Abstract
Translocation of game species is a widespread tool for hunting and conservation, but for some species there is a lack of information, being this the case for the Iberian hare (Lepus granatensis). We studied the survival and spatial behaviour of 12 wild Iberian hares translocated in Northwestern Spain, in a hunting ground with a combination of arable land, vineyards and other habitats where game management and hare coursing were conducted. The average hare survival time was 102 days, with adults showing higher survival than juveniles, the overall survival probability being reduced during the hunting season. Presumed predation was the most important cause of death (ranging 45–77% of causalities), and 23% of hares were hunted. Hares showed a higher resting place selection at daytime for arable land, selecting however to settle in areas with a combination of habitats. The average core area considering all animals (MCP 50%) was 7.4 ha and the home range (MCP 95%) was 27 ha, and males showed significant higher values of home range compared to females. When compared to previous studies on wild hares, the survival of translocated hares in the present study was in general lower but there were similarities on spatial behaviour, and hunting was considered a major factor driving survival and spatial behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three hare species occur in the Iberian Peninsula: the brown hare (Lepus europaeus), distributed in Northeastern Spain, the broom hare (Lepus castroviejoi) only found in the Cantabrian Mountains and the Iberian hare (Lepus granatensis), which is widespread in most of the Iberian Peninsula and the island of Majorca (Rodríguez et al. 1997). The Iberian hare (hereafter just ‘hare’) is one of the most important small game species in Spain and Portugal, especially in the open lands (northern and southern plateaus) where they are hunted with greyhounds (Canis lupus familiaris). In the period 2005–2017, an average of 884,000 hares were harvested through shooting and coursing (Garrido et al. 2020) and in 2019 there were at least 12,000 hunters devoted to coursing (Consejo Superior de Deportes 2019).
Game managers from different areas of Central Spain, where hares were abundant decades ago, have recorded declines on populations in recent years. Thus, in 2019 hare densities in the region of Castilla-La Mancha were 50–60% lower than the ones recorded in early 2000s (Lázaro et al. 2019), and even local hare extinctions may had occurred, owing to a combination of factors such as habitat change, intensive farming, inadequate game management and disease (Rodríguez et al. 1997). In fact, in the late 1990s, a first outbreak of tularemia in hares was detected in Castilla y Leon region (Reviriego et al. 2000), and from July 2018, outbreaks of a new myxomatosis virus have been detected in several regions of Spain and Portugal (Águeda-Pinto et al. 2019; Dalton et al. 2019), affecting to populations on Central and Southern Spain (García-Bocanegra et al. 2019).
Hare translocation for hunting purposes in certain areas of Spain seems to be a widespread technique, though there is a lack of studies on the survival, spatial behaviour, habitat selection, contribution to the hunting bag and effects on the resident population. To the best of our knowledge, only one study evaluated these variables in 47 translocated and radio-tagged hares and concluded that 52% of hares survived during the closed season and 36% during the hunting season (Rodríguez et al. 1997).
There are mixed results on the output of translocations for brown hares, which occur naturally in Eurasia. Fischer and Tagand (2012) considered that the translocation of brown hares in Switzerland was a useful technique, because translocated hares managed to settle and reproduce, not showing significant variations compared to the source population. However, Ferretti et al. (2010) found differences on home range and habitat selection between translocated and resident brown hares in Italy, while Sokos et al. (2014) found that the survival rates in the wild of translocated hares were higher than hares intensively and extensively reared.
It is likely that hare translocation will be used for hunting and conservation purposes, especially in some areas where hares are at low densities or recently extinct, especially after the outbreaks of myxomatosis. Aiming to help those game managers and conservationist working on hares’ recovery through this technique, this work evaluated the survival, home range, resting place selection and causes of death of translocated hares in a typical coursing ground in Northwestern Spain.
Material and methods
Study area
The field work was carried out from July 2010 to February 2011 in the private hunting ground of Pajares de los Oteros (province of Leoón, Northwestern Spain, UTM ETRS89 X:296200 Y:4689400), which covers 5950 ha. The area has an average altitude of 791 m asl and is mainly flat, being the climate Mediterranean dry continental according to Papadakis (1966). The study area was partially included in the Special Protection Area for Birds ‘Oteros-Campos’ (ES0000194), which holds populations of farmland birds of conservation concern (www.rednatura.jcyl.es). Although agriculture was intensive at the study site, the landscape retained uncultivated land and grasslands, and no arable land consolidation had been conducted.
Three main types of habitats were distinguished inside the hunting ground using the Spanish Land Parcel Information System (SIGPAC), with the following percentages: arable land (hereafter AL), which were fields of barley (Hordeum distichon), winter wheat (Triticum aestivum) and chick peas (Cicer arietinum), covering 72% of the hunting ground; traditional vineyards (Vitis vinifera) (VI), 20%; and “other” (OT), including uncultivated land, grasslands and forest, the remaining 8% (Fig. 1).
Small game species were managed by local hunters and a part-time gamekeeper, involving 20 water troughs located in the vineyards and uncultivated land area; five 100 ha areas acting as hunting reserves together with a specific ground of 1500 ha where coursing was the only type of hunting allowed; and control of predators, with 7 red foxes (Vulpes vulpes) culled in March 2010 and 3 during the hunting season, 24 magpies (Pica pica) and 11 carrion crows (Corvus corone) culled in April 2010. We could not conduct a proper estimation of predator density, but this data showed that foxes were present at the area during the whole study. In March 2010, we conducted a hare count in the whole hunting ground using a spotlight from a vehicle (Carro and Soriguer 2017), aiming to choose the source and release areas. The average density for the study site was 3.4 hares/100 ha.
During the open season 2010–2011 (12 October–15 January), hunting was only allowed in Sundays, on an average of 10 people/day hunted hare with greyhounds in the open lands. Red-legged partridges (Alectoris rufa) and wild rabbits (Oryctolagus cuniculus) were hunted through walking-up shooting, not being allowed hare shooting anywhere.
Translocation and radio-tracking
To study the survival and behaviour of the translocated hares, they were first caught in a source area with relatively high density (> 7 hares/100 ha) and released afterwards in an area with a lower density (under 3 hares/100 ha), with similar proportions of habitat types and keeping a minimum distance of 5 km away from the catching point. Hares were translocated on 2nd and 12th July 2010, allowing enough time before the beginning of the hunting season (October). We followed the same methodology and equipment described by Sánchez-García et al. (2012a), using long nets and beaters, starting captures at 8:00 a.m. and finishing not later than 11:00 a.m. In all captured hares, the sex, age and body weight were determined. We captured and released nine hares on the first day and three on the second, discarding leverets to avoid radio-tagging problems. Hares were marked with a Biotrack Ltd. (TW-5), the radio-tag weighing 30 g and kept in a box in darkness individually until releasing (Paci et al. 2012), which occurred 60 min after capture.
The selected releasing area included the habitat types where hare coursing was conducted. We aimed to replicate the on-site translocations conducted by game managers to increase hunting bags and prevent crop damage. At the releasing area, we selected four releasing points, each of them within plots of 20–40 ha which combined cereals and chick peas, the latter sown in January–February 2010 and harvested in the coming autumn-winter (Fig. 1). We avoided releasing hares straight in vineyards, though releasing points were close to them to provide a combination of habitats offering food, resting places and shelter. In each of the four plots, three hares were released individually.
Radio-tracking was conducted from the day after releasing until the animal was found dead. Hunters were warned about the translocation of radio-tagged hares to increase tag recovery. In the case the signal was lost, we expanded radio-tracking outside the study area as dispersion may have occurred. When a hare was no longer located, it was classified as dead in the survival analysis. Radio-tracking was carried out during daytime, avoiding harsh weather, and the frequency varied across the study period: from July to September between 8:00–11:00 a.m. and 5:00–8:00 p.m., and from October onwards from 9:00 a.m. to 4 p.m., avoiding weekends when hunting occurred. To locate hares on their resting place, we followed the triangulation method as described by Sánchez-García et al. (2012a) for the same species in a similar landscape of Spain. To avoid unnecessary disturbance, we aimed to locate and see hares at least once every 7–10 days, trying not to flush them.
At the study area, cereal harvesting was conducted by the end of July, and stubbles remained until mid-September, when ploughing was conducted, the fields being sown afterwards in October–November. As hunting started in October, we considered two main study periods: July–September, with no hunting and partial vegetation cover in the arable fields, and October–January, when hunting was conducted with limited vegetation cover in the arable fields (with the exception of plots of chick peas).
Data analysis
Multistate capture-recapture models were used to analyse the survival and habitat selection of hares. A multistate model is a model for stochastic process which each individual at any time occupies one state from a set of discrete states. Also, we used a hierarchical model because the detection process is not perfect and usual survival analysis does not consider this process separately (Houggard 1999; Gimenez et al. 2012). Note that ‘dead’ cases correspond to those hares in which death was confirmed and those that dispersed from the study site.
Because the radio-tracking was always conducted in the absence of harsh weather, we assumed that the detection probability (Po) was constant during the course of the study. The survival of hares was analysed using gender (male, female) and age (subadult, adult) as the two components of the individual survival. Thus, the survival probability (Ps) for each individual (i = 1,....., 12) at time (t = 1,....., 18 dates of radio-tracking) was estimated as:
where Pssex was the sex-specific and Psage the age-specific probability of survival. Because the days of sampling were not equally spaced from each to other, we transformed the resulting probabilities of survival to weekly survival probabilities making the assumption that the probability of survival was constant within dates of radio-tracking.
The multistate model considers a survival process (Sv) with two states (1: alive, 0: dead) and an observation process (Ob) with two states (1: located, 0: not located). Thus, the states of each individual (i) at time (t) were estimated using the Bernoulli probability distribution as:
being Psi,t and Po the parameters to be estimated.
The resting place selection was analysed using movement probabilities between the habitats where hares were located. We consider a state process (St) with four states (present in AL, VI, OT or dead) and an observation process (Ob) with four states (located in AL, VI, OT or not located). Then, the model included two four-dimensional matrices: a state-transition matrix (S) and an observation matrix (O), with the transition probabilities from row-state to column-state (for more detail see Kéry and Schaub 2012). We made the assumption that there was a general probability of survival (Ps) between sampling dates, because we did not want to investigate sex/age covariate effects for each specific transition between two habitats. Thus, the transition probabilities of each individual (i) at time (t) to move from habitat k = 1, ...., 4, were estimated using the generalized Bernoulli (Categorical) probability distribution as:
The home range was computed using a bivariate kernel function applied to the utilization distribution concepts and the minimum convex polygon (MCP) estimator. The utilization distribution (UD) represents the probability of finding an animal at a given point. Then, the home range size was estimated using the 95% MCP and the core area using the 50% MCP.
The statistical analyses were conducted using the R 3.4 software (R Development Core Team, 2017), in particular the ‘adehabitatHR’ package (Calenge 2006) to the home range analysis. The Bayesian analysis applied to the survival and resting place analysis was performed using JAGS 4.3.0 (Plummer 2017). The first 10,000 iterations were treated as a burn-in period and the following 500,000 iterations with thinning interval of 10 were saved. The prior distributions used to estimate the survival and observation probabilities were uniform distributions while in the estimation of the movement probability, a Dirichlet distribution was used. The convergence was tested using Gelman and Rubin (1992) from three chains generated with different initial values. Following Mc Elreath (2018), the highest posterior density interval (HPDI) was estimated using 89% interval while in the classical confidence intervals (CI), the usual 95% interval was used.
Results
A total of 12 hares were captured, radio-tagged and released: eight females (five adults and three subadults) and four males (three adults and one subadult). The radio-tracking was conducted during 25 weeks (197 days after the first release), from the 2nd of July 2010 until the 15th of January 2011. A total of 99 radio-locations were obtained. The estimated probability of detection for the whole study was 0.872 (HPDI: 0.833-0.901).
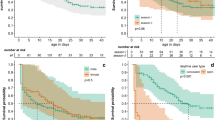
Figure 2a shows the changes of the estimated survival probabilities by sex and age. The median survival of adults was 129 days and 99 for subadults, and the median survival of females was 104 days and 85 days for males. For the whole study, the mean survival probability of adults (Pad = 0.879, HPDI: 0.838–0.921) was higher than subadults (Psa =0.807, HPDI: 0.740–0.873), being this the case for both males and females.
Estimated survival probabilities of hares by sex and age. The vertical dashed dot line shows the moment of opening of the hunting season. (a) The lines show the general survival probability along study (left vertical axis in log scale) and the bars show the number of radio-located hares along study (right vertical axis). (b) Survival time changes of the estimated weekly survival probabilities by sex (lower subfigure) and age (upper subfigure) in relation to opening of the hunting season. The horizontal lines show the median value
Figure 2b shows the changes of the estimated weekly survival probabilities in relation to the hunting season. The survival probability during the closed season was higher for both adults and subadults (Psa = 0.835, Pad = 0.894) when compared to the one recorded during the open season. As soon as the season was open, the survival probability of subadults decreased (Psa = 0.719, HPDI: 0.548–0.896) while in adults, it remained relatively high (Pad = 0.860, HPDI: 0.785–0.933). Quite the opposite, the survival probability during the close season was significantly lower in males (Pf = 0.905, Pm = 0.798), while during the open season no significant differences were found between males and females (Pf = 0.822, Pm = 0.831).
From the 12 hares released, it was not possible to determine the probable cause of death in three individuals (which had less than 2 radio-locations); hence the probable cause of death was determined in nine hares: two were hunted (radio-tag retrieved from hunters) and four were presumably predated (one by fox and three by unknown predators, i.e. carcass found or radio-tag with marks), not discarding those eaten as carrion. In the remaining hares, radio-tags were found during the hunting season but it was not possible to address whether they had been predated or hunted. Hence, in a first scenario, 77% of hares may have been predated (n = 7) and 23% hunted (n = 2), and in a second scenario 44% predated (n = 4), 33% ‘unknown’ (n = 3) and 23% hunted (n = 2).
With regard to resting place selection, we found that the probability of movement between the three types of habitats varied significantly (p < 0.01) (Fig. 3). Once hares were located in AL, they were less keen to move to VI (PVI = 0.190, HPDI: 0.110–0.271) or OT (POT = 0.168, HPDI: 0.096–0.250). Thus, this behaviour resulted in a higher selection of AL (PAL = 0.636, HPDI: 0.531–0.732) compared to the other habitats.
Probability of hare movement between the three types of habitats considered: arable (AL), vineyards (VI) and other (OT). Significant differences with the equal probability of movement (1/3) are marked using solid lines when significant differences existed and dashed lines when not significant differences were found
The 8 hares with more than 4 radio-locations were used to the home range analysis (Fig. 4). The average core area was 7.37 ha (CI: 4.03–10.70 ha, median 6.35 ha) and the average home range size was 27.12 ha (CI: 18.54–35.69 ha, median 24.98 ha). No significant differences were found between adults (26.2 ± 87.1 ha) and subadults (48.5 ± 13.7 ha) (χ2 = 0.43, df = 1, P = 0.47), while significant differences were reported between males (57.1 ± 18.7 ha) and females (27.9 ± 6.9 ha) (χ2 = 3.43, df = 2, P < 0.05). In general, hares settled their territories in ecologically complex areas where the three types of habitats considered were present, and there was a core area overlap across seven hares (Fig. 4).
Contour plot showing the estimated use of space by the 8 radio-tracked hares using the bivariate probability density functions according to the geographic coordinates. The areas may be considered a home range (equivalent to the 95% MCP) when the probability is upper than 0.1 and as core areas (equivalent to the 50% MCP) when the probability is upper than 0.5 (in grey). The white dots represent the centroid and the black dots correspond to the radio-locations of translocated hares. In the background, the three types of habitats are represented (see Fig. 1)
Discussion
To the best of our knowledge, this is the second study dealing with translocation of Iberian hares, an understudied small game species in Iberia; hence, our results could fill research gaps (Alzaga et al. 2013). The interpretation of our results should be done with caution, as the sample size does not allow generalizations.
Our survival values were halved when compared to results from Rodríguez et al. (1997), who recorded in translocated hares 52% while the hunting season was closed (240 days) and 36% during hunting season (90 days), and also compared to the results from Sánchez-García et al. (2012a), who recorded in wild hares 13% of survival in 300 days in an area with no hunting. Interestingly enough, Schultz (1980) recorded severe mortality (83% in 16 days) in translocated snowshoe hares (Lepus americanus).
As three hares were lost during the first 2 weeks after releasing, it seems that in the short term, some hares dispersed or were predated owing to stress induced by handling as it has been reported in many species of mammals and birds (Letty et al. 2007), though we cannot discard natal dispersal occurring in juveniles as described by Bray et al. (2007) in brown hares. It is interesting to note that patterns of survival varied among open/close hunting periods: during the close season, survival was higher in adults and females while during the open season, survival of subadults remained lower, with no significant differences between males and females. In this way, studies conducted in resident brown hares in France showed that exploration movements were more likely to be explained by hunting and related disturbances rather than natal dispersal, which often coincides with the opening of the hunting season (Avril et al. 2014), and hunting induces higher dispersion in hares when compared to areas with no hunting (Bray et al. 2007). Owing to the fact that the majority of deaths occurred after the opening of the hunting season, we are led to believe that the overall low survival recorded in our study could be explained mainly by disturbances from hunting, though further research should confirm this hypothesis with a large sample size. Despite the releasing area was managed for hares and predator removal was conducted, we cannot rule out that predation affected the spatial behaviour and subsequent hare survival.
Presumed predation was identified as the main cause of death, although in three hares it was not possible to determine whether they had been predated or hunted. This is in agreement with previous studies conducted on wild and translocated Iberian hares (Rodríguez et al. 1997; Carro 2005; Sánchez-García et al. 2012a) and also in wild and captive-reared brown hares which are often subject to high rates of predation, especially from foxes (Angelici et al. 2000; Schmidt et al. 2004; Panek 2009). The low number of hares hunted (n = 2) indicates that the contribution of translocated hares to the game bag was limited, as previously proved by Rodríguez et al. (1997) in Iberian hare and Santilli and Galardi (2006) in a brown hare population of Italy. As we did not observe leverets within the territories of females during the field study and none of the hares survived the hunting season, we could not confirm successful reproduction.
Hares showed a higher selection for arable when compared to vineyards and other lands, and the areas where hares settled down had a combination of the three main habitats considered. In studies on wild hares, Sánchez-García et al. (2012a) found a high selection for uncultivated land in a study site dominated by shrubland/forest and arable crops, while Rodríguez et al. (1997) at a similar habitat found a different selection of habitat across the year, with an overall selection for grasslands and crops. Thus, in our study translocated hares selected the more open habitat (arable) but settled in patches where there was a combination of habitats, a similar pattern than the one described for wild hares, including hares occurring at different habitats in southern Spain (Duarte et al. 2002; Carro 2005). Also, in brown hares, it has been demonstrated that the wild ones use more diverse patches when compared to translocated (Ferretti et al. 2010), though with no significant differences in some cases (Fischer and Tagand 2012). Although we did not radio-track resident hares, these patterns may correspond to the behavioural patterns of wild Iberian and brown hares aiming to reduce predation risk and ensure feeding and breeding performance.
The average values of home range were significantly lower compared to the translocated hares studied by Rodríguez et al. (1997), ranging 123–300 ha, and also lower but closer to the values recorded in wild hares by Sánchez-García et al. (2012a) and Carro (2005) at different habitats (range 32–40 ha). If we look at studies on translocated brown hares, there are mixed results, with either no significant differences compared to resident hares (Fischer and Tagand 2012) or significant higher home range (Ferretti et al. 2010). The fact that males had a significant higher home range could be explained by their need to explore new territories while females may tend to reduce their home range to save energy for reproduction, a similar conclusion found for wild hares by Sánchez-García et al. (2012a), and partially in agreement with the findings of Rodríguez et al. (1997), showing in general higher values of home range for males compared to females. One of the highlights of our study was that there was a core area overlap across the majority of individuals (n = 7), which agrees with previous findings in the same species (Carro 2005). We are led to believe that this overlapping is explained by the social structure of the species, as suggested for the brown hare by Rühe and Hohmann (2004), and we speculate that more diverse habitats may favour higher hare densities (Alzaga et al. 2013) and subsequent overlapping, though further research is needed.
Taking our results and looking at previous studies on wild hares, can translocation be considered a useful tool when aiming to increase hunting bags or hare densities? With regard to hunting, translocated hares are the only possibility to increase hunting bags in some regions in Spain because there are not many farms, and the number of hares produced in captivity is limited; in 2012 only 1000 individuals were produced (Sánchez-García et al. 2012b). Also, hare translocations are not permitted in some regions to reduce disease transmission (even on-site translocations with short distances between the source and release areas). The low number of radio-tracked hares in which hunted was confirmed points towards the idea that translocation may not be a cost-effective practice owing to the resources needed, but this may not be the case in hunting grounds where hares may damage crops, being translocation recommended rather than culling.
As no hares survived the hunting season and we could not confirm successful reproduction within the areas where hares settled, it is not clear whether translocation can be recommended to increase hare densities in the wild. However, the spatial behaviour was in general quite similar to wild hares, and it is likely that with no hunting, survival and breeding success may have been improved.
If we look at hare hunting bags during the last 20 years (Garrido et al. 2020) and studies at regional scales (Lázaro et al. 2019), it seems that the Iberian hare is declining. However, like with other small game species, we think that efforts conducted by managers and hunters should mainly focus on increasing wild hare densities through monitoring, habitat improvement, targeted predator removal and adaptative hunting (Reynolds et al. 2010; Weber et al. 2019), rather than releasing captive-reared or translocated hares. However, translocated hares may ensure hunting and associated management, which is also important, and probably reduce hunting pressure on resident hares.
If hares are at very low densities or extinct, special care should be taken to first address the limiting factors before any translocation attempt. This may help to recover wild hare populations and ultimately increase hunting bags, though further studies in different management and hunting contexts should be conducted to address whether translocation could have conservation implications.
References
Águeda-Pinto A, de Matos AL, Abrantes M et al (2019) Genetic characterization of a recombinant Myxoma virus in the Iberian hare (Lepus granatensis). Viruses 11:530. https://doi.org/10.3390/v11060530
Alzaga V, Torres J, Villanúa D et al (2013) Conocimientos científicos importantes para la conservación y gestión de las tres especies de liebre de la Península Ibérica: deficiencias y retos para el futuro. Ecosistemas 22:13–19. https://doi.org/10.7818/ecos.2013.22-2.03
Angelici FM, Riga F, Boitani L, Luiselli L (2000) Fate of captive-reared brown hares Lepus europaeus released at a mountain site in central Italy. Wildl Biol 6:173–178. https://doi.org/10.2981/wlb.2000.013
Avril A, Letty J, Léonard Y, Pontier D (2014) Exploration forays in juvenile European hares (Lepus europaeus): dispersal preludes or hunting-induced troubles? BMC Ecol 14:6. https://doi.org/10.1186/1472-6785-14-6
Bray Y, Devillard S, Marboutin E et al (2007) Natal dispersal of European hare in France. J Zool 273:426–434. https://doi.org/10.1111/j.1469-7998.2007.00348.x
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Carro F (2005) Historia natural de la liebre ibérica (Lepus granatensis Rosenhauer, 1856) en el Parque Nacional de Doñana. PhD Thesis, Universidade de Santiago de Compostela (Spain)
Carro F, Soriguer RC (2017) Long-term patterns in Iberian hare population dynamics in a protected area (Doñana National Park) in the southwestern Iberian Peninsula: effects of weather conditions and plant cover. Integr Zool 12:49–60. https://doi.org/10.1111/1749-4877.12212
Consejo Superior de Deportes (2019) Anuario de estadísticas deportivas 2019. http://www.culturaydeporte.gob.es/dam/jcr:dc406096-a312-4b9d-bd73-2830d0affb2d/anuario-de-estadisticas-deportivas-2019.pdf. Accessed 13 March 2020
Core Team R (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna: Austria. Available from: http://www.R-project.org/
Dalton KP, Martín JM, Nicieza I et al (2019) Myxoma virus jumps species to the Iberian hare. Transbound Emerg Dis 66:2218–2226. https://doi.org/10.1111/tbed.13296
Duarte J, Vargas JM, Farfán M (2002) Biología de la liebre ibérica (Lepus granatensis). In: Bases técnicas para la gestión cinegética. Aportaciones de la gestión sostenible de la caza. FEDENCA-EEC, Madrid, pp 29–59
Ferretti M, Paci G, Porrini S et al (2010) Habitat use and home range traits of resident and relocated hares (Lepus europaeus, Pallas). Ital J Anim Sci 9:278–284. https://doi.org/10.4081/ijas.2010.e54
Fischer C, Tagand R (2012) Spatial behaviour and survival of translocated wild brown hares. Anim Biodivers Conserv 35:189–196
García-Bocanegra I, Camacho-Sillero L, Risalde MA et al (2019) First outbreak of myxomatosis in Iberian hares (Lepus granatensis). Transbound Emerg Dis 66:2204–2208. https://doi.org/10.1111/tbed.13289
Garrido J, Ferreres J, Gortázar C (2020) Las especies cinegéticas españolas en el siglo XXI.
Gelman A, Rubin DB (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472. https://doi.org/10.1214/ss/1177011136
Gimenez O, Lebreton JD, Gaillard JM et al (2012) Estimating demographic parameters using hidden process dynamic models. Theor Popul Biol 82:307–316. https://doi.org/10.1016/j.tpb.2012.02.001
Houggard P (1999) Multi-state models: a review. Lifetime Data Anal 5:239–264
Kéry M, Schaub M (2012) Bayesian population analysis using WinBUGS: a hierarchical perspective. Academic Press Limited, Cambridge
Lázaro C, Sánchez-Garćia C, Guzmán J (2019) Population status of the Iberian hare (Lepus granatensis) after the mixomatosis outbreak in Castilla-La Mancha. In: First Iberian Congress of Applied Science on Game Resources. (CICARC), Ciudad Real, p 14
Letty J, Marchandeau S, Aubineau J (2007) Problems encountered by individuals in animal translocations: lessons from field studies. Ecoscience 14:420–431. https://doi.org/10.2980/1195-6860(2007)14[420:pebiia]2.0.co;2
Mc Elreath R (2018) Statistical rethinking: a Bayesian course with examples in R and Stan. Chapman and Hall Ltd, London, UK
Paci G, Ferretti M, Bagliacca M (2012) Reducing visual stimulations in European hares (Lepus europaeus Pallas) captured for translocation. Ital J Anim Sci 11:275–278. https://doi.org/10.4081/ijas.2012.e51
Panek M (2009) Factors Affecting predation of red foxes Vulpes vulpes on brown hares Lepus europaeus during the breeding season in Poland. Wildl Biol 15:345–349. https://doi.org/10.2981/07-042
Papadakis J (1966) Climates of the world and their agricultural potentialities. Buenos Aires, Argentina.
Plummer M (2017) JAGS Version 4.3.0 user manual
Reviriego F, Reques F, Álvarez F et al (2000) Tularemia en Castilla y León. Brote epidemilógico. Junta de Castilla y León, Valladolid, Spain
Reynolds JC, Stoate C, Brockless MH et al (2010) The consequences of predator control for brown hares (Lepus europaeus) on UK farmland. Eur J Wildl Res 56:541–549. https://doi.org/10.1007/s10344-009-0355-3
Rodríguez M, Palacios J, Martín J et al (1997) La liebre. Mundiprensa, Barcelona, España
Rühe F, Hohmann U (2004) Seasonal locomotion and home-range characteristics of European hares (Lepus europaeus) in an arable region in central Germany. Eur J Wildl Res 50:101–111. https://doi.org/10.1007/s10344-004-0049-9
Sánchez-García C, Alonso ME, Bartolomé DJ et al (2012a) Survival, home range patterns, probable causes of mortality, and den-site selection of the Iberian hare (Lepus, Leporidae, Mammalia) on arable farmland in north-west Spain. Ital J Zool 79:590–597. https://doi.org/10.1080/11250003.2012.685109
Sánchez-García C, Alonso ME, Díez C et al (2012b) An approach to the statistics of wild lagomorph captive rearing for releasing purposes in Spain. World Rabbit Sci 20:49–56. https://doi.org/10.4995/wrs.2012.1030
Santilli F, Galardi L (2006) Factors affecting brown hare (Lepus europaeus) hunting bags in Tuscany region (central Italy). Hystrix, Ital J Mammal 17:143–153. https://doi.org/10.4404/hystrix-17.2-4372
Schmidt NM, Asferg T, Forchhammer MC (2004) Long-term patterns in European brown hare population dynamics in Denmark: effects of agriculture, predation and climate. BMC Ecol 4:15. https://doi.org/10.1186/1472-6785-4-15
Schultz WC (1980) Extent and causes of mortality in stocked snowshoe hares. J Wildl Manag 44:716. https://doi.org/10.2307/3808027
Sokos C, Birtsas P, Papaspyropoulos KG et al (2014) Conservation considerations for a management measure: an integrated approach to hare rearing and release. Environ Manag 55:19–30. https://doi.org/10.1007/s00267-014-0388-6
Weber D, Roth T, Kohli L (2019) Increasing brown hare (Lepus europaeus) densities in farmland without predator culling: results of a field experiment in Switzerland. Eur J Wildl Res 65:75. https://doi.org/10.1007/s10344-019-1306-2
Acknowledgements
We are grateful to the hunting syndicate ‘Sociedad de Cazadores Pajares de los Oteros’ for their help. We thank M.E. Alonso for her support with equipment, and we are indebted to the people who kindly helped in hares’ captures, despite 12nd July 2010 was the day after the Spanish football squad won its first World Cup.
Funding
This project was funded by the Universidad de León and the Regional Government of Castilla y León (Consejería de Agricultura y Ganadería), the latter also providing the permission for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sánchez-García, C., Pérez, J.A., Armenteros, J.A. et al. Survival, spatial behaviour and resting place selection of translocated Iberian hares Lepus granatensis in Northwestern Spain. Eur J Wildl Res 67, 22 (2021). https://doi.org/10.1007/s10344-021-01464-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-021-01464-8