Abstract
Tonkin wingnut is the rarest species within the relict tree genus Pterocarya (Juglandaceae), known from only a few isolated stands in the Indo-Burman hotspot. Despite its classification as a vulnerable species, Pterocarya tonkinensis has received comparatively less attention than other wingnut species, leading to a limited understanding of its distribution patterns. In this study, we utilize spatial distribution modeling with MAXENT software and conservation prioritization methods implemented in ZONATION to estimate the potential range of species, identify the key environmental variables influencing its habitat, and designate potential areas for conservation. We used a set of 45 known species populations, the set of bioclimatic variables, and the proximity of watercourses to create the model of the potential range. The results indicate two main centers of potential climatically suitable areas for the species in the future - in southern Yunnan (China) and Vietnam. The calculated total suitable area (292,365.67 km2) is similar to the estimated extent of species occurrence but may decline in the future. Highly suitable areas near the rivers cover around 19,000 km2. The most important factors shaping species occurrence were those related to temperature amplitude (around 60% of contribution to the models). The seasonality of precipitation and distance from watercourses also have a significant impact. Assessment of potential reserves has identified the need for protected areas in southern China and points to the possibility of expanding reserves in Vietnam. The fact that river valleys are often densely populated can be an obstacle to the conservation of species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The area of Southeast Asia, including the southern part of China, represents one of the centers of biodiversity in the world. The Indo-Burman biodiversity hotspot is home to approximately 7,000 endemic vascular plants, including many Cenozoic relics (Myers et al. 2000; van Dijk 2004). At the same time, this region is densely populated, especially in the river valleys, where there are many farmlands; therefore, natural, undisturbed areas are sparse and constitute only approximately 5% of its previous extent (Myers et al. 2000). For this reason, areas on sparsely populated peripheries, often in difficult terrain far from major urban centers, are gaining significant ecological importance. The transboundary region between China, Myanmar, Laos, and Vietnam, which is part of the hotspot, constitutes a zone of high priority for environmental protection (Tordoff 2012; Wang et al. 2021). Additionally, it is exceptionally rich in terms of not only plant species but also vertebrate taxa (Wang et al. 2021). Unfortunately, the remarkable biological richness of this region is endangered by habitat destruction, strong human pressure, and climate change (Xu and Wilkes 2004; Tordoff 2012; Hughes 2017). Considering the scale and rapidity of these changes, determining the impact of disturbances in environmental conditions on the distribution of rare species is urgent. This is largely the case for rare and relict species, which make an important contribution to biodiversity.
One of the endangered species from the Indo-Burman hotspot is Pterocarya tonkinensis (Franch.) Dode (Tonkin wingnut, also known as Cơi in Vietnam), a member of the walnut family (Juglandaceae). It is the southernmost-distributed species of the genus Pterocarya, and the area in which it occurs constitutes the northern edge of the tropical region of Southeast Asia (Zhu 2017). Other species from the genus occur in more northern areas of East Asia (five species), with one representative (Pterocarya fraxinifolia (Lam.) Spach) in the western part of this continent (Song et al. 2020). Known populations of Tonkin wingnut occur on riverbanks, mainly in lowlands between 100 and 700 m a.s.l. in Vietnam, Laos, and China (Yunnan Province), often as monodominant communities (Kozlowski et al. 2019; Van Sam et al. 2021). This species prefers sandy and moist soils. It can survive floods, similarly to the close relative Pterocarya stenoptera D. DC., which occurs mainly in China (Zhang et al. 2020). Tonkin wingnut is the rarest species in the genus Pterocarya, with only approximately 3000 mature individuals and an estimated area of occupancy of only approximately 80 km2 (Kozlowski et al. 2019). Since the species is often found in inaccessible regions, undiscovered populations may exist. The number of seedlings is usually low (Kozlowski et al. 2019); however, natural regeneration in some stands is described as good (Van Sam et al. 2021). Tonkin wingnut is currently endangered due to habitat destruction and fragmentation, especially in riparian areas (Kozlowski 2018). It is listed on the IUCN Red List under the category Vulnerable (VU) (Kozlowski 2019). Since the species is strongly associated with watercourses, any changes related to water management (dam construction, agricultural development) as well as climate impacts on rivers (water level changes, flooding episodes) could threaten natural sites (Kozlowski 2019). In addition, the species only rarely occurs in botanical collections and gardens. However, some natural populations are located in already protected areas, such as Cuc Phuong National Park and Pu Mat National Park in Vietnam (Kozlowski et al. 2019). The species may be important in pharmacology, as it exhibits antifungal properties (Thi Ngo et al. 2021). It is also traditionally used as a poison for fishing and as an insect repellent (Van Sam et al. 2021); it may have potential medical applications (Liu et al. 2006).
One of the tools used for the description of species’ potential distribution is spatial distribution modeling (SDM). This approach uses the location of the species and a set of environmental variables to estimate the potential range under different climate scenarios and determine which factors shape the species’ range (Guisan and Zimmermann 2000). Various SDM methods are invaluable in increasing knowledge of species’ occurrence patterns and in planning conservation strategies (Franklin 2023). One of the most popular programs used for this purpose is MAXENT (Elith et al. 2011; Philips et al. 2006), which uses the maximum entropy algorithm (Philips et al. 2004) and works with presence-only data. Its advantages include the robust performance of models calculated on the basis of a small number of stands and rather high insensitivity to the approximation of the location of some populations (Baldwin 2009). For this reason, it is often used in studies of rare, endemic species with a small number of stands (for example Williams et al. 2009; Rhoden et al. 2017; Ye et al. 2021). Additionally, it is possible to predict whether a species is at high risk of range fragmentation and which areas will provide potential corridors between populations (Nguyen 2021; Gao et al. 2022; Sękiewicz et al. 2022) using software such as GuildosToolbox (Vogt and Riitters 2017). Estimation of the species’ potential range, coupled with knowledge of additional factors (such as human impact, competition with other species, or the presence of barriers), makes it possible to select the best areas that can serve as refugia for endangered species and to choose the areas that should be protected with the highest priority.
Determining the impact of environmental factors is a key challenge to address in the face of rapid climate change and increasing human pressure associated with industrial and agricultural development. Despite the fact that the Tonkin wingnut is rare and occurs in a biodiversity hotspot, it attracts less attention than other members of the genus. Conservation of this species is important due to its rarity, importance to local biodiversity and potential medical use. To date, the potential range and influence of environmental variables on its dynamics have not been evaluated for P. tonkinensis, as has been done for other species in the genus, namely, P. fraxinifolia (Song et al. 2021) and stenoptera (Qian et al. 2019; Zhang et al. 2020). Forecasting the range dynamics of the species is extremely important, as its populations are sparse and ex situ cultivation is scarce (Kozlowski et al. 2019). The main objective of this study was to fill knowledge gaps and achieve the following aims: (1) estimate the potential range of the species under current climatic conditions and future climate scenarios to recognize areas with an appropriate climate; (2) estimate the fragmentation of the species’ potential range and connectivity between northern and southern stands; and (3) identify the areas of higher conservation priority, taking into account the potential range according to the climatic conditions as well as the influence of human pressure (land transformation).
Materials and methods
MAXENT v. 3.3.2 was applied to estimate the potential range of Pterocarya tonkinensis (Elith et al. 2011; Philips et al. 2006). This software is often used for rare species because it is robust even with a small number of locations (Baldwin 2009; Williams et al. 2009; Rhoden et al. 2017; Ye et al. 2021). Models were built using 45 localities of the species (Fig. 1) and a set of 19 bioclimatic variables from the CHELSA database with a resolution of 30 arc-sec (Karger et al. 2017, 2018). In addition, considering the ecological preferences of the species, a raster of the distance from the nearest river was used. This raster was calculated using the detailed map of the watercourses obtained from the HydroSHEDS database (Lehner et al. 2006). The used species’ locations cover the entire range of the species and all currently known populations, thus avoiding bias (Merrow et al. 2013).
According to the recommendation of Merrow et al. (2013), we removed overly correlated variables before the modeling procedure. The correlations between variables were evaluated in the ENMTOOLS package in R with using “raster.cor.matrix” function (Warren 2021; Table S1). Finally, 11 uncorrelated variables were used for the estimations of the potential range of the species (Table S1). Analyses were performed for the current conditions as well as for three future projections for the years 2071–2100, including three shared socioeconomic pathways (SSPs): SSP1.26 (CO2 emissions cut to net zero by approximately 2075), SSP3.70 (CO2 emissions doubled by 2100) and SSP5.85 (CO2 emissions tripled by 2075; Meinshausen et al. 2020, IPCC 2021). The model MPI-ESM-P (Max Planck Institute for Meteorology) was used (Giorgetta et al. 2013); for each future scenario, the current climatic conditions were used as environmental layers. Each SDM analysis was performed as a bootstrap procedure with 100 replicates; for each model run, 20% of the data were set aside as test points with the ‘random seed’ option. The convergence threshold was 0.00001, the maximum number of iterations was set to 10,000, and the output was set to logistic. Four feature classes were used: linear, quadratic, product and hinge. Clamping and MESS projection were used for the projecting; regularization multiplier was set to 1.0 and default prevalence to 0.5. The value of the area under the curve (AUC) was used for model evaluation (Wang et al. 2007; Mas et al. 2013).
To estimate the fragmentation of the species’ potential range, we performed an Entropy Map analysis in GuidosToolbox software (Vogt and Riitters 2017) for the part of the potential range with suitability above 0.25. In this method, the spatial entropy is calculated while assuming connectivity of the foreground pixels. The result is a map with the study area divided into cores and edges. In the case of the potential range of a species, the map allows us to determine which populations are located on the borders, which are in the center, and which are in detached smaller cores. In the same software, we estimated the structure of the species’ potential range with the MSPA (morphological spatial pattern analysis) method (Soille and Vogt 2009), which allows division of the potential range into several spatial classes: cores, edges, perforations, loops (connections of the same core areas), bridges (connections of different core areas) and branches (connections to one end of a bridge, a loop, an edge or a perforation), facilitating the identification of the links between different parts of the potential range.
ZONATION 5.0 (Moilanen et al. 2022) was used to choose the best areas that could be protected to save the species. This software uses a set of features (such as habitat type or suitability), as well as potential threats, to estimate a map of areas that should be protected with the highest priority. Analysis was performed with the MAXENT outputs as features with a weight of 1.0 for the current potential range and 0.5 for each future scenario. Vegetation type from the Copernicus database (Buchhorn et al. 2020) was used as a condition raster with a weight for forest of 1.0, shrublands of 0.75, grassland of 0.25, and other types (croplands, barren lands, cities) of 0.01; thus, forest areas had the highest priority in the model. The locations of reserves and national parks from the Protected Planet database (UNEP-WCMC and IUCN 2023) were used as a hierarchic mask to exclude already protected areas, whereas proximity to the closest known population of species was used as a retention raster to prioritize areas where natural stands exist. The results were visualized using QGIS 3.16.4 ‘Hanover’ (QGIS Development Team 2020).
Results
For all tested SDM models, the AUC was high (0.943 for the current scenario, 0.946 for SSP1.26, 0.942 for SSP3.70, and 0.941 for SSP5.85), indicating that the estimated models were robust. The most important variables in each tested model were temperature seasonality (bio 4), with a 33.4–39.7% contribution depending on the model (Table 1), temperature annual range (bio7, 20.1–26.9% contribution), precipitation seasonality (bio15, 10.6–11.4% contribution), and distance to the closest river (8.6–11.1% contribution). The optimal conditions according to the obtained model were an annual temperature amplitude between 18 and 22 °C, a seasonality of precipitation between 700 and 800 (coefficient of variation), and a distance from a watercourse not more than 2 km.
Under the current conditions, the most suitable areas cover the northern part of Vietnam and southern Yunnan, where most of the stands are located (Fig. 2A). The total suitable area is 292,356.74 km2, including 46,955.39 km2 with the highest suitability (> 0.75; Table 2). Considering only areas in close proximity to watercourses (less than 1 km), the potential suitable area includes 73,375.36 km2, including 20,457.83 km2 with high suitability and 19,044.33 km2 with very high suitability (Fig. S1). The SDM model based on the optimistic SSP1.26 scenario shows a potential range expansion of the species reaching approximately 4,000 km2 and a slight shift to the northeast. However, the difference between this model and the current potential range is rather small (Fig. 2B). The other two models of future climate (SSP3.70 and SSP5.85) both assume a significant reduction in the potential range of the species (loss of 34% and 56% of area, respectively) but predict different areas as future potential refugia. Under the SSP3.70 model, the most climatically favorable areas are predicted in the Vietnam-Laos border area and southern Yunnan (Fig. 2C). According to the SSP5.85 scenario, the area with the highest suitability is expected to be in the Hong River (Red River) basin in the northern part of Vietnam (Fig. 2D). Of all known populations of the species, only 26 (approx. 58%) may occur in areas that remain climatically suitable in the future.
The spatial structure of the potential range differs between the tested scenarios (Fig. 3, Figure S2). Taking into account the contemporary potential range, there is a good connection between range cores in Vietnam and southern Yunnan. Under the SSP1.26 scenario, suitability in the northern part of the range may even be higher than today. The next model, SSP3.70, predicts fragmentation and bursting of the potential range into two main areas - northern and southern - which will be quite poorly connected to each other along river valleys (Fig. 3C). Under the most pessimistic scenario SSP5.85, only the southern part of the range forms a strong core, while areas in the north become edges (Fig. 3D). Both major range cores and minor areas with favorable conditions are connected by a network of rivers that act as bridges and branches (Figure S2).
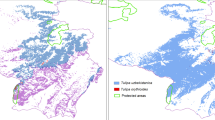
ZONATION software designated areas in Vietnam and southern Yunnan (China), which are similar to the refugia identified in the SSP3.70 model, suggesting these regions as suitable for creating potential reserves (Fig. 4). The most important is the zone located between the Hong (Red) River and Da (Black) River in Vietnam, where many stands of the species exist.
Discussion
Potential range of the species
China and Vietnam constitute the center of diversity for the family Juglandaceae (Kozlowski et al. 2018). The genus Pterocarya has a disjunct range, with one species in West Asia (P. fraxinifolia) and five species in East Asia. Of these species, P. tonkinensis has the southernmost range, occurring in tropical monsoon forests. According to the estimated model, suitable climatic conditions occur in most of northern Vietnam, in a small part of northeastern Laos, and in the southeastern part of the Chinese province of Yunnan. However, the MAXENT model has some limitations (Lissovsky and Dudov 2021), and the results should be interpreted with caution. Species occurrence depends not only on environmental factors but also on species interactions, which are difficult to estimate in the model. For species with a limited number of occurrences, a small number of locations can cause bias in the output map. However, the maximum entropy algorithm can produce a robust model even for a small number of observations (van Proosdij et al. 2016). The current potential range of the species is much larger than the known range. The estimated area of occupancy covers less than 100 km2 (Kozlowski et al. 2019), whereas the potential range with very high suitability is more than 46,000 km2 (including around 19,000 km2 in close proximity to the rivers). The discrepancy between these values may be due, among other things, to the fact that the MAXENT model does not take into account additional factors such as human pressure. The purpose of using this method was to identify areas with climates suitable for the species under study. However, the estimated total suitable area (292,365.67 km2) is similar to the estimated extent of species occurrence (262,358 km2, Kozlowski et al. 2019). Analysis in the ZONATION program, which also used data on vegetation type and incorporated the impact of human-transformed areas, significantly reduced the raw output from the MAXENT. Second, Pterocarya must compete with other species with similar habitat requirements, which may limit its occurrence, especially since natural regeneration is rather low (Kozlowski et al. 2019). Moreover, with the fragmentation of riparian habitats, it is difficult for the species to colonize new suitable habitats (Fink and Scheidegger 2018). Phenomena such as facilitation or complementarity between taxa are also possible, further complicating the species’ response to environmental conditions (Forrester 2014). Finally, there is the possibility that there are still some natural populations that have not yet been discovered because they are located in inaccessible areas of northern Vietnam and southeastern Yunnan, which are characterized by high suitability. The mountainous Sino-Vietnamese borderland is a humid and sparsely populated region that is very rich in biodiversity (Wang et al. 2021). The MAXENT results suggest that in the remote and poorly studied parts of this area, unknown species locations may exist. However, this border region is currently undergoing intensive land transformations (Turner and Pham 2015). Although the area of closed-canopy forests is increasing in some parts of this region, the acreage of the open-canopy forest is decreasing at the same time, and urbanization is increasing (Turner and Pham 2015). Thus, there is a risk that potential stands of the species may disappear before they are described.
The most important factors impacting the P. tonkinensis potential range were those related to temperature amplitude (bio4 - temperature seasonality and bio7 - temperature annual range). Pterocarya tonkinensis prefers moderate annual amplitudes of approximately 18–20 degrees Celsius, typical for the subtropics. Unfortunately, models of the future climate assume both an increase in the average annual temperature and a change in the temperature amplitude. Under the most severe (SSP5.85) scenario, the change in annual amplitude is approximately 4 degrees in the areas of occurrence of the studied species (Karger et al. 2018), which would negatively affect habitat suitability. Precipitation variables have lesser importance, even though the species is closely associated with moist environments. This may be connected to the fact that P. tonkinensis prefers to draw water from deeper soil layers (below 50 cm), which are a good source even during drier periods (Wang et al. 2019). The obtained results are clearly different than in the case of the closely related P. stenoptera, for which precipitation-related variables were the most important for the shape of the potential range (Zhang et al. 2020). In contrast, temperature factors are significant for adaptive genetic variation in both P. stenoptera (Li et al. 2022) and another closely related species found in southern China, P. macroptera (Wang et al. 2023). For the latter species, the most significant factors in shaping genetic variation are related to temperature amplitude: temperature seasonality (bio4), isothermality (bio3), and temperature annual range (bio7).
In the case of P. tonkinensis, two water-connected variables, the seasonality of precipitation and the distance from the nearest watercourse, also have a significant impact on the potential range. Southeastern Asia experiences periodic monsoons, which strongly modify the precipitation pattern during the year and have an impact on the local flora. The importance of distance from a watercourse is related to the fact that P. tonkinensis is a typical riparian species (Kozlowski et al. 2019). Periodic inundation does not pose a threat to these trees, similar to in P. stenoptera (Li et al. 2010; Zhang et al. 2020). According to the model, the highest suitability is in the immediate vicinity of rivers, and as the distance increases, habitat suitability decreases rapidly. At a distance of greater than 2 km to a watercourse, conditions become almost completely unfavorable. Although P. tonkinensis is predominantly an anemochoric species (Knörr et al. 2012), hydrochory may influence its distribution, as suggested by genetic analyses of other species from the genus Pterocarya (Yousefzadeh et al. 2018). Soil type was not tested in the model, as it is not a limiting factor for the species studied; P. tonkinensis occurs on clay-rich acrisols, which are clearly dominant in the humid, subtropical climate of Southeast Asia (Hengl et al. 2017).
The results obtained indicate that in the case of Tonkin wingnut, we will not observe an expansion of the potential range, as previously described for other thermophilic species of the genus, P. stenoptera and P. fraxinifola, which are likely to expand northward (Zhang et al. 2020; Song et al. 2021). Under the SSP1.26 scenario, the potential range of P. tonkinensis was almost the same as that under the current conditions; however, in the other two scenarios (SSP3.7 and SSP5.85), the loss of suitable habitat for the species was observed. Such a difference may be related to the different responses of individual species to environmental factors; for P. tonkinensis, the most important are the seasonality of precipitation and temperature as well as temperature amplitude; for P. fraxinifolia, annual mean temperature and precipitation of the wettest month (Song et al. 2021); and for P. stenoptera, precipitation of the driest month (Zhang et al. 2020). Additionally, in the case of the presented research, SSP models were used (Meinshausen et al. 2020; IPCC et al. 2021), while previous studies used older representative concentration pathway (RCP) models (Collins et al. 2013). It should also be remembered that the shape of the range is affected by other factors (such as the biological traits of species or human impact), as mentioned above; additionally, the knowledge of potential new sites for such a rare species may change the overall picture of the potential range. Despite these limitations, a certain trend can be identified, which clearly indicates that P. tonkinensis, unlike other representatives of the genus, will not be a beneficiary of future climate change. Therefore, it is advisable to strengthen conservation efforts, taking into account the potential fragmentation of the species’ range. There is a particular urgency to protect populations in the edge of the species’ range, which are the most threatened and, due to their isolation from the range core, may represent an important reservoir of genetic variability.
Range fragmentation and conservation remarks
The connectivity between the potential range cores in Vietnam and Yunnan is good both in the current climatic model and in the SSP1.26 scenario. The core of the range covers northern Vietnam and southeastern Yunnan, while sites in western Yunnan represent the edge. The riparian nature of the species is clearly evident in the MSPA, in which individual parts of the range are connected by watercourses acting as bridges and branches. However, under the SSP3.70 scenario, the potential range became significantly fragmented, whereas under the SSP5.85 scenario, the Yunnanese part of the range declined. Unfortunately, in this most pessimistic scenario, the potential range of the species shifts eastward to areas dominated by cities and farmland, which may significantly reduce the possibility of species conservation. In addition, cultivated areas may expand into hitherto wild areas, a common pattern in the lowlands that is associated with the withdrawal of woody species (Weinzettel 2018). A serious threat to riparian habitats is not only the increasing urbanization and expansion of agricultural fields but also the construction of dams, which can lead to the transformation of riverine ecosystems on a large section of the river (Turner and Pham 2015).
Populations far from the center of the potential range are in a worse situation compared to stands in the core of the range, as the areas where they occur will lose their suitability. Marginal populations may be more vulnerable because they are far from the other regions where species occurs, which limits migration; they may also be less well adapted to climate change, as in the case of P. macroptera (Wang et al. 2023). Stands of Tonkin wingnut located in the western part, near the border of China and Laos, in all analyses constitute the edge of the range, although they are a relatively large group. This region, known as Xishuangbanna, is very rich in tropical flora; P. tonkinensis forms communities along the riverbanks in this area (Zhu 2006). These stands are particularly threatened by climate change, although some of them are located in protected areas (UNEP-WCMC and IUCN 2023). However, most of the species’ populations throughout the range are located outside the reserves. The results of the ZONATION analysis, which combined climatic data and human impact, suggest that the highest conservation priority should be given to populations located in Vietnam close to existing protected areas (possibility of expansion of reserve borders) and sites in southern Yunnan that are far from current reserves (possibility of establishing new protected areas).
When planning effective conservation methods for endangered species, the existing genetic variability within the species should be taken into account. Its maintenance is crucial for adaptive potential and resilience to negative factors (Ostfeld and Keesing 2012; Frankham et al. 2014). In the case of a species as rare as P. tonkinensis, the loss of each site is a loss of invaluable variability. Unfortunately, to date, no analysis has been carried out to estimate gene resources in natural populations of this endangered taxa; existing work focuses on phylogenetic analyses (Song et al. 2020). Given the modeling results, as well as the rarity of the species under study, genetic analysis of the Tonkin wingnut is urgent for maintaining and preserving its resources. Currently, P. tonkinensis is found in only approximately 10 collections worldwide; therefore, more extensive ex situ cultivation should be planned (BGCI 2023, Kozlowski et al. 2019).
Conclusions
Predictions of the future potential range of P. tonkinensis show the possibility of populations declining due to environmental changes. More than half of known stands occur in areas that could lose suitability. This process could be further accelerated by human pressure in the region, which is already very strong. Populations from Yunnan are the most endangered, as they constitute a range edge and are located outside the protected areas. Some of the stands from the China–Vietnam border could be protected in situ; reserves from central Vietnam could also be enlarged to protect populations from the range core. However, further research is needed so that information on the species’ gene resources can be incorporated into conservation strategies.
References
Baldwin RA (2009) Use of maximum entropy modeling in wildlife research. Entropy 11:854–866. https://doi.org/10.3390/e11040854
BGCI (2023) Botanic Gardens Conservation International (BGCI) - Plant Search. Available at: https://www.bgci.org/plant_search.php. Access 06.10.2023
Buchhorn M, Smets B, Bertels L, De Roo B, Lesiv M, Tsendbazar N-E, Herold M, Fritz S (2020) Copernicus global land service: land cover 100m: collection 3: epoch 2019: Globe 2020
Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver AJ, Wehner MF, Allen MR, Andrews T, Beyerle U, Bitz CM, Bony S, Booth BBB (2013) Long-term climate change: projections, commitments and irreversibility. In: Climate Change 2013: The Physical Science Basis. IPCC Working Group I Contribution to AR5. Eds. IPCC, Cambridge: Cambridge University Press. 2013
Elith J, Phillips SJ, Hastie T, Dudik M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Divers Distrib 17:43–57. https://doi.org/10.1111/j.1472-4642.2010.00725.x
Fink S, Scheidegger C (2018) Effects of barriers on functional connectivity of riparian plant habitats under climate change. Ecol Eng 115:75–90. https://doi.org/10.1016/j.ecoleng.2018.02.010
Forrester DI (2014) The spatial and temporal dynamics of species interactions in mixed-species forests: from pattern to process. Ecol Manag 312:282–292. https://doi.org/10.1016/j.foreco.2013.10.003
Frankham R, Bradshaw CJ, Brook BW (2014) Genetics in conservation management: revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol Conserv 170:56–63. https://doi.org/10.1016/j.biocon.2013.12.036
Franklin J (2023) Species distribution modelling supports the study of past, present and future biogeographies. J Biogeogr 50:1533–1545. https://doi.org/10.1111/jbi.14617
Gao X, Liu J, Huang Z (2022) The impact of climate change on the distribution of rare and endangered tree Firmiana kwangsiensis using the Maxent modeling. Ecol Evol 12(8):pe9165. https://doi.org/10.1002/ece3.9165
GEBCO Compilation Group (2022) GEBCO_2022 Grid, https://doi.org/10.5285/e0f0bb80-ab44-2739-e053-6c86abc0289c
Giorgetta MA, Jungclaus J, Reick CH, Legutke S, Bader J et al (2013) Climate and carbon cycle changes from 1850 to 2100 in MPI-ESM simulations for the coupled model intercomparison project phase 5. J Adv Model Earth Syst 5:572–597. https://doi.org/10.1002/jame.20038
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Modell 135:147–186. https://doi.org/10.1016/S0304-3800(00)00354-9
Hengl T, Mendes de Jesus J, Heuvelink GB, Ruiperez Gonzalez M, Kilibarda M, Blagotić A, Shangguan W, Wright MN, Geng X, Bauer-Marschallinger B, Guevara MA (2017) SoilGrids250m: global gridded soil information based on machine learning. PLoS ONE 12(2):e0169748. https://doi.org/10.1371/journal.pone.0169748
Hughes AC (2017) Understanding the drivers of southeast Asian biodiversity loss. Ecosphere 8:e01624. https://doi.org/10.1002/ecs2.1624
IPCC (2021) In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JB, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Summary for policymakers. Climate change 2021: the physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge (UK)
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2017) Climatologies at high resolution for the earth’s land surface areas. Sci Data 4:1–20. https://doi.org/10.1038/sdata.2017.122
Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW, Zimmermann NE, Linder HP, Kessler M (2018) Data from: climatologies at high resolution for the earth’s land surface areas (Version 1, p. 7266827510 bytes). Dryad
Knörr UC, Kovar-Eder J, Mazouch P, Roth-Nebelsick A (2012) Fruit dispersal ecology of woody taxa in temperate to tropical forests of China and Japan. Palaios 27(8):523–540
Kozlowski G, Bétrisey S, Song YG (2018) Wingnuts (Pterocarya) and walnut family. Relict trees: linking past, present and future. Natural History Museum Fribourg, Fribourg, p 128
Kozlowski G, Song Y, Bétrisey S (2019) Pterocarya tonkinensis. IUCN Red List Threatened Species 2019:eT191414A1978789
Lehner B, Verdin K, Jarvis A (2006) : HydroSHEDS Technical Documentation. World Wildlife Fund US, Washington, DC. Available at http://hydrosheds.cr.usgs.gov
Li C, Wei H, Lü Q, Zhang Y (2010) Effects of water stresses on growth and contents of oxalate and tartarate in the roots of Chinese wingnut (Pterocarya stenoptera) seedlings. Scientia Silvae Sinicae 46:81–88
Li LF, Cushman SA, He YX, Ma XF, Ge XJ, Li JX, Qian ZH, Li Y (2022) Landscape genomics reveals genetic evidence of local adaptation in a widespread tree, the Chinese wingnut (Pterocarya stenoptera). J Syst Evol 60(2):386–397. https://doi.org/10.1111/jse.12699
Lissovsky AA, Dudov SV (2021) Species-distribution modeling: advantages and limitations of its application. 2. MaxEnt. Biol Bull Rev 11(3):265–275. https://doi.org/10.1134/S2079086421030087
Liu HB, Cai B, Cui CB, Gu QQ, Zhao QC, Guan H (2006) Pterocaryquinone, a novel naphthoquinone derivative from Pterocarya tonkinesis. Chin J Chem 24(12):1683–1686
Mas J-F, Soares Filho B, Pontius RG, Farfán Gutiérrez M, Rodrigues H (2013) A suite of tools for ROC analysis of spatial models. ISPRS Int J Geoinf 2:869–887. https://doi.org/10.3390/ijgi2030869
Meinshausen M, Nicholls ZR, Lewis J, Gidden MJ, Vogel E, Freund M, Beyerle U, Gessner C, Nauels A, Bauer N, Canadell JG (2020) The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci Model Dev 13(8):3571–3605
Merow C, Smith MJ, Silander JA Jr (2013) A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36(10):1058–1069. https://doi.org/10.1111/j.1600-0587.2013.07872.x
Moilanen A, Lehtinen P, Kohonen I, Virtanen E, Jalkanen J, Kujala H (2022) Novel methods for spatial prioritization with applications in conservation, land use planning and ecological impact avoidance. Methods Ecol Evol 13:1062–1072. https://doi.org/10.1111/2041-210X.13819
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772):853–858
Nguyen TT, Gliottone I, Pham MP (2021) Current and future predicting habitat suitability map of Cunninghamia Konishii Hayata using MaxEnt model under climate change in Northern Vietnam. Eur J Ecol 7(2):1–17. https://doi.org/10.17161/eurojecol.v7i2.15079
Ostfeld RS, Keesing F (2012) Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst 43:157–182
Phillips SJ, Dudík M, Schapire RE (2004) A maximum entropy approach to species distribution modeling. ACM Int Conf Proc Ser 69:655–662. https://doi.org/10.1145/1015330.1015412
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Modell 190:231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
QGIS Development Team (2020) QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://qgis.org/en/site/
Qian ZH, Li Y, Li MW, He YX, Li JX, Ye XF (2019) Molecular phylogeography analysis reveals population dynamics and genetic divergence of a widespread tree Pterocarya stenoptera in China. Front Genet 10:1089. https://doi.org/10.3389/fgene.2019.01089
Rhoden CM, Peterman WE, Taylor CA (2017) Maxent-directed field surveys identify new populations of narrowly endemic habitat specialists. PeerJ 5:e3632. https://doi.org/10.7717/peerj.3632
Sękiewicz K, Danelia I, Farzaliyev V, Gholizadeh H, Iszkuło G, Naqinezhad A, Ramezani E, Thomas PA, Tomaszewski D, Walas Ł, Dering M (2022) Past climatic refugia and landscape resistance explain spatial genetic structure in oriental beech in the South Caucasus. Ecol Evol 12(9):pe9320. https://doi.org/10.1002/ece3.9320
Soille P, Vogt P (2009) Morphological segmentation of binary patterns. Pattern Recognit Lett 30:456–459. https://doi.org/10.1016/j.patrec.2008.10.015
Song YG, Fragnière Y, Meng HH, Li Y, Bétrisey S, Corrales A, Manchester S, Deng M, Jasińska AK, Văn Sâm H, Kozlowski G (2020) Global biogeographic synthesis and priority conservation regions of the relict tree family Juglandaceae. J Biogeogr 47(3):643–657. https://doi.org/10.1111/jbi.13766
Song YG, Walas Ł, Pietras M, Sâm HV, Yousefzadeh H, Ok T, Farzaliyev V, Worobiec G, Worobiec E, Stachowicz-Rybka R, Boratyński A (2021) Past, present and future suitable areas for the relict tree Pterocarya fraxinifolia (Juglandaceae): integrating fossil records, niche modeling, and phylogeography for conservation. Eur J Res 140:1323–1339. https://doi.org/10.1007/s10342-021-01397-6
Thi Ngo M, Han JW, Van Nguyen M, Le Dang Q, Kim H, Choi GJ (2021) Antifungal properties of natural products from Pterocarya tonkinensis against phytopathogenic fungi. Pest Manag Sci 77(4):1864–1872. https://doi.org/10.1002/ps.6211
Tordoff AW, Baltzer MC, Fellowes JR, Pilgrim JD, Langhammer PF (2012) Key biodiversity areas in the Indo-Burma hotspot: process, progress and future directions. J Threat Taxa 4:2779–2787. https://doi.org/10.11609/JoTT.o3000.2779-87
Turner S, Pham TTH (2015) Nothing is like it was before: the dynamics between land-use and land-cover, and livelihood strategies in the northern Vietnam borderlands. Land 4(4):1030–1059. https://doi.org/10.3390/land4041030
UNEP-WCMC IUCN (2023) Protected planet: the World database on protected areas (WDPA) and World database on other effective area-based Conservation measures (WD-OECM). UNEP-WCMC and IUCN, Cambridge, UK. Available at: www.protectedplanet.net
van Dijk PP, Tordoff AW, Fellowes J, Lau M, Ma JS (2004) Indo-Burma, pp. 323–330. In: Mittermeier RA, Robles-Gil P, Hoffmann M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreaux J, da Fonseca GAB (eds.). Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions. CEMEX, Monterrey; Conservation International, Washington D.C.; and Agrupación Sierra Madre, Mexico, 390pp
van Proosdij AS, Sosef MS, Wieringa JJ, Raes N (2016) Minimum required number of specimen records to develop accurate species distribution models. Ecography 39(6):542–552. https://doi.org/10.1111/ecog.01509
Van Sam H, Quang Tung D, Jasińska AK, Rion F, Tuyen PT, Ngoc DTB, Tam DT, Betrisey S, Song YG, Kozlowski G (2021) Diversity, distribution and threats of the Juglandaceae in Vietnam. Dendrobiology 86:39–55. https://doi.org/10.12657/denbio.086.005
Vogt P, Riitters K (2017) GuidosToolbox: universal digital image object analysis. Eur J Remote Sens 50(1):352–361. https://doi.org/10.1080/22797254.2017.1330650
Wang Z, Chang YI, Ying Z, Zhu L, Yang Y (2007) A parsimonious threshold-independent protein feature selection method through the area under receiver operating characteristic curve. Bioinformatics 23:2788–2794. https://doi.org/10.1093/bioinformatics/btm442
Wang P, Liu W, Zhang J, Yang B, Singh AK, Wu J, Jiang X (2019) Seasonal and spatial variations of water use among riparian vegetation in tropical monsoon region of SW China. Ecohydrology 12(4):e2085. https://doi.org/10.1002/eco.2085
Wang L, Yang B, Bai Y, Lu X, Corlett RT, Tan Y, Chen XY, Zhu J, Liu Y, Quan RC (2021) Conservation planning on China’s borders with Myanmar, Laos, and Vietnam. Conserv Biol 35(6):1797–1808. https://doi.org/10.1111/cobi.13733
Wang TR, Meng HH, Wang N, Zheng SS, Jiang Y, Lin DQ, Song YG, Kozlowski G (2023) Adaptive divergence and genetic vulnerability of relict species under climate change: a case study of Pterocarya macroptera. Ann Bot 132(2):241–254. https://doi.org/10.1093/aob/mcad083
Warren DL, Matzke NJ, Cardillo M, Baumgartner JB, Beaumont LJ, Turelli M, Glor RE, Huron NA, Simões M, Iglesias TL, Piquet JC, Dinnage R (2021) ENMTools 1.0: an R package for comparative ecological biogeography. Ecography 44(4):504–511. https://doi.org/10.1111/ecog.05485
Weinzettel J, Vačkář D, Medková H (2018) Human footprint in biodiversity hotspots. Front Ecol Environ 16:447–452. https://doi.org/10.1002/fee.1825
Williams JN, Seo C, Thorne J, Nelson JK, Erwin S, O’Brien JM, Schwartz MW (2009) Using species distribution models to predict new occurrences for rare plants. Divers Distrib 15(4):565–576. https://doi.org/10.1111/j.1472-4642.2009.00567.x
Xu JC, Wilkes A (2004) Biodiversity impact analysis in northwest Yunnan, Southwest China. Biodivers Conserv 13:959–983. https://doi.org/10.1023/B:BIOC.0000014464.80847.02
Ye P, Zhang G, Zhao X, Chen H, Si Q, Wu J (2021) Potential geographical distribution and environmental explanations of rare and endangered plant species through combined modeling: a case study of Northwest Yunnan, China. Ecol Evol 11(19):13052–13067. https://doi.org/10.1002/ece3.7999
Yousefzadeh H, Rajaei R, Jasińska A, Walas Ł, Fragnière Y, Kozlowski G (2018) Genetic diversity and differentiation of the riparian relict tree Pterocarya fraxinifolia (Juglandaceae) along altitudinal gradients in the Hyrcanian forest (Iran). Silva Fennica 52(5):10000
Zhang K, Liu H, Pan H, Shi W, Zhao Y, Li S, Liu J, Tao J (2020) Shifts in potential geographical distribution of Pterocarya stenoptera under climate change scenarios in China. Ecol Evol 10(11):4828–4837. https://doi.org/10.1002/ece3.6236
Zhu H (2006) Forest vegetation of Xishuangbanna, south China. Forestry Stud China 8(2):1–58. https://doi.org/10.1007/s11632-006-0014-7
Zhu H (2017) A biogeographical study on tropical flora of southern China. Ecol Evol 7(23):10398–10408. https://doi.org/10.1002/ece3.3561
Acknowledgements
This research was financially supported by the Institute of Dendrology, Polish Academy of Sciences, Poland, under statutory activity. We thank Anna K. Jasińska (former researcher at the Institute of Dendrology, Polish Academy of Sciences) for support in conceptualization. Felipe Bravo’s collaboration thanks to projects 'CLU-2019-01-iuFOR Institute Unit of Excellence’ of the University of Valladolid (funded by the Junta de Castilla and co-financed by the European Union: ERDF "Europe drives our growth").
Author information
Authors and Affiliations
Contributions
Łukasz Walas: Conceptualization, Methodology, Software, Validation, Formal analysis, Visualization, Writing - Original Draft, Writing – review & editing, Supervision. Do Quang Tung: Investigation, Resources, Data curation, Writing – review & editing. Katarzyna Sękiewicz: Conceptualization, Validation, Writing – review & editing. Marcin Pietras: Writing – review & editing. Felipe Bravo: Resources, Writing – review & editing. Gregor Kozlowski: Writing – review & editing. Hoàng Văn Sâm: Investigation, Resources, Data curation, Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gediminas Brazaitis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Walas, Ł., Tung, D.Q., Sękiewicz, K. et al. Risk assessment of habitat suitability decline for the endangered riparian tree Pterocarya tonkinensis (Juglandaceae): conservation implications. Eur J Forest Res 143, 1057–1068 (2024). https://doi.org/10.1007/s10342-024-01679-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-024-01679-9