Abstract
The negative effects of plant protection chemicals on the environment and human health have led scientists to research alternative control methods. Fungi, especially Trichoderma have an important place in biological control, one of the most common alternative methods. Licensed as an agricultural product, it has been effectively used in agricultural lands. This study aimed to evaluate long-lasting carriers of the ET 4 and ET 14 Trichoderma harzianum isolates, proven effective against different pathogens in previous studies, to allow licensing for their commercial and mass production in the industry. The efficacy of ET 4 and ET 14 isolates against Alternaria solani (isolate ET 66) was initially confirmed by testing in vitro and in vivo conditions. Then, spores of the fungus isolate developed on potato dextrose agar (PDA) were transferred to five different liquid media (soybean oil, neem oil, canola oil, paraffin oil, and glycerine) prepared as the carrier formulations. They were kept at room (22 ℃) and refrigerator (+4 ℃) temperatures for 10 months. The viability tests of the bioagent fungus were performed by sowing into PDA from the monthly samples taken from the formulations. In addition, at the end of the tenth month, the efficacy of the bioagents was tested against the pathogenic fungus in vitro and in vivo conditions. Even though the most successful carrier was neem oil with a very intense bioagent development, the bioagent maintained its viability in all carriers even at the end of the 10th month, with percent inhibition rates varying between 37.85% and 52.33% in vitro and between 14.26% and 3.95% in vivo conditions. It was concluded that paraffin, glycerin, and especially neem oil were good carriers for the T. harzianum bioagent, that the shelf life could be extended even more with further studies, and that it could be licensed as a biopesticide after toxicological and ecotoxicological evaluations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trichoderma species belong to the subphylum Ascomycota, the order Hypocreales, and the family Hypoceaceae (Aydın 2015). These species are commonly found in natural habitats and all types of soils and can be isolated from plant roots, decomposed bark, sclerotia, or other reproductive organs of fungi (Papavizas 1985). The antagonistic effects of Trichoderma species, achieved through various mechanisms such as the production of antifungal metabolites, competition for nutrients and space (Sivan 1989; Güçlü and Özer 2022), mycoparasitism (Mukherjee et al. 2022) and the synthesis of secondary metabolites with antibiosis properties (Kredics et al. 2003; El-Debaiky 2017; Mironenka et al. 2021), have increased their significance in agriculture. Furthermore, these species also promote plant growth by producing hormone-like metabolites and by solubilizing nutrients from soil or organic matter (Vinale et al. 2006; Verma et al. 2007). Studies have determined that these species can suppress the growth of numerous phytopathogenic fungi and oomycetes, including but not limited to Fusarium solani, Sclerotinia sclerotiorum, Botrytis cinerea, Macrophomina phaseolina, Cordana musae, Rhizoctonia solani, and Pythium ultimum (Samuelian 2016; Zhang et al. 2016; Hewedy et al. 2020; Erazo et al. 2021). Additionally, Trichoderma species can indirectly prevent pathogen infection by enhancing plant resistance (Woo et al. 2022).
Among the Trichoderma species, Trichoderma harzianum Rifai, in particular, possesses multiple mechanisms of action, including the production of chitinases, β‑1‑3 glucanases, and β‑1‑4 glucanases, competition, antibiotics, solubilization of inorganic plant nutrients, inactivation of pathogenic enzymes, and promotion of resistance (Elad and Kapat 1999; Harman 2006). In addition to these mechanisms, T. harzianum also produces volatile metabolites and non-volatile antibiotics, suppresses soil-borne fungal pathogens, and competes in terms of space and nutrients, among other antagonistic mechanisms (Harman et al. 2004; Akrami and Yousefi 2015). This way, it suppresses the development of pathogens. Indeed, the T. harzianum isolates ET 4 and ET 14 used in this study were found to inhibit Alternaria alternata, A. solani, Botrytis cinerea, Colletotrichum gloesporoides, F. oxysporum, F. solani, Geotrichum candidum and Sclerotinia sclerotiorum (Tozlu et al. 40,41,a, b, Tekiner et al. 37,38,a, b, Çamlıca and Tozlu 2019, Çakar and Tozlu 2022, Tekiner et al. 2020).
T. harzianum has several bio-pesticides, and they are available in various formulations such as liquid, semi-solid, and solid. The effectiveness of this species is stated to be highly related to the composition and quality of carriers. Therefore, determining suitable and effective carrier compositions is of great importance (Ramanujam et al. 2010; Singh et al. 2014). The choice of which formulation to prefer in practice depends on the purpose and conditions of the application. The selection of an appropriate formulation is a crucial component in controlling plant pathogens and requires careful consideration.
Transforming the formulations of microorganisms that effectively control plant pathogens in biological control into products and delivering them to the fields are of great importance. They are expected to be produced on a large scale through mass production systems and should also be compatible with field applications. Therefore, it is necessary to determine suitable environments for T. harzianum formulations. This constitutes the first step in creating a biocontrol agent formulation. Consequently, taking into account the significant characteristics of T. harzianum, it is crucial to commercially produce it for the benefit of humanity and extend its shelf life. This study aims to determine the shelf life of T. harzianum isolates ET 4 and ET 14 in various liquid carrier formulations against the plant pathogen A. solani (Çamlıca and Tozlu 2019), which has been previously determined to be effectively controlled by these isolates in tomato cultivation worldwide and causes significant product losses (79%) (Bektaş 2022).
Material and Methods
Bioagents and Pathogenic Fungal Isolate
In this study, ET 4, ET 14 Trichoderma harzianum isolates and ET 66 Alternaria solani isolate were used (Table 1).
Cultures of T. harzianum ET 4 and ET 14 isolates and A. solani ET 66 isolate, stored in a solid medium at +4 °C in the Mycology Laboratory of the Department of Plant Protection, Faculty of Agriculture, Atatürk University (Türkiye) were used in this study. For these isolates, a disk of mycelium. (6 mm) was transfered to 9 cm diameter Petri dishes containing Potato Dextrose Agar (PDA, Difco) medium using a scalpel, and then they were incubated at 27 °C. Stored pure cultures were used in the subsequent stages of the study.
Carriers
-
Soybean oil: 100% soybean oil obtained from the soy plant (MINDIVAN Limited Company, TR-41-K-006718)
-
Neem oil: Azadirachta Indica Cas no: 8002-65‑1 (DOGASA Pharmaceutical Food Industry Trade Limited Company).
-
Canola oil: It contains canola oil, coconut oil, date oil, and dimethyl silicone (PAM).
-
Paraffin oil: Food industry compliant paraffin oil has been used (WÜRTH).
-
Glycerin: 85% glycerin has been used (ISOLAB).
In vitro Assay
The effectiveness of the bioagents ET 4 and ET 14, previously tested against numerous pathogens was assessed against the pathogen A. solani ET 66 isolate at the beginning and end of the study. For this purpose, mycelial disks with a diameter of 6 mm from the respective bioagents and the pathogenic fungus A. solani ET 66 isolate were placed in Petri dishes containing PDA, with a 7.5 cm distance between them, and then incubated at 27 °C until the plates were completely covered with fungal growth in the Petri dishes where only bioagents were placed. In contrast, the control Petri dish only contained the pathogenic fungus isolate. In the study, 3 Petri dishes were used for each bioagent, and the study was conducted with 3 repetitions.
The “hyperparasite level” refers to the density or impact level of a secondary parasite that is effective on a primary parasite in an ecosystem or organism. To determine the hyperparasitic effect, the radial growth inhibition percentages of fungal bioagents were calculated according to Skidmore and Dickinson (1976) by measuring the radius of the pathogen in the control Petri dish (R1) and the radius of the pathogen in the Petri dish where the bioagent and pathogen were co-cultured (R2). The formula is presented in Fig. 1. It has been modified based on the Skidmore and Dickinson (1976). This process helped to reaffirm the effectiveness of the bioagent fungi.
- PIRG:
-
= Percentage inhibition of radial growth (%)
- R1:
-
= The semi-diameter of the pathogen mycelium in the Petri dish control
- R2:
-
= The semi-diameter of the pathogen mycelium in the double culture Petri dish (ET 4 or ET 14 and pathogen fungus)
- +:
-
: PIRG ≤ 50%: Low
- ++:
-
: 50% < PIRG ≤ 60%: Medium
- +++:
-
: 60% < PIRG ≤ 75%: High
- ++++:
-
: PIRG > 75%: Very high
- −:
-
: Ineffective
Pre-storage In vivo Assay
Tomato seedlings were inoculated with 30 µl fungal suspensions containing 1 × 106 conidia/ml from T. harzianum isolates, prepared with the addition of Tween 80, into wounds made on their stems located 10 cm above the soil surface. Afterward, a mycelial disk with a diameter of 6 mm, obtained from the pathogenic fungus A. solani ET 66 isolate, which was cultured on PDA for 10 days, was placed on top of the wound and sealed with parafilm. In the study, sterile water was used as the negative control, and the pathogenic fungus A. solani ET 66 without bioagent was used as the positive control. Three pots received the pathogen and another three the bioagent fungus, three pots received both the pathogenic fungus and the bioagent fungus isolate together, and three pots received sterile water. The study was conducted in growth cabinets set at 24 °C with a 12-hour light and 12-hour dark cycle for a duration of 8 days, until severe symptoms were observed only in control plants subjected to pathogen application.
Evaluation of Results
Until disease symptoms appeared, the plants were examined daily for one week. The evaluation of diseased plants was conducted on a 0–4 scale based on the plant’s symptom condition.
0–4 scale
-
0: No disease symptoms were observed
-
1: 25% disease symptoms were observed
-
2: 50% disease symptoms were observed
-
3: 75% disease symptoms were observed
-
4: 100% disease symptoms were observed (The plant displayed severe symptoms and was entirely affeced)
The obtained values were converted to the percentage of disease severity using the disease severity (DS) formula (Viriyasuthee et al. 2019). An average of 25 leaves per plant was assessed.
- DS:
-
: Disease severity
- S:
-
: Scale value
- L:
-
: Number of plant leaves evaluated on the scale
- M:
-
: Total number of plant leaves
- Smax:
-
: Represents the highest scale value
Formulation of Conidia in Different Carriers
The conidia of T. harzianum ET 4 and ET 14 were mixed with the carrier soybean oil, neem oil, canola oil, paraffin oil, and glycerin. All tubes of 15 ml each were prepared for each carrier, and each tube was labeled and filled with 10 ml of the respective carrier. All tubes were autoclaved at 121 °C for 20 min to sterilize them. On the other hand, conidia of ET 4 and ET 14 isolates, cultivated for 10 days on 9 cm diameter Petri dishes containing PDA medium at 27 °C, were collected using a spatula. 1 × 106 conidia/ml of T. harzianum isolates were then individually placed in the tubes with carriers, and to ensure homogeneous distribution within the carrier, they were homogenized using a vortex. Of these prepared tubes, 300 tubes (150 ET 4 and 150 ET 14) were stored at room temperature (22 °C), and another 300 tubes (150 ET 4 and 150 ET 14) were stored at +4 °C. Every month, 60 tubes (30 ET 4 and 30 ET 14) were taken from their storage location, and viability tests were conducted. This study was conducted in three replicates. For this purpose, the conidia stored in the tubes were transferred to media containing PDA, and their growth was monitored in Petri dishes kept in a sterile environment for six days. In the shelf life study conducted using five different carriers, the growth of bioagents was monitored by transferring conidia, obtained from the suspension of bioagent were transfered to PDA using a sterile needle at 30-day intervals from the first day, to PDA for ten months.
Post-storage In vivo Assay
After being stored for an extended period in five different media, the effectiveness of T. harzianum ET 4 and ET 14 isolates was re-evaluated on tomato seedlings at the end of the 10th month. Using a micropipette, 30 µl of bioagent suspensions containing 1 × 106 conidia/ml were applied to wounds made on the stems of tomato seedlings, located 10 cm above the soil surface, with 30 µl applied to each wound. A mycelial disk with a diameter of 6 mm, obtained from the A. solani ET 66 isolate, was placed on top of the wound and sealed with parafilm. Sterile water was used as the negative control, and only the ET 66 isolate was used as the positive control. The study was repeated three times and conducted in growth chambers set at 24 °C with a 12-hour light and 12-hour dark cycle.
Results Analysis
Analysis of variance was applied to the values regarding the obtained in vitro test results, and the differences between the averages were compared with the LSMeans Differences Student’s test at a significance level of P < 0.01. Data analysis was made via the utilization of JMP IN (SAS Institute, cary, NC,%.0 PC version) statistics software.
Results
At the beginning of the study, the efficacy of the original stock cultures of ET 4 and ET 14 isolates as bioagents against A. solani’s ET 66 isolate was tested under in vitro conditions, and the radial growth of the pathogenic fungus was measured to calculate the percentage inhibition rates according to Skidmore and Dickinson (1976) (Table 2, Fig. 2).
In vitro conditions, the percentage inhibition rates of ET 4 and ET 14 isolates were determined, and their effectiveness on tomato seedlings was also evaluated in pot trials. Disease severity was calculated according to Viriyasuthee et al. (2019) (Table 3).
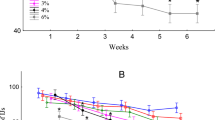
The results of the viability tests of the biocontrol fungus obtained from samples taken every month for ten months from the carriers stored at room temperature and +4 °C are provided in Table 4.
T. harzianum isolates stored for 10 months were subjected to pairwise tests against A. solani (ET 66), and the effectiveness results on the pathogen under in vitro conditions are presented in Table 5.
In the conducted study, the percentage inhibition rate of T. harzianum ET 4 isolate stored in different liquid carriers ranged between 37.85% and 52.33% at the end of the 10th month. The highest percentage inhibition rate was obtained from neem oil (52.33%), followed by paraffin (49.19%), canola (48.01%), soybean oils (47.26%), and glycerin (37.85%), respectively (Table 5). The percentage inhibition rate of T. harzianum ET 14 isolate at the end of the 10th month ranged between 44.92% and 51.56%. The highest percentage inhibition rate was achieved with canola oil (51.56%), followed by neem oil (49.60%), glycerin (46.48%), paraffin (45.30%), and soybean oils (44.92%) (Table 5). It was observed that ET 4 and ET 14 isolates showed similar results in terms of their percentage inhibition rates (Table 5).
The ET 4 and ET 14 isolates, which were stored for an extended period in carrier media, and their continued effectiveness under in vitro conditions were determined. Their effectiveness on tomato seedlings was also evaluated in pot trials, and disease severity was calculated according to Viriyasuthee et al. (2019) (Table 6).
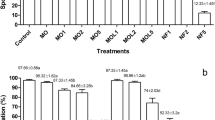
The study revealed that the T. harzianum ET 4 and ET 14 isolates had varying effects on the disease severity of A. solani ET 66 in tomato plants, either reducing or increasing it to some extent (Table 6, Fig. 3). Furthermore, it was noted that isolates stored in liquid carriers reduced disease severity when compared to isolates stored in the long-term storage medium of PDA. The best results were obtained by ET 4 stored in glycerin at +4 °C and ET 14 stored in neem oil at room temperature (Table 6). For ET 4 isolate, glycerin at +4 °C followed by neem, paraffin at +4 °C, and soybean oil at room temperature and +4 °C, for ET 14 isolate, neem oil at room temperature and +4 °C, glycerin, canola oil and soybean oil at room temperature provided the best results (Table 6).
Discussion
T. harzianum is one of the most commonly used species for the management of plant diseases (Meher et al. 2020; Rush et al. 2021). It has been employed in the production of more than twenty commercial biocontrol agents worldwide (Woo et al. 2014). It has been employed in the production of more than twenty commercial biocontrol agents worldwide (Woo et al. 2014). These commercial formulations are available in both solid and liquid forms. Studies have evaluated the different aspects of these two formulations. Indeed, in a study conducted by Hiralkar (2012), it was mentioned that the solid-based formulation of Trichoderma species, despite its significance in environmentally friendly disease management due to its ability to enhance root growth and secrete enzymes like viridin and gleotoksin, is not practical for commercial production due to its shorter shelf life. On the other hand, it was noted that the liquid Trichoderma formulation has a better shelf life, maintains its properties through sub-culturing, is not affected by high temperatures, and can be characterized by its typical fermented odor. Additionally, it was stated that the liquid formulation can better survive when applied to both seeds and soil.
In a study conducted by Jayaraj et al. (2006), they evaluated the shelf life of various formulations of T. harzianum M1 isolate, including talc, lignite, lignite + fly ash-based powder formulation, wettable powder, bentonite paste, polyethylene glycol paste, and gelatin-glycerol-gel. They assessed the shelf life over nine months at 24 °C and found that the propagule population was optimum in all formulations up to three months of storage. When these Trichoderma formulations were applied to seeds, the impact of the pathogen on tomatoes was reduced by up to 74%. Additionally, they observed an increase in plant biomass under greenhouse and field conditions. In another study, it was found that microencapsulation with sugars such as sucrose, molasses, and glycerol significantly increased the survival percentage of Trichoderma conidia, with the highest survival percentage achieved with a 2% sucrose solution for microencapsulation (Jin and Custis 2011). Furthermore, the addition of glycerol as an osmotic agent to the production medium was investigated, and it was observed that the addition of 3% or 9% glycerol reduced the water activity in the medium (Sriram et al. 2011). Both in shake flask culture and fermentor production, the addition of 3% and 6% glycerol to the production medium extended the shelf life of the talc formulation of Trichoderma. Compared to formulations obtained without glycerol, those with added glycerol exhibited shelf lives extended from 4–5 months to 7–12 months. In bioefficacy tests, it was determined that even after 12 months of storage, formulations with added glycerol could still protect tomato plants from Fusarium wilt by 44–50% (Sriram et al. 2011). In this study, the disease severity in plants treated with the ET 66 was 66%, while in plants treated with the biocontrol isolates, it ranged between 13.91–14.26% (ET 4) and 8.36–8.35% (ET 14). For the biocontrol agents stored in glycerol medium for 10 months, the disease severity varied between 3.34–7.23% (ET 4) and 3.96–10.76% (ET 14). The study suggested that the addition of osmotic agents to the production medium could be employed to extend the shelf life of fungal formulations produced through liquid fermentation (Sriram et al. 2011).
Celaya et al. (2012) noted that microencapsulation of biological control agents in biopolymer matrices through spray drying could provide an alternative method to produce formulations with longer shelf lives. In their study, they used maltodextrin DE10 (MD10), maltodextrin DE20 (MD20), gum arabic (GA), and a 1:1 mixture of MD10-GA as the matrix materials. They also evaluated the activation energy (Ea) for the viability of T. harzianum conidia after spray drying. They achieved the highest conidial viability of 86% immediately after spray drying and 40% after 8 weeks of storage at 4 °C. In another study that examined the effects of thermal stress and dehydration levels (water activity, aw = 0.1–0.7) on the survival of T. harzianum conidia during drying and storage, it was found that conidia produced in liquid culture were highly sensitive to thermal stress but did not experience significant mortality due to dehydration (Sandoval et al. 2012).
Kumar et al. (2014) determined that oil-based formulations of Trichoderma were suitable for foliar sprays under dry conditions and had a long shelf life. They found that when the spores were coated with oil that protected against desiccation at 5 °C, they could survive on plant surfaces for an extended period even in dry conditions. The researchers also developed an emulsion formulation of T. harzianum for controlling post-harvest rot caused by Botrytis cinerea in apples. In another study comparing the effectiveness of three different formulations, solid, semi-solid, and liquid formulations of T. harzianum were tested for the control of Tilletia laevi. The solid formulation reduced infection by 39.07%, showing better efficacy than the semi-solid formulation. However, the liquid formulation yielded almost the same results as the solid formulation in terms of yield and yield components, and it outperformed the semi-solid formulation (Booshehri 2014). In this study, biocontrol agents were preserved in 5 different liquid media, and in vivo tests conducted after 10 months revealed that the antifungal effect of the biocontrol agents had not diminished.
In a study conducted on T. harzianum using liquid culture fermentation, it was found that a higher carbon concentration in the liquid medium supported the formation of microsclerotia (MS) at all tested C:N ratios. Additionally, it was determined that MS granules dried in the air (< 4% moisture content) maintained an excellent shelf life without any loss in conidial production under cold and non-cooled storage conditions (Kobori et al. 2015).
In a study conducted by Batta (2005), various oils (soybean, coconut, corn) were prepared separately as 50%-50% water and oil phases using glycerol, distilled sterile water, and/or water-soluble wax. These nine different formulations were then investigated for their effectiveness against B. cinerea in apples by adding the water phase onto the oil phase. The formulation that used water-soluble wax was found to have a shelf life of up to 36 months. It was also reported that the same formulation was effective against soil-borne diseases in peanuts (Kumar et al. 2014).
T. harzianum liquid formulations were found to have a good shelf life when stored at room temperature for 540 days against Botryotinia ricini and Alternariaster helianthi, with live spore counts ranging from 8.0 to 7.4 log CFU (Navaneetha et al. 2015). In another study that used various oils and their combinations such as canola oil, paraffin oil, soybean oil, neem oil, and glycerol, it was determined that glycerol-based formulations with live conidia counts of +3.00 × 10^4 CFU/ml and 2.00 × 10^4 CFU/ml provided the best shelf life results during twelve months of storage when compared to paraffin oil-based formulations (Ahamedemujtaba and Kulkarni 2017). However, in this study, the best results were obtained from formulations based on glycerol and neem oil.
In this study, attempts were made to identify liquid environments in which T. harzianum, crucial for biological control, could be preserved for an extended period without losing its effectiveness. In the initial phase, isolates ET 4 and ET 14, previously found effective against different pathogens, were retested for their effectiveness on A. solani. Subsequently, these isolates were transferred to five different liquid carriers and preserved for an extended period. Their development was then monitored by transferring them to PDA under in vitro conditions. At the end of ten months of in vitro testing, effectiveness tests were conducted on A. solani under in vitro and in vivo conditions to determine whether their effectiveness was maintained. The percentage inhibition rate of T. harzianum ET 4 isolate, preserved in different liquid carriers, ranged from 37.85% to 52.33% after ten months, while the ET 14 isolate exhibited a percentage inhibition rate ranging from 44.92% to 51.56%. The results showed that the percentage inhibition rates of ET 4 and ET 14 isolates were similar to each other. These findings are consistent with the studies conducted by Batta (2005), Sriram et al. (2011), and Ahamedemujtaba and Kulkarni (2017).
In this study, it was observed that bioagents stored in liquid carriers reduce disease severity more than those stored in the flat agar containing PDA, which serves as a long-term storage medium. This suggests that bioagents in liquid environments may respond more rapidly to environmental changes, allowing conidia to develop antagonist properties more quickly and effectively when exposed to stress. Further investigation into this potential relationship could provide valuable insights into the mechanisms behind the observed differences in disease severity and contribute to the development of more effective biocontrol strategies.
Conclusion
In conclusion, it has been demonstrated that T. harzianum, used as a biocontrol agent to manage plant diseases, can be successfully stored for 10 months in liquid carriers such as neem oil, glycerol, and paraffin oil. In light of the obtained data, future studies in this direction should explore the potential use of these three liquid carriers for the preservation of other fungal bioagents. This research opens up possibilities for further investigations into the preservation of biocontrol agents, which is crucial for the sustainable management of plant diseases.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not available
References
Ahamedemujtaba V, Kulkarni S (2017) Shelf life of Trichoderma harzianum an antagonist in different oil based formulations. IRA Int J Appl Sci 6(2):34–40. https://doi.org/10.21013/jas.v6.n2.p2
Akrami M, Yousefi Z (2015) Biological control of Fusarium wilt of tomato (Solanum lycopersicum) by Trichordema spp. as antagonist fungi. Biol Forum 7(1):887
Aydın MH (2015) Trichoderma in Biological Warfare Against Plant Fungal Diseases. Turk J Agric Res 2(2):135–148
Batta YA (2005) Postharvest biological control of apple gray mold by Trichoderma harzianum formulated in an invert emulsion. Crop Prot 23(1):19–26
Bektas Y (2022) Promising plant defense elicitor, controls early blight (Alternaria solani) disease in the tomato by inducing host resistance associated gene expression. Horticulturae 8(6):484. https://doi.org/10.3390/horticulturae8060484
Booshehri SMS (2014) Effects of physical state of formulations on the potential of Trichoderma harzianum 199 against wheat common bunt. J Crop Prot 3:615–624
Çakar G, Tozlu E (2022) The biological control of Fusarium oxysporum the causal agent of potato rot. Gesunde Pflanz 74(2):305–315. https://doi.org/10.1007/s10343-021-00610-1
Çamlıca E, Tozlu E (2019) Biological control of Alternaria solani in tomato. Fresenius Environ Bull 28(10):7092–7100. https://doi.org/10.3390/ijms20081950
Celaya ALM, Garciaa MO, Carter EJV, Rinconb JJ, Galindoa E, Carreona LS (2012) Spray-drying microencapsulation of Trichoderma harzianum conidias in carbohydrate polymers matrices. Carbohydr Polym 88:1141–1148
El-Debaiky SA (2017) Antagonistic studies and hyphal interactions of the new antagonist Aspergillus piperis against some phytopathogenic fungi in vitro in comparison with Trichoderma harzianum. Microb Pathog 113:135–143. https://doi.org/10.1016/j.micpath.2017.10.041
Elad Y, Kapat A (1999) The role of Trichoderma harzianum protease in the biocontrol of Botrytis cinerea. Eur J Plant Pathol 105:177–189
Erazo JG, Palaccios SA, Pastor N, Giordano FD, Rovera M, Reynoso MM, Venisse JS, Torres AM (2021) Biocontrol mechanisms of Trichoderma harzianum ITEM 3636 against peanut brown root rot caused by Fusarium solani RC 386. Biol Control 164:104774. https://doi.org/10.1016/j.biocontrol.2021.104774
Güçlü T, Özer N (2022) Trichoderma harzianum antagonistic activity and competition for seed colonization against seedborne pathogenic fungi of sunflower. Lett Appl Microbiol 74:1027–1035. https://doi.org/10.1111/lam.13698
Harman GE (2006) Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96(2):190–194
Harman GE, Howel CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Hewedy OA, Abdel Lateif KS, Seleiman MF, Shami A, Albarakaty FM, El-Meihy RM (2020) Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology 9:189. https://doi.org/10.3390/biology9080189
Hiralkar PM (2012) Studies on production technology of liquid formulation of Trichoderma. Department of Plant Pathology and Agricultural Microbiology, Post Graduate Institute, Rahuri-Maharashtra State, p 59 (Master Thesis)
Jayaraj J, Radhakrishnan NV, Velazhahanet R (2006) Development of formulations of Trichoderma harzianum strain M1 for control of damping-off of tomato caused by Pythium aphanidermatum. Arch Phytopathol Plant Prot 39(1):1–8. https://doi.org/10.1080/03235400500094720
Jin X, Custis D (2011) Microencapsulating aerial conidia of Trichoderma harzianum through spray drying at elevated temperatures. Biol Control 56:202–208
Kobori NN, Mascarin GM, Jackson MA, Schisler DA (2015) Liquid culture production of microsclerotia and submerged conidia by Trichoderma harzianum active against damping-off disease caused by Rhizoctonia solani. Fungal Biol 119(4):179–190. https://doi.org/10.1016/j.funbio.2014.12.005
Kredics L, Antal Z, Manczinger L, Szekeres A, Kevei F, Nagy E (2003) Trichderma strains with biocontrol potential. Food Technol Biotechnol 41(1):37–42
Kumar S, Thakur M, Rani A (2014) Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr J Agric Res 9(53):3838–3852
Meher J, Rajput RS, Bajpai R, Teli B, Sarma BK (2020) Trichoderma: A globally dominant commercial biofungicide. In: Manoharachary C, Singh HB, Varma A (eds) Trichoderma: Agricultural applications and beyond. Springer, New York Cham, pp 195–208
Mironenka J, Różalska S, Soboń A, Bernat P (2021) Trichoderma harzianum metabolites disturb Fusarium culmorum metabolism: metabolomic and proteomic studies. Microbiol Res 249:126770. https://doi.org/10.1016/j.micres.2021.126770
Mukherjee PK, Mendoza-Mendoza A, Zeilinger S, Horwitz BA (2022) Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol Rev 39:15–33. https://doi.org/10.1016/j.fbr.2021.11.004
Navaneetha T, Prasad RD, Venkateswar RL (2015) Liquid formulation of Trichoderma species for management of gray mold in castor (Ricinus communis L.) and alternariaster leaf blight in sunflower (Helianthus annuus L.). J Biofertilizers Biopestic 6:1. https://doi.org/10.4172/2155-6202.1000149
Papavizas GC (1985) Trichoderma and Glocladium: biology, ecology, and potential for biocontrol. Annu Rev Phytopathol 23:23–54
Ramanujam B, Prasad RD, Sriram S, Rangeswaran R (2010) Mass production, formulation, quality, and delivery of Trichoderma for plant disease management. J Plant Prot Sci 2(2):1–8
Rush TA, Shrestha HK, Gopalakrishnan Meena M, Spangler MK, Ellis JC, Labbé JL, Abraham PE (2021) Bioprospecting Trichoderma: a systematic roadmap to screen genomes and natural products for biocontrol applications. Front Fungal Biol 2:716511. https://doi.org/10.3389/ffunb.2021.716511
Samuelian S (2016) Potential of Trichoderma harzianum for control of banana leaf fungal pathogens when applied with a food source and an organic adjuvant. 3 Biotech 6:8. https://doi.org/10.1007/s13205-015-0327-0
Sandoval MTF, García MO, Galindo E, Carreón LS (2012) Cellular damage during drying and storage of Trichoderma harzianum spores. Process Biochem 47(2):186–194
Singh AS, Panja B, Shah J (2014) Evaluation of suitable organic substrates based Trichoderma harzianum formulation for managing Rhizoctonia solani causing collar rot disease of cowpea. Int J Curr App Sci 3(8):127–134
Sivan A (1989) The possible role of competition between Trichoderma harzianum and Fusarium oxysporum on rhizosphere colonization. Phytopathology 79:198–203. https://doi.org/10.1094/Phyto-79-198
Skidmore AM, Dickinson CM (1976) Colony interactions and hyphal interference between Septoria nodorum and Phylloplane fungi. Trans Br Mycol Soc 66:57–64
Sriram S, Roopa KP, Savitha MJ (2011) Extended shelf-life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol in the production medium. Crop Prot 30:1334–1339
Tekiner N, Tozlu E, Kotan R, Dadaşoğlu F (2019a) Determination of some biological control agents against Alternaria fruit rot in quince. Alinteri J Agr Sci 34(1):25–31
Tekiner N, Tozlu E, Kotan R (2019b) Investigation of biological control possibility of anthracnose disease agent, Colletotrichum gloeosporioides, on orange. Res Agric Sci 50(3):282–291
Tekiner N, Kotan R, Tozlu E, Dadaşoğlu F (2020) Biological control of Botrytis cinerea and Alternaria alternata with bioagent bacteria and fungi under in vitro conditions. Fresenius Environ Bull 29(1):640–649
Tozlu E, Tekiner N, Kotan R (2017) Screening of Trichoderma harzianum Rifai (1969) isolates of domestic plant origin against different fungal plant pathogens for use as biopesticide. International Conference on Advences in Natural and Applied Science (ICANAS), Antalya, 18–21 April, p 187
Tozlu E, Tekiner N, Kotan R (2018a) Screening of Trichoderma harzianum Rifai (1969) isolates of domestic plant origin against different fungal plant pathogens for use as biopesticide. Fressenius Environ Bull 27(6):4232–4238
Tozlu E, Tekiner N, Kotan R, Örtücü S (2018b) Investigation on the biological control of Alternaria alternata. Indian J Agric Sci 88(8):93–99
Verma M, Brar SK, Tyagi RD, Surampalli RY, Valéro JR (2007) Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng J 37:1–20. https://doi.org/10.1016/j.bej.2007.05.012
Vinale F, Marra R, Scala F, Ghisalberti EL, Lorita M, Sivasithamparam K (2006) Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett Appl Microbiol 43:143–148
Viriyasuthee V, Saepaisan S, Saksirirat W, Gleason ML, Chen RS, Jogloy S (2019) Effective plant ages for screening for field resistance to Alternaria leaf spot (caused by Alternaria spp.) under natural infection in jerusalem artichoke (Helianthus tuberosus L.). Agronomy 9:754. https://doi.org/10.3390/agronomy9110754
Woo SL, Ruocco M, Vinale F, Nigro M, Marra R, Lombardi N, Pascale A, Lanzuise S, Manganiello G, Lorito M (2014) Trichoderma-based products and their widespread use in agriculture. Open Mycol J 8:71–126. https://doi.org/10.2174/1874437001408010071
Woo SL, Hermosa R, Lorito M, Monte E (2022) Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat Rev Microbiol. https://doi.org/10.1038/s41579-022-00819-5
Zhang F, Ge H, Zhang F, Guo N, Wang Y, Chen L, Xiue J, Chengwei L (2016) Biocontrol potential of Trichoderma harzianum isolate T‑aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol Biochem 100:64–74. https://doi.org/10.1016/j.plaphy.2015.12.017
Acknowledgements
This study was supported by Department of Scientific Research Project Management of Atatürk University (BAP), with the project number FYL-2021-9694. We are thank to the Department of Scientific Research Project Management of Atatürk University (BAP).
Funding
This research was supported by funds of Department of Scientific Research Project Management of Atatürk University (BAP) with the project number FYL-2021-9694.
Author information
Authors and Affiliations
Contributions
ET conceived and designed research. YEK conducted experiments. YEK studied fungal experiments. ET and YEK studied controlled assays. ET analyzed data. ET and YEK wrote manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Y.E. Kara and E. Tozlu declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study is part of Yasemin Esra KARA’s master thesis accepted by Atatürk University Graduate School of Natural and Applied Sciences.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kara, Y.E., Tozlu, E. Effect of Different Liquid Carriers On the Shelf-life and Antifungal Effectiveness of Trichoderma harzianum. Journal of Crop Health 76, 425–435 (2024). https://doi.org/10.1007/s10343-024-00971-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-024-00971-3