrusted by mamillated microbialites that terminate the preceding community replacement sequence and trap the reef-associated fauna.
The community replacement sequence recorded here resembles that described from Thamnasteria dendroidea thickets of the Süntel Mts (Helm and Schülke 1998, 1999; Helm 2005). Probably, the settling sequence is also typical for other Late Jurassic coral reefs that are composed of tightly branched corals: The cosmopolitan Lithocodium/Troglotella-consortium corresponds to the first encrusting stage of the reefal framework in shallow-water settings. It commonly encrusts external surfaces of corals (Kołodziej 1997; Laternser 2001). Hence, Schmid and Leinfelder (1996) and Kołodziej (1997) suggest a light dependence and that the alveola side of the tests of L. aggregatum was the preferred position of the photoautotrophic symbionts. Also, Koskinobullina socialis and Iberopora bodeuri appear to depend on light exposure, because they commonly develop folious (?)colonies that are attached directly to skeletal hard tissues (mostly coral skeletons). The Lithocodium/Troglotella-consortium, K. socialis and I. bodeuri are pioneering organisms that colonize rapidly, but that are poorly competitive concerning overgrowth processes (Dupraz and Strasser 1999; Shiraishi and Kano 2004). They demise during the ensuing development of the epifaunal community and are only able to survive if reef growth continues and coral skeletons provide new substrate for colonization (Kobluk 1988).
The presence of small patelloid gastropods (limpets) in the patch reefs is of special interest. The limpets belong to the mobile dwellers in small reef cavities where they feed on microbial coatings. Similar observations were made in coral thickets composed of Th. dendroidea (florigemma-Bank Member, Süntel Mts) and in reef rubble (Hainholz reef complex, Osterwald Mts) where a variety of additional species occur (Helm 2004, 2005). The ecological niche of the patelloids in the reefs of the Korallenoolith Formation corresponds to that of the contemporaneous coral thrombolite reefs of the northern Paris Basin where the patelloids are only a single taxon among a variety of other herbivorous raspers that are restricted to hard substrate (Bertling and Insalaco 1998). However, this difference can be interpreted as a sampling bias because small gastropods, roughly assigned to the trochoids, are apparent on several polished slabs, but were not yet identified in hand specimens.
The cryptic epifauna (non-rigid sponges, thecideidinid brachiopods, bryozoans, a few foraminifers) recognized in the Deister patch reefs are considered as fossil analogues of recent micromorphic communities of coelobites in marine caves (Asgaard and Stentoft 1984; Rasmussen and Brett 1985; Scoffin 1993) and within coral rubble (Gischler and Ginsburg 1996). Similar fossil epifaunal communities are known from Middle Jurassic hardgrounds (Wilson 1998) and from Kimmeridgian reef cavities (Taylor and Palmer 1994). Even from mudmound environments a similar scenario has been described (Wood 2001).
Although the preservation potential of such non-rigid sponges is considered low (Rasmussen and Brett, 1985 put them into the non-preservable category) in coral reefs (Bertling and Insalaco 1998: 172) and a significant information loss should be expected, non-rigid demosponges seem to be common in Late Jurassic coral reefs. Fürsich et al. (1994a) reported small non-rigid sponges as interstitial fauna within bivalve-dominated patch reefs of southern England. Lauxmann et al. (1998: 127) emphasise that sponge spicules of non-rigid sponges are very common in reefal limestone of the Late Jurassic from the Swabian Alb in southern Germany. In conclusion, these sponges abundantly contribute to the reef-dwelling fauna (cf. Dupraz and Strasser 1999: 113, pl. 13/3). The common co-occurrence of coral limestone and sponge-rich interstitial sediment in reef cavities and aprons around the reef framework (e.g., Herrmann 1996: Oxfordian coral reefs in Dobrogea/eastern Romania; Insalaco 1999: Oxfordian coral reefs in England) further supports this assumption. Further Jurassic occurrences are known from sponge-algal mounds of southern Germany (Pisera 1997).
Due to the broad spectrum of possible dwelling sites of such sponges, it is not yet clear whether Late Jurassic non-rigid sponges are generally coelobites. The fact that non-rigid sponges are mostly found in reef cavities may have two reasons either they are true coelobites who only live in such cavities with restricted physical and biological disturbance, or there is a preservational bias, i.e., the sponges that lived outside the protected microenvironments were destroyed following post mortem decomposition of the sponge soft tissues.
The possible source of rhaxes in the peri-reefal deposits remains unclear. Rhaxes are also well known from Jurassic micritic deposits that lack reefal build-ups (e.g., Gramann 1963). This implies that they did not derive from decomposed reef-inhabiting sponges. According to Schweigert et al. (2000) and Dietl and Schweigert (2002), it seems reasonable that the rhaxes originate from a special kind of “Rhaxella” sponges. These non-rigid sponges are presumed to have pillow-like “bodies” with finger-like protuberances, and their skeleton possibly consist exclusively of rhaxes (Krautter, oral communication 2005). The individual bodies presumingly dwelled directly on soft to firm substrate adjacent to the patch reefs.
Like their non-rigid counterparts, “coralline sponges” occupy cryptic habitats in Late Jurassic reefs (Taylor and Palmer 1994; Leinfelder 2001: 265). This habitat preference is thought to reflect escape from detrital input (Werner et al. 1994). The secondary rigid calcareous skeleton enhances the preservation potential of these small to even micromorphic sponges.
The increase of borings in the patch reefs towards the final microbialite surface indicates enhanced boring activity after the final coating of coral colonies or thickets with thick microbial crusts. This coincides well with Late Jurassic bivalve-dominated patch reefs of southern England (Fürsich et al. 1994a) and with Oxfordian coral thrombolite reefs in the Suiss Jura (Dupraz and Strasser 1999). Even in modern coral reefs, microbial crusts commonly form as the final stage of an encrustation succession around the coralgal framework (Wood 2001, and references therein). Dupraz and Strasser (1999) emphasised that activity of boring organisms is strongest before (borings within coral skeletons) and after formation of microbial crusts. This observation agrees with a suspected increased eutrophication during final patch reef growth (discussed below) because enhanced nutrient fluxes can enforce bioerosional processes (e.g., Chazottes et al. 1995).
The composition of reef faunas changes along a bathymetric gradient, resulting in a depth zonation of reef organisms. Such a bathymetric distribution pattern has been elaborated for the Iberian Peninsula by Leinfelder et al. (1996, and references therein). To our astonishment, the shallow-water Speckhals patch reefs comprise both organisms of shallow and deeper water settings as defined by Leinfelder et al. (1996). However, organisms that are confined to deeper reefal settings (thecideidinid brachiopods, bryozoans, demosponges, Terebella lapilloides) according to Leinfelder et al. (1996), are restricted to cryptic habitats in the examined patch reefs. Thus, the cave-dwelling mode of life extends the bathymetric range of these faunal components into shallow water. On the other hand, photoautotrophic organisms are stenobathic and can thus be regarded as bathymetric markers.
According to these observations, an analysis of environmental conditions on the basis of reef organisms should be executed carefully (Werner et al. 1994) and should take into account that coelobites do not show a clear depth stratification and are able to flourish even in small patch reefs within well oxygenated lagoons. Moreover, in a few exceptional situations, e.g., after a breakdown of the reefal framework following a storm and the subsequent accumulation of loose coral branches (Scoffin and Hendry 1984), the sheltering epifauna and coelobites are able to exist directly attached to the coral skeleton, and the community replacement sequence described above is not valid anymore.
Late Jurassic coral reefs mainly composed of branched corals
The success of bushy corals in forming reefs in Late Jurassic lagoonal environments even under a high-energy condition has frequently been recognized. Except for a few studies (Beauvais et al. 1974; Werner 1986; Fookes 1995; Bertling and Insalaco 1998; Helm and Schülke 1998; Helm 2005), reefs composed mainly of branched corals were not subjected to crucial investigations concerning the composition of coral fauna, their growth fabrics, the guild structure, the overall shape, the interstitial sediments, the sedimentation rates, etc. A lot of these reefs are developed as coral thrombolite reefs. Such reefs, however, do not fit a certain type as defined by Insalaco et al. (1997). They expose features of both type III (reefal thickets dominated by tall dense phaceloid colonies developed within pure carbonate muds), and type IV (microbial-coral reefs dominated by massive, branching ramose and phaceloid colonies). Other bioconstructions composed of branched corals suffered from detrital influx and lack microbial crusts and encrusting organisms (Werner 1986). Such sediment-stressed reefs with constratal growth fabric partly belong to the type VIII following the classification of Insalaco et al. (1997).
In contrast to recent reef corals, Late Jurassic branched corals were largely restricted to lower-energy settings (Leinfelder et al. 2002: 469). According to, e.g., Brett (1990), Scoffin (1992), and Insalaco et al. (1997), branched corals are interpreted less resistant to mechanical destruction and, thus, good indicators for the intensity of water turbulence (Leinfelder 1992: 18). Following these authors, the fraction of framework in growth position versus that of reef rubble can be used for estimations of water energy. In particular, phaceloid and dendroid corals that lack soft tissue on their branches are subjected to enhanced penetration by boring organisms (Scoffin 1992) that arose during Late Jurassic times (Bertling 1999; Perry and Bertling 2000).
In contrast to the statements above, bushy corals and reefs mainly composed of such corals are frequently preserved in situ in less protected settings (Leinfelder 2001: 265). Single colonies are even able to survive in high-energy reefs (e.g., Leinfelder 1992; Reuter et al. 2001). The preservation processes remain unclear (Leinfelder et al. 2002). One explanation is that many of these bioconstructions are buried in terrigenous and/or carbonate sediment that stopped the mechanical breakdown and bioerosion (e.g., Beauvais et al. 1974: Thamnasteria dendroidea mud banks; Werner 1984: “Calamophyllia” meadows). In most cases, only the terminal tips of the coral branches raised above the sea floor (constratal growth fabric), because they continuously baffled sediment during their growth (Geister 1995). Leinfelder (1994) and Nose (1995) emphasise that sediment-stressed environments favour bushy corals that are adapted to such environmental conditions, because they are able to keep pace with sediment supply (Leinfelder et al. 1994, 1996; Fookes 1995; Nose and Leinfelder 1997; Laternser 2001: 155; Lathuilière et al. 2005: 553). Thus, occurrences of (Late Jurassic) branched corals are largely connected with such environments (Leinfelder 1994), forming the reef type “clayey coral meadows” (Leinfelder 2001).
However, many settings with bioconstructions mainly composed of branched corals lack such enhanced sediment supply during coral growth (e.g., Olivier et al. 2003). The Northwest German reefs demonstrate that the excellent reef preservation is also favoured by tightly branched corals with anastomosing growth of corallites and enveloping microbial crusts.
A further example of such coral thickets consisting of the ramose Thamnasteria dendroidea was given by Helm and Schülke (1998, 1999, 2000a) and Helm (2005). The patch reefs of the Deister Mts similarly consist of branched corals among which the phaceloid coral St. corallina prevails. Its tightly branched colonies are quite similar to those of Th. dendroidea concerning their shape, superstratal growth fabric and internal architecture. In contrast, each (phaceloid) branch of St. corallina is terminated with a calice, whereas the calices of Th. dendroidea are arranged around the branches. This growth type, the intraspecific reactions of the soft tissue, and fusion of adjacent coral branches, that are well known from Th. dendroidea (Helm and Schülke 1998, 2000a) should be absent with St. corallina, because of the lack of wrapping tissue. Nevertheless, coalescent (phaceloid) corallites of St. corallina colonies are very common and produce a lattice of branches. On microscopic scale, well-preserved specimens reveal a diffuse dark and sometimes jagged line that encircles the single branches (Fig. 2C). This feature reflects a slightly micritized margin of the corallite prior to encrustation by additional coral skeleton that connected adjacent corallites. It suggests that – in contrast to most recent phaceloid corals (Barnes 1973) – the soft tissue of St. corallina extends even along the sides of each branch and possibly enclosed the whole skeleton. Therefore, the subsequent increase in skeletal mass in radial direction resulted in submassive coralla consisting of fused branches and extensively enhanced the rigidity of single colonies (Fig. 14). Such colonies incorporate both features of the massive stylinid coral genus Stylina and of the branched stylinid Goniocora.
Coral morphology and reef development
Growth fabric, patch reef growth, encrustation by microbialite and reef dwellers, and resistance against biotic or abiotic destruction are strongly influenced by the coral morphology, which itself is subjected to the above and other synecological constraints (e.g., Insalaco 1998). In the following, we aim to demonstrate the unique position of the Northwest German bioconstructions. The first aspect is the connexion between branched corals and the initiation of reefal growth. The breaking of branches with living soft tissue and the transport into the peri-reefal environment promotes the development of either new scattered patch reefs or vertical accretion of the patch reefs (Helm 2005, and references therein). Such corals are considered pioneer species (Leinfelder 2001), because they enhance reef development by building their own substrate (Roberts et al. 1988) and flourish in particular in lagoonal settings (Geister and Lathuilière 1991).
As shown by Vytopil and Willis (2001), the epifaunal communities on branched coral species and coral thickets are mainly controlled by the coral morphology. They observed a greater abundance and species richness of epifauna on tightly branched coral species when compared to their rarity or absence on open-branched species in fringing reefs of the Great Barrier Reef. In fossil analogues, similar observations can be made. The patch reefs of the Süntel and Deister Mts consist of a thicket of Th. dendroidea and St. corallina/G. socialis, respectively. The coral species belong to the tightly branched coral type and, consequently, the epifaunal community is well developed (Helm 2005, this work). In contrast, coral thrombolite reefs from the northern Paris Basin, mainly composed of corals with widely spaced branches developing open colonies (Dendrohelia coalescens, Th. dendroidea type A, Allocoenia sp.), largely lack any encrusting invertebrates (Bertling and Insalaco 1998). Bertling and Insalaco (1998) suggest that microbial films may hinder larval settlement. However, encrusting organisms and microbial crusts are often closely related in coral thrombolite reefs (Leinfelder et al. 1993; Nose 1995). On the other hand, there are sediment-stressed coral meadows (Actinastrea/Stylina-thickets) that bear veneers of attached organism, but largely lack accompanying microbial crusts (Leinfelder 1994: 164). Hence, increased sediment supply can also hamper encrusting organisms and microbial crusts (Leinfelder 1994), but to which extent different sedimentation rates account for this phenomenon and which factors finally control the process of encrustation (epifauna versus microbial crusts) is hard to define (Olivier et al. 2006). In addition, the non-sheltered epifauna, attached to “exposed” substrates, suffers greatly from grazers (e.g., echinoids) and other predators (Ruggiero 1996: fishes).
As it is shown in modern counterparts (Vytopil and Willis 2001), the coral morphology apparently controls the epifaunal community structure (trigger versus hamper) and the replacement sequence (Fig. 14) in the Northwest German reefs. Thus, the existence of a certain microhabitat governs the composition of the epifaunal community and enhances microbial growth (Fürsich et al. 1994a; Leinfelder and Schmid 2000; Laternser 2001; Wood 2001, and references therein). Even the space between the branches of different coral species significantly affected the size distribution patterns of nestlers and mobile dwellers (Vytopil and Willis 2001: certain crab species in different Acropora species). The tightly branched coral species appear to even stimulate lush epifaunal development and promote the evolution of cavity-dwellers, e.g., small limpets or thecideidinid brachiopods, probably by providing a variety of small microhabitats.
Moreover, the formation of a reefal framework depends on the growth rate of the coral branches in relation to the growth rate of microbial coatings (Dupraz and Strasser 1999). In particular, branched corals are characterized by high linear growth rates (Geister and Lathuilière 1991; Fookes 1995; Geister 1995). This growth enhances the microbial activity and leads to extensive carbonate precipitation and promotes framework rigidity (Leinfelder 1992; Nose 1995; Bertling and Insalaco 1998; Helm and Schülke 1998; Dupraz and Strasser 1999; Laternser 2001; Olivier et al. 2003). However, the growth capacity of both should be balanced, and microbial crust growth should keep pace with coral growth (Nose 1995; cf. Laternser 2001). Otherwise, in case of an excessive microbial growth, the outpaced corals suffer from thriving microbes (Olivier et al. 2006), so that microbial crusts finally cover the whole coral colonies/thickets (Nose 1995: 76). On the other hand, accelerated coral growth leads to a lack of microbial coatings (in particular open-branched coral species, see above), which makes the respective colonies prone to bioerosion and mechanical breakdown (e.g., Laternser 2001).
The Speckhals patch reefs are increasingly encrusted by microbialite up to their tops. Whether this is due to excessive microbial growth outpacing the corals or a post mortem microbialite encrustation both of single colonies and the whole thickets (mamillated microbialites) can only be assumed. These crusts, however, provide additional rigidity so that the patch reefs remained intact after their final demise.
Several environmental triggers have been proposed to explain such a scenario of disproportionate microbial growth. A possible explanation is an increased nutrient level that favours microbial activity (e.g., Hallock and Schlager 1986). The vertical succession of the Speckhals patch reefs gives rise to the assumption that the environment changed towards eutrophication and thus favoured microbialite rather than coral growth (Nose 1995; Dupraz and Strasser 1999, 2002; Olivier et al. 2006).
This interpretation agrees with the appearance of thin platy microsolenid corals towards the top of the florigemma-Bank Member. These corals are known to occur in deeper water, where they build a special kind of reef types (type I reefs after Insalaco et al. 1997). However, mass occurrences of microsolenid corals are also known from Late Jurassic shallow-water settings, e.g., the Ota Reef (Leinfelder 1994), the Lusitanian Basin (Nose 1995; Nose and Leinfelder 1997), the Swiss Jura Range (Dupraz and Strasser 1999) or the Dobrogea, eastern Romania (Herrmann 1996: fungiid biostromes). Flourishing of microsolenids reflects reduced illumination (cf. Insalaco 1996). Hence, occurrences of microsolenids in comparably shallow water emphasise increased presence of suspended matter that is mostly connected with intensified nutrition (Hallock and Schlager 1986; Herrmann 1996). Such a mesotrophic to eutrophic environment prevents branched coral growth (e.g., Nose 1995) and stimulates growth of opportunistic microsolenid corals instead (Fürsich et al. 1994b) as well as of microbial crusts (Olivier et al. 2006, and references therein).
The growth of reefs is strongly influenced by the rate of sedimentation as shown by Schmid et al. (2001). High sedimentation rates lead to progressive burial of reefs that are composed of branched corals. Following Leinfelder et al. (1993) and Schmid (1996), such conditions hamper microbial growth in reefal settings. Hence, the lack or decrease of microbial crusts on coral skeletons would generally suggest a high sediment supply. However, this model cannot be applied to branched corals, because fast growing species rise above the sea floor and can therefore promote microbial growth even under increased sedimentation rates.
Sea-level development
Water depth is an important controlling factor for the development and evolution of Jurassic reefs (Leinfelder et al. 2002). The studied succession reveals a deepening-shallowing trend of the sea level.
In several sections in the Lower Saxony Basin, the top of the pre-florigemma-Bank Member is developed as a lag deposit (e.g., oncolite, bioclasts encrusted by porostromate algae) or as an erosional surface that indicates the flooding surface of a major transgression (Helm 2005), possibly representing fourth-order or lower-order transgression within the framework of a third-order sea-level rise. In the studied sections, no evidence was found for a sedimentary unconformity at the base of the florigemma-Bank Member, but the depositional sequence reveals a gradual change from grainstone/packstone to wackestone facies that indicates the transgressive sea level. At the top of the florigemma-Bank Member, a widespread erosional surface is developed (Helm 1998, 2005; Helm et al. 2002) that is present in the Deister Mts as a minor facies shift.
The reefal community structure reflects the sea-level trend. The oligotrophic conditions at the beginning of the patch reef growth are related to a rising relative sea level (Leinfelder et al. 2002: 500), whereas the more mesotrophic to eutrophic conditions during advanced patch reef growth (intense microbial coatings, enhanced bioerosion, thriving of microsolenid corals) are linked to a falling sea level (see Dupraz and Strasser 1999 for discussion).
Possibly, the transgressive-regressive sequence described above corresponds to a cycle of orbital eccentricity (100 ka: smale-scale sequence after Strasser et al. 1999) or to the 20-ka precession cycle of the equinoxes as proposed by Dupraz and Strasser (1999) for similar successions in Oxfordian shallow-water coral bioherms. According to these authors (Dupraz and Strasser (1999: 125), such “20-ka orbital cycles did not directly influence the reef system, but probably opened and closed lagoons and thus modified water circulation and oxygenation, and through climate changes controlled terrigenous run-off and nutrient availability.”
Palaeobiogeography
The close palaeobiogeographical relationship of the florigemma-faunas to the contemporaneous Tethyan ones are underpinned by the presence of a variety of faunal elements, especially the chaetetids and coral-chaetetid-solenoporid-microbial crust-reefs, respectively. Chaetetides are represented by Ptychochaetetes polyporus that is common in deposits of the florigemma-Bank Member exposed in the Deister area (Helm and Schülke 2000b; Helm et al. 2001). Recent findings of this species in reef debris of the Hainholz Member in the Osterwald Mts indicate a more widespread distribution in the Korallenoolith Formation of the Lower Saxony Basin. The chaetetid specimens from the Deister Mts represent the northernmost occurrence of the taxon known until today (Fischer 1977). Southward shifted occurrences are known from the Paris Basin (Oxfordian of Lorraine; Geister and Lathuilière 1991) and Poland (Swietokrzyskie in Holy Cross Mts; B. Kołodziej, written comment 2002). The chaetetids are also known from southern Germany, but there they appear in Late Kimmeridgian strata for the first time. They are described from the Arnegg Reef exposed in the Swabian Alb (P. polyporus; Paulsen 1964; Laternser 2001) and from the southern Franconian Alb (Ptychochaetetes grimmeri Flügel; Flügel 1979).
During the Late Jurassic, chaetetids generally reach a peak in specific diversity (e.g., Ernstbrunn and Stramberg limestone from Lower Austria and Czech Republic; Bachmayer and Flügel 1961) and are highly abundant in wave-agitated reefal settings or even in high-energy coral reefs (Pümpin 1965; Flügel 1979; Steiger and Wurm 1980; Flügel et al. 1993; Nose 1995). However, P. polyporus is by far the most common and widespread species.
The enigmatic serpulid worm genus Carpathiella is known up to now only from limestone deposits of Latest Jurassic to Aptian age from the Western Carpathians and Eastern Alps and seemed to be endemic in this region. A few species (C. triangulata, C. perforata, and C. sp.) were described from loose tubes in thin-sections (Mišik et al. 1999). Additional species derive from the Northern Calcareous Alps, e.g., Late Jurassic to Early Cretaceous Plassen Limestone (Schlagintweit et al. 2003). Our specimens represent the earliest (Middle Oxfordian) evidence of Carpathiella and extend its regional distribution to the Lower Saxony Basin. Specimens are restricted to peri-reefal deposits (wackestone) of the Süntel Mts (Helm 2005) and Deister Mts, and have never been observed in the reef facies.
Conclusions
-
A variety of coral reef types are developed in the Oxfordian sedimentary succession of the Lower Saxony Basin, NW Germany. The patch reefs considered here belong to the coral-chaetetid-solenoporid-microbialite-reefs and represent a reef type that was hitherto unknown so far north of the Tethys.
-
The coral fauna of the Speckhals area consists of more than 20 species belonging to 17 genera, three of which mainly occur in peri-reefal deposits. Bushy corals are represented by only five species, and the framework of the patch reefs is dominated by two of them (Stylosmilia corallina and Goniocora socialis).
-
The phaceloid Stylina corallina combine fast and anastomosing growth of branches that provoke both the formation of rigid submassive colonies and the strengthening of the overall reef framework. These features are crucial for the success of this species in lagoonal settings. Moreover, this coral species is even able to conquer high-energy settings, because the anastomosing growth enables the colonies to tolerate increased water turbulence.
-
The interspecific differences in coral gross morphology of bushy corals strongly governs the habitat structures of reefs and epifaunal communities they host. Tightly branched corals like Stylosmilia corallina stimulate epifaunal development whereas loosely branched coral are hostile substrates and hamper the development of a complex community structure.
-
Coral reefs that are composed of tightly branched coral species typically show community replacement sequences as a result of their habitat complexity. These successions are controlled by increasing space restriction between the branches (gradual change from photophilic to sciaphilic organisms). The assemblage of cryptobionts is similar to those observed on Jurassic hardgrounds and reef cavities.
-
Phototrophic organisms such as Lithocodium aggregatum belong to the pioneer community and are directly attached to the coral skeletons whereas sheltered microhabitats in the coral thicket are characterized by prolific cryptic non-rigid sponges. Such soft sponges with a generally low preservation potential seam to be common in Late Jurassic coral reefs which provide a variety of tiny reef caves. Their fossil record suffered from post mortem decay. Therefore, it is not clear how important their role is as Jurassic reef constituents.
-
The Speckhals patch reefs’ faunal association encloses both organisms of shallow and deeper water settings. Thecideidinid brachiopods, bryozoans, non-rigid sponges, Terebella, and nubeculariids are eurybath but become increasingly important towards deeper water. In shallow-water settings they were restricted to caves in hardgrounds and coral reef cavities. In contrast, phototrophic organisms such as Lithocodium aggregatum are stenobath and restricted to shallow-water settings.
References
Asgaard U, Stentoft N (1984) Recent micromorph brachiopods from Barbados: palaeoecological and evolutionary implications. Géobios Mém Spéc 8:29–33
Bachmayer F, Flügel E (1961) Die “Chaetetiden” aus dem Ober-Jura von Ernstbrunn (Niederösterreich) und Stramberg (CSR). Palaeontographica A 116:144–174
Barnes DJ (1973) Growth in colonial scleractinians. Bull Mar Sci 23:280–298
Beauvais L (1985) The Madreporaires jurassiques indicateurs de paleoenvironments: quelques exemples. Palaeogeogr Palaeoclimatol Palaeoecol 49:207–215
Beauvais L, Beauvais M, Bourrouilh F (1974) A study of the reef complex of Bellême (Normandy, France). Proc 2nd Int Coral Reef Symp 2:639–652
Bertling M (1989) Die korallengebundenen Choriozönosen des norddeutschen Malm. Unpublished PhD thesis, University of Münster, p 167
Bertling M (1993) Riffkorallen im Norddeutschen Oberjura – Taxonomie, Ökologie, Verteilung. Palaeontographica A 226:77–123
Bertling M (1994) Ökologie und Taxonomie koralleninkrustierender Bryozoen des Norddeutschen Malm. Paläont Z 68:419–435
Bertling M (1997a) Structure and function of coral associations under extreme siltation stress – a case study from the Northern German Upper Jurassic. Proc 8th Int Coral Reef Symp 2:1749–1754
Bertling M (1997b) Bioerosion of Late Jurassic reef corals – implications for reef evolution. Proc 8th Int Coral Reef Symp 2:1663–1668
Bertling M (1999) Late Jurassic reef bioerosion – the dawning of a new era. Bull Geol Soc Denmark 45:173–176
Bertling M, Insalaco E (1998) Late Jurassic coral/microbial reefs from the northern Paris Basin – facies, palaeoecology and palaeobiogeography. Palaeogeogr Palaeoclimatol Palaeoecol 139:139–175
Brachert TC (1986) Kontinuierliche und diskontinuierliche Sedimentation im süddeutschen Oberjura (unteres Kimmeridge; Ludwag/Oberfranken, Nördliche Frankenalb). Facies 15:233–284
Brett CE (1988) Paleoecology and evolution of marine hard substrate communities: an overview. Palaios 3:374–378
Brett CE (1990) Destructive taphonomic processes and skeletal durability. In: Briggs DEG, Crowther PR (eds) Palaeobiology – a synthesis. Blackwell, Oxford, pp 223–226
Bucur I, Senowbari-Daryan B, Abate B (1996) Remarks on some foraminifera from the Upper Jurassic (Tithonian) reef limestone of Madonie Mountains (Sicily). Boll Soc Paleont Ital 35:65–80
Chazottes V, Le Campion-Alsumard T, Peyrot-Clausade M (1995) Bioerosion rates on coral reefs: interactions between macroborers, microborers and grazers (Moorea, French Polynesia). Palaeogeogr Palaeoclimatol Palaeoecol 113:189–198
Delecat S, Peckmann J, Reitner J (2001) Non-rigid sponges in oyster patch reefs (Lower Kimmeridgian, Langenberg/Oker, Germany). Facies 45:231–254
Dietl G, Schweigert G (2002) Im Reich der Meerengel. Der Nusplinger Plattenkalk und seine Fossilien. Pfeil, München, p 144
Dupraz C, Strasser A (1999) Microbialites and micro-encrusters in shallow coral bioherms (Middle to Late Oxfordian, Swiss Jura Mountains). Facies 40:101–130
Dupraz C, Strasser A (2002) Nutritional modes in coral-microbolite reefs (Jurassic, Oxfordian, Switzerland): evolution of trophic structure ads a response to environmental change. Palaios 17:449–471
Edinger EN, Risk MJ (1996) Sponge borehole size as a relative measure of bioerosion and paleoproductivity. Lethaia 29:275–286
Eliášová H (1981) The Tithonian Reef of Stramberk Limestone (Czechoslovakia, West Carpathians). Čas Mineral Geol 26:113–124
Errenst C (1990) Das korallenführende Kimmeridgium der nordwestlichen iberischen Ketten und angrenzender Gebiete (Fazies, Paläogeographie und Beschreibung der Korallenfauna). Teil 1. Palaeontographica A 214:121–207
Fischer JC (1977) Biogéographie des chaetetides et des tabulospongida post-paléozoiques. Proc 2nd Int Symp Corals and Coral Reef. Mém Bur Rech Géol Minèr 89:530–534
Flügel E (1979) Ptychochaetetiden aus dem oberen Malm der südlichen Frankenalb. Geol Bl NO-Bayern 29:1–11
Flügel E, Alt T, Joachimski MM, Riemann V, Scheller J (1993) Korallenriff-Kalke im oberen Malm (Unter-Tithon) der Südlichen Frankenalb (Laisacker, Marching): Mikrofazies-Merkmale und Fazies-Interpretation. Geol Bl NO-Bayern 43:33–56
Fookes E (1995) Development and eustatic control of an Upper Jurassic reef complex (Saint Germain-de-Joux, Eastern France). Facies 33:129–150
Fürsich FT, Sykes RM (1977) Palaeobiogeography of the European boreal realm during Oxfordian (Upper Jurassic) times: a quantitative approach. N Jb Geol Paläont Abh 154:137–161
Fürsich FT, Palmer TJ, Goodyear KL (1994a) Growth and disintegration of bivalve-dominated patch reefs in the Upper Jurassic of Southern England. Palaeontology 37:131–171
Fürsich FT, Pandey DK, Oschmann W, Jaintly AK, Singh IB (1994b) Ecology and adaptive strategies of corals in unfavourable environments: examples from the Middle Jurassic of the Kachchh Basin, Western India. N Jb Geol Paläont Abh 194:269–303
Geister J (1995) Sclerochronology of Jurassic corals: geological applications. Publ Serv Géol Lux 29:150–152
Geister J, Lathuilière B (1991) Jurassic coral reefs of the northeastern Paris Basin (Luxembourg and Lorraine). Excursions-Guidebook, 6th Int Symp Fossil Cnidaria, p 112
Gischler E, Ginsburg RN (1996) Cavity dwellers (Coelobites) under coral rubble in southern Belize barrier and atoll reefs. Bull Mar Sci 58:570–589
Gramann F (1963) Schwamm-Rhaxen und Schwamm-Gesteine (Spongiolithe, Spiculite) aus dem Oxford NW-Deutschlands. Geol Jb 80:213–220
Gramann F, Heunisch C, Klassen H, Kockel F, Dulce G, Harms F-J, Katschorek T, Mönnig E, Schudack M, Schudack U, Thies D, Weiss M, Hinze C (1997) Das Niedersächsische Oberjura-Becken – Ergebnisse interdisziplinärer Zusammenarbeit. Z Dt Geol Ges 148:165–236
Hallock P, Schlager W (1986) Nutrient excess and the demise of coral reefs carbonate platforms. Palaios 1:389–398
Helm C (1998) Paläokarst-Erscheinungen im Oberjura (Oxfordium, Dachfläche der florigemma-Bank, Korallenoolith, Hauptdiskontinuität) von NW-Deutschland (Süntel). Ber Naturhist Ges Hannover 140:99–120
Helm C (2004) Napfschnecken als Riffbewohner (Korallenoolith, Oberjura, NW-Deutschland). In: Reitner J, Reich M, Schmidt G (eds) Geobiologie. 74 Jahrestag Paläont Ges, Kurzfassungen Vorträge und Poster, Göttingen, pp 105–106
Helm C (2005) Riffe und fazielle Entwicklung der florigemma-Bank (Korallenoolith, Oxfordium) im Süntel und östlichen Wesergebirge (NW-Deutschland). Geol Beitr Hannover 7:3–339
Helm C, Schülke I (1998) A coral-microbialite patch reef from the Late Jurassic (florigemma-Bank, Oxfordian) of NW Germany (Süntel Mountains). Facies 39:75–104
Helm C, Schülke I (1999) Ein “Tethys-Riff” im Korallenoolith (Oxfordium) von Nordwestdeutschland. Zbl Geol Paläont I 1999:399–414
Helm C, Schülke I (2000a) Contact reactions and fusion of Upper Jurassic ramose coral Thamnasteria dendroidea in a patch reef environment. Coral Reefs 19:89–92
Helm C, Schülke I (2000b) Der Korallenoolith (Oxfordium) im Deister (NW-Deutschland): Eine Re-Evaluation der Fazies, Stratigraphie und Mächtigkeit. Ber Naturhist Ges Hannover 142:149–168
Helm C, Schülke I, Fischer I (2001) Paläobiogeographie des Korallenooliths (Mittleres Oxfordium – Unteres Kimmeridgium): Tethyale Faunen- und Florenelemente auf höherer Paläobreite (Niedersächsisches Becken, NW-Deutschland). Geol Beitr Hannover 2:51–64
Helm C, Fischer R, Schülke I (2002) Paleokarst formation on a Late Jurassic lowstand unconformity (Korallenoolith Formation, Süntel and Wesergebirge Mountains, NW Germany). In: Hüssner R, Hinderer M, Götz AE, Petschick R (eds) Sediment 2002. 17 Sedimentologen-Treffen, Abstracts and Programme. Schriftenr Dt Geol Ges 17:85–86
Helm C, Reuter M, Schülke I (2003a) Der Korallenoolith im Osterwald (NW-Deutschland): Fazielle Entwicklung und Ablagerungsdynamik. Z Dt Geol Ges 153:159–186
Helm C, Reuter M, Schülke I (2003b) Die Korallenfauna des Korallenooliths (Oxfordium, Oberjura, NW-Deutschland): Zusammensetzung, Stratigraphie und regionale Verbreitung. Paläont Z 77:55–72
Helm C, Schülke I, Schlagintweit F (2003c) Calcareous algae (“Porostromata“, Rhodophyta, Dasycladales) and microproblematica with algal affinity from the NW German Korallenoolith Formation (Oxfordian, Süntel Mountains). Facies 49:61–86
Herrmann R (1996) Entwicklung einer oberjurassischen Karbonatplattform: Biofazies, Riffe und Sedimentologie im Oxfordium der Zentralen Dobrogea (Ost-Rumänien). Berliner Geowiss Abh E 19:1–101
Hitchings VH (1980) Tentilenticulina latens, n. gen., n. sp., a new foraminifer from the Corallian (Jurassic), Great Britain. Micropaleontology 26:216–221
Hoyer P (1965) Fazies, Paläogeographie und Tektonik des Malm im Deister, Osterwald und Süntel. Beih Geol Jb 61:1–249
Insalaco E (1996) The use of Late Jurassic coral growth bands as palaeoenvironmental indicators. Palaeontology 39:413–431
Insalaco E (1998) The descriptive nomenclature and classification of growth fabrics in fossil scleractinian reefs. Sediment Geol 118:159–186
Insalaco E (1999) Facies and palaeoecology of Upper Jurassic (Middle Oxfordian) coral reefs in England. Facies 40:81–100
Insalaco E, Hallam A, Rosen B (1997) Oxfordian (Upper Jurassic) coral reefs in Western Europe: reef types and conceptual depositional model. Sedimentology 44:707–734
Jaintly AK, Szabo J, Fürsich FT (2000) Contributions to the Jurassic of Kachchh, Western India. VII. The gastropod fauna. Part I. Pleurotomarioidea, Fissurelloidea, Trochoidea and Eucyloidea. Beringerina 27:31–61
Jacquin T, Dardeau G, Durlet C, de Graciansky PC, Hantzpergue P (1998) The North Sea cycle: an overview of 2nd-order transgressive/regressive facies cycles in Western Europe. SEPM Spec Publ 60:445–466
Keupp H, Jenisch A, Herrmann R, Neuweiler F, Reitner J (1993) Microbial carbonate crusts – a key to the environmental analysis of fossil spongiolites? Facies 29:41–54
Kiessling W, Flügel E, Golonka J (1999) Paleoreef maps: evaluation of a comprehensive database on Phanerozoic reefs. AAPG Bull 83:1552–1587
Klüpfel W (1931) Stratigraphie der Weserkette (Oberer Dogger und Malm unter besonderer Berücksichtigung des Ober-Oxford). Abh Preuß Geol Landesanst, NF (129):13–423
Kobluk DR (1988) Cryptic faunas in reefs: Ecology and geological importance. Palaios 3:379–390
Kołodziej B (1997) Boring foraminifera from exotics of the Stramberk Limestone (Tithonian-Lower Berriasian). Ann Soc Geol Polon 67:249–256
Lambelet E (1968) Korallen im Korallenoolith mit besonderer Berücksichtigung der Gattungen Montlivaltia und Thecosmilia. Unpublished PhD thesis, University of Hamburg, p 235
Laternser R (2001) Oberjurassische Korallenriffe von Nordostfrankreich (Lothringen) und Südwestdeutschland. Unpublished PhD thesis, University of Stuttgart, p 300 [http://elib.uni-stuttgart.de/opus/volltexte/2001/877/pdf/Diss_Laternser.pdf]
Lathuilière B, Gaillard C, Habrant N, Bodeur Y, Boullier A, Enay R, Hanzo M, Marchand D, Thierry J, Werner W (2005) Coral zonation of an Oxfordian reef tract in the northern French Jura. Facies 50:545–559
Lauxmann U (1991) Revision der oberjurassischen Korallen von Württemberg (SW-Deutschland), exclusive Fungiina. Palaeontographica A 219:107–175
Lauxmann U, Schweigert G, Kapitzke M (1998) Die Schwamm- und Korallenriffe der Schwäbischen Alb. In: Heinzmann EPJ (ed) Vom Schwarzwald zum Ries, vol 2. Erdgesch Mitteleurop Reg, pp 117–128
Leinfelder RR (1992) A modern-type Kimmeridgian reef (Ota Limestone, Portugal): implications for Jurassic reef models. Facies 26:11–34
Leinfelder RR (1994) Karbonatplattformen und Korallenriffe innerhalb siliziklastischer Sedimentationsbereiche (Oberjura, Lusitanisches Becken, Portugal). Profil 6:1–207
Leinfelder RR (2001) Jurassic reef ecosystem. In: Stanley GD Jr (ed) The history and sedimentology of ancient reef systems, vol 17. Topics Geobiol Ser, pp 251–309
Leinfelder RR, Nose M (1999) Increasing complexity – decreasing flexibility. A different perspective of reef evolution through time. Profil 16:135–147
Leinfelder RR, Schmid DU (2000) Mesozoic reefal thrombolites and other microbolites. In: Riding R, Awramik SM (eds) Microbial sediments. Springer, Berlin, pp 289–294
Leinfelder RR, Nose M, Schmid DU, Werner W (1993) Microbial crusts of the Late Jurassic: composition, paleoecological significance and importance in reef construction. Facies 29:195–230
Leinfelder RR, Krautter M, Laternser R, Nose M, Schmid DU, Schweigert G, Werner W, Keupp H, Herrmann R, Reinfeld-Kiefer U, Schroeder JH, Reinhold C, Koch R, Zeiss A, Schweizer V, Christmann H, Menges G, Luterbacher H (1994) The origin of Jurassic reefs: current research developments and results. Facies 31:1–56
Leinfelder RR, Werner W, Nose M, Schmid DU, Krautter M, Laternser R, Takacs M, Hartmann D (1996) Paleoecology, growth parameters and dynamics of coral, sponge and microbolite reefs from the Late Jurassic. In: Reitner J, Neuweiler F, Gunkel F (eds) Global and regional controls on biogenic sedimentation. I. Reef evolution. Research reports, vol 2. Göttinger Arb Geol Paläontol, Sb, pp 227–248
Leinfelder RR, Schmid DU, Nose M, Werner W (2002) Jurassic reef patterns – the expression of a changing globe. SEPM Spec Publ 72:465–520
Mišik M, Sotak J, Ziegler V (1999) Serpulid worms Mercierella Fauvel, Durandella Dragastan and Carpathiella nov. gen. from the Jurassic, Cretaceous and Paleogene of the Western Carpathians. Geol Carpathica 50:305–312
Mönnig E, Bertling M (1995) Exkursion C. Mittlerer und oberer Jura zwischen Weser und Leine mit besonderer Berücksichtigung des Oxfordiums (Stratigraphie, Fazies). In: Boetzkes M, Vespermann J (eds) 65 Jahrestag Paläont Ges (Exkursionsführer), vol 95. Terra Nostra, pp 84–124
Nose M (1995) Vergleichende Faziesanalyse und Palökologie korallenreicher Verflachungsabfolgen des iberischen Oberjura. Profil 8:1–237
Nose M, Leinfelder R (1997) Upper Jurassic coral communities within siliciclastic settings (Lusitanian Basin, Portugal): implications for symbiotic and nutrient strategies. Proc 8th Int Coral Reef Symp 2:1755–1760
Olivier N, Hantzpergue P, Gaillard C, Pittet B, Leinfelder RR, Schmid DU, Werner W (2003) Microbialite morphology, structure and growth: a model of the Upper Jurassic reefs of the Chay Peninsula (Western France). Palaeogeogr Palaeoclimatol Palaeoecol 193:383–404
Olivier N, Carpentier C, Martin-Garin B, Lathuilière B, Gaillard C, Ferry S, Hantzpergue P, Gaillard C, Geister J (2004) Coral-microbialite reefs in pure carbonate versus mixed carbonate-siliciclastic environments: the example of the Pagny-sur-Meuse section (Upper Jurassic, northeastern France). Facies 50:229–255
Olivier N, Lathuilière B, Thiry-Bastien P (2006) Growth models of Bajocian coral-microbialite reefs of Chargey-lès-Port (eastern France): palaeoenvironmental interpretations. Facies 52:113–127
Ourribane M, Chellai EH, Zaghbib-Turki D (2000) Rôle des microbialites et des “micro-encroûtants” dans la lithification récifale: exemples du Jurassique supérieur de l´Atlas maghrébin (Maroc et Tunisie). C R Sci Paris 330:407–414
Paulsen S (1964) Aufbau und Petrographie des Riffkomplexes von Arnegg im höheren weißen Jura der Schwäbischen Alb (Württemberg). Arb Geol Paläont Inst TH Stuttgart 42:1–98
Perrin C (2000) Changes of palaeozonation patterns within Miocene coral reefs, Gebel Abu Shaar, Gulf of Suez, Egypt. Lethaia 33:253–268
Perry CT (2001) Storm-induced coral rubble deposition: Pleistocene records of natural reef disturbance and community response. Coral Reefs 20:171–183
Perry CT, Bertling M (2000) Spatial temporal patterns of macroboring within Mesozoic and Cenozoic coral reef systems. In: Insalaco E, Skeleton PW, Palmer TJ (eds) Carbonate platform systems: components and interactions, vol 178. Geol Soc London Spec Publ, pp 33–50
Pisera A (1997) Upper Jurassic siliceous sponges from the Swabian Alb: taxonomy and paleoceology. Palaeontol Polon 57:1–216
Pümpin VF (1965) Riffsedimentologische Untersuchungen im Rauracien von St-Ursanne und Umgebung (Zentraler Schweizer Jura). Eclogae Geol Helv 58:799–876
Rasmussen KA, Brett CE (1985) Taphonomy of Holocene cryptic biotas from St. Croix, Virgin Islands: informations loss and preservational biases. Geology 13:551–553
Reitner J (1993) Modern cryptic microbialite/metazoan facies from Lizard Island (Great Barrier Reef, Australia) formation and concepts. Facies 29:3–40
Reitner J (1994) Mikrobialith-Porifera Fazies eines Exogyren/Korallen Patchreefs des Oberen Korallenooliths im Steinbruch Langenberg bei Oker (Niedersachsen). Berliner Geowiss Abh E 13:397–417
Reuter M, Fischer R, Helm C, Schülke I (2001) Entwicklung und Faziesverteilung eines Riffkomplexes im Korallenoolith (Oberjura) des Osterwaldes (Niedersachsen). Geol Beitr Hannover 2:31–50
Roberts HH, Lugo A, Carter B, Simms M (1988) Across reef flux and shallow subsurface hydrology in modern coral reef. Proc 6th Int Coral Reef Symp 2:509–515
Roniewicz E, Roniewicz P (1971) Upper Jurassic coral assemblages of the Central Polish Uplands. Acta Geol Polon 21:399–422
Rosendahl S (1985) Die oberjurassische Korallenfazies von Algarve (Südportugal). Arb Inst Geol Paläont Univ Stuttgart, NF 82:1–125
Ruggiero ET (1996) Notes on living brachiopod ecology in a submarine cave off the Campania cost, Italy. In: Copper P, Jin J (eds) Brachiopods. Proc 3rd Int Brachiopod Congress 1995, pp 227–231
Schlagintweit F, Gawlick H-J, Sanders D (2003) Serpulid tubes of the genus Carpathiella Mišík, Sotak and Ziegler, 1999 from the Upper Jurassic to Paleogene of the Northern Calcareous Alps (Austria, Germany). Mitt Ges Geol Bergbaustud Österr 46:91–110
Schmid DU (1996) Marine Mikrobolithe und Mikroinkrustierer aus dem Oberjura. Profil 9:101–251
Schmid DU, Leinfelder RR (1996) The Jurassic Lithocodium aggregatum – Troglotella incrustans foraminiferal consortium. Palaeontology 39:21–52
Schmid DU, Leinfelder RR, Nose M (2001) Growth dynamics and ecology of Upper Jurassic mounds, with comparisons to Mid-Palaeozoic mounds. Sediment Geol 145:343–376
Schülke I, Helm C (2000) A new thecideidinid species (Brachiopoda, Spiriferida) from the Late Jurassic (Oxfordian) of northwestern Germany. N Jb Geol Paläont Mh 2000(5):257–270
Schülke I, Delecat S, Helm C (1998) Oberjura-Riffe in NW-Deutschland: Ein Überblick. Mitt Geol Inst Univ Hannover 38:191–202
Schülke I, Helm C, Kästner M, Winsemann J (2004) The basal Upper Jurassic of the Süntel area: a key for facies development and sequence stratigraphic analysis of the Lower Saxony Basin. Terra Nostra 2004(5):112–114
Schweigert G, Dietl G, Krautter M (2000) Schwämme im Nusplinger Plattenkalk. 70 Jahrestag Paläont Ges (Vorträge und Poster). Terra Nostra 2000(3):111
Scoffin TP (1992) Taphonmy of coral reefs: a review. Coral Reefs 11:57–77
Scoffin TP (1993) Microfabrics of carbonate muds in reefs. In: Rezak R, Lavoie DL (eds) Carbonate microfabrics. Springer, Berlin, pp 65–74
Scoffin TP, Hendry MD (1984) Shallow-water sclerosponges on Jamaican reefs and a criterion for recognition of hurricane deposits. Nature 307:728–729
Shiraishi F, Kano A (2004) Composition and spatial distribution of microencrusters and microbial crusts in upper Jurassic-lowermost Cretaceous reef limestone (Torinosu Limestone, southwest Japan). Facies 50:217–227
Steiger T, Wurm D (1980) Faziesmuster oberjurassischer Plattform-Karbonate (Plassen-Kalke, Nördliche Kalkalpen, Steirisches Salzkammergut, Österreich). Facies 2:241–284
Strasser A, Pittet B, Hillgärtner H, Pasquier JB (1999) Depositional sequences in shallow carbonate-dominated sedimentary systems: concepts for a high-resolution analysis. Sediment Geol 128:201–221
Taylor PD, Palmer T (1994) Submarine caves and their biotas in a Jurassic reef (La Rochelle, France) and the evolution of cave biotas. Naturwissenschaften 81:357–360
Turnšek D (1975) Malmian Corals from Zlobin, southwest Crotia. Palaeontol Jugoslavia 16:1–23
Vytopil E, Willis BL (2001) Epifaunal community structure in Acropora spp. (Scleractinia) on the Great Barrier Reef: implications of coral morphology and habitat complexity. Coral Reefs 20:281–288
Walter B (1969) Les bryozoaires jurassique. Doc Lab Geol Fac Sci Lyon 35:1–328
Werner W (1986) Palökologische und biofazielle Analyse des Kimmeridge (Oberjura) von Consolacao, Mittelportugal. Zitteliana 13:1–109
Werner W, Leinfelder RR, Fürsich FT, Krautter M (1994) Comparative palaeoecology of marly coralline sponge-bearing reefal associations from the Kimmeridgian (Upper Jurassic) of Portugal and Southwestern Germany. Cour Forschinst Senckenberg 172:381–397
Wilson MA (1998) Succession in a Jurassic marine cavity community and the evolution of cryptic marine faunas. Geology 26:379–380
Wood R (2001) Are reefs and mud mounds really so different? Sediment Geol 145:161–171
Wright VP (1985) Seasonal banding in the alga Solenopora jurassica from the Middle Jurassic of Gloucestershire, England. J Paleont 59:721–732
Ziegler PA (1990) Geological Atlas of Western and Central Europe. Shell Int Petrol Maatschappij BV, Den Haag, 239 pp
Acknowledgements
Heartfelt thanks are extended to A. Broschinski (Hannover) who provided the drawing of Fig. 5. We are grateful to A. Strasser (Fribourg) and R. Henrich (Bremen) for careful reviews and helpful comments on the manuscript. The study was financially supported by the Deutsche Forschungsgemeinschaft (DFG, Project-Nos.: Fi 136/27-1; Schu 1214/6-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Helm, C., Schülke, I. Patch reef development in the florigemma-Bank Member (Oxfordian) from the Deister Mts (NW Germany): a type example for Late Jurassic coral thrombolite thickets. Facies 52, 441–467 (2006). https://doi.org/10.1007/s10347-006-0078-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10347-006-0078-9