Abstract
The aim of this study was to determine some physical properties and biochemical contents of myrtle fruits at three different maturity stages. For this purpose, the fruits were harvested on 3 October (pre-maturity), 1 November (beginning of maturity) and 29 November (fully mature) in 2015. In the study, L* and b* values, which are among the skin color values of the fruits, did not change with maturity, while the a* value increased with maturity from −8.21 (1st period) to 4.40 (3rd period). While the total soluble solid content of the fruits increased with maturity, the titratable acid content decreased. Total phenolic content of myrtle fruit was found to be 1408, 794 and 757.6 mg 100 g−1 in the 1st, 2nd and 3rd periods, respectively. The quercetin content of myrtle decreased significantly with maturity. While the quercetin content in fruits was 10.20 µg g−1 in the 1st period, it decreased to 6.33 µg g−1 in the 3rd period. It was determined that the tannic acid content of myrtle fruits also decreased statistically significantly with maturity. In addition, malic and citric acids contents in fruits increased statistically significantly with maturity, from 790.5 and 86.6 µg g−1 in the 1st period, respectively, to 1037.6 and 219.8 µg g−1 in the 3rd period, respectively. The tartaric acid content in myrtle fruits, on the other hand, decreased statistically significantly with maturity, from 2063.3 µg g−1 (1st period) to 754.1 µg g−1 (3rd period). As a result, it was determined that myrtle fruits differed significantly in terms of biochemical content according to the ripening level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myrtle (Myrtus communis L.) is a shrub-shaped, evergreen fruit species with medicinal and aromatic properties in the Myrtaceae family. In addition to the coastlines of Mediterranean countries, it naturally spreads in temperate regions of Austria, the Middle East and North America (Baytop 1999; Jamoussi et al. 2005; Mendes et al. 2001). The myrtle plant, which grows wild in the coastal regions of Morocco, Turkey and Tunisia, is cultivated in Spain, Italy, Iran and Corsica (Jamoussi et al. 2005). Myrtle, which has a wide ecology, can grow in Turkey at an altitude of 500–600 m above sea level, on the entire coastline, especially in the Mediterranean and Aegean Regions (Kaya and Aladağ 2009; Yildirim et al. 2015).
Myrtle, which has black and white fruits, is referred to as ‘common myrtle’, ‘murt’ and ‘hambeles’ in the southern parts of Turkey. Its fruits are consumed fresh or dried. Myrtle is a fruit that has positive effects on health thanks to the tannins, essential oils, organic acids, anthocyanins and phenolic compounds it contains. Phenolic compounds are known to have antibacterial, antidepressant, antidiabetic, antiviral, anti-inflammatory and immunostimulatory properties. Due to its antioxidant properties, it also reduces the risk of many diseases such as cancer (Proestos et al. 2006; Aydın and Özcan 2007; Şan et al. 2015a, 2016; Yanguia et al. 2021).
Some researchers state that the nutrient content of fruit species is affected by genotype, environmental factors, cultural practices and processing methods (Howell et al. 2001; Pehluvan and Güleryüz 2004; Gao et al. 2012; Alkaltham et al. 2021). In recent years, research on increasing the nutritional content of fruits has been given importance (Rossi et al. 2003; Küçüker et al. 2015; Kui et al. 2020). It is also known that the biochemical contents of fruits change with the time of harvest and the degree of ripening. For this reason, it is important to harvest the fruits at the appropriate time when they are rich in biochemical content. Phenolic compounds are one of the most important biochemical substances in fruits, which are affected by genetic or external factors. It has been reported that phenolic components decrease during fruit ripening, while the amount of anthocyanins increases in fruit species such as jujube, pomegranate and myrtle (Wang et al. 2016; Babou et al. 2016; Bayar and Şan 2017). In this respect, consumers and food industry organizations need important information about the changes in the bioactive substance content of fruit species and their antioxidant activities at different maturity stages. As in all fruit species, it is necessary to know the changes in biochemical contents of myrtle fruits according to different maturity levels. In this study, changes in some physical properties and biochemical contents of myrtle fruits harvested at different maturity periods were investigated.
Materials and Methods
Materials
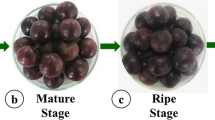
In the study, the fruits of the myrtle genotype in the farmer’s garden in the Serik district of Antalya province were used as material. The genotype of myrtle used in the study is a type with white and large fruit, propagated by grafting by producers and sold especially in local markets. This genotype is locally referred to as ‘Aşı Mersin’ (Şan et al. 2015b). Fruits belonging to the genotype were harvested on 3rd October (pre-maturity), 1st November (beginning of maturity) and 29th November (fully mature) in 2015.
Method
Determination of Physical Properties
Fruit width and fruit length were measured with a digital caliper in harvested fruits, and fruit weight was measured with precision scales. Skin color values of fruits were measured with Minolta CR-400 colorimeter (Osaka, Japan) and expressed as L*, a*, b*.
Determination of Biochemical Contents
In order to determine their biochemical properties, the fruits were squeezed and the juice was filtered with the help of filter paper. The pH of the juice was determined with the help of a pH meter. The water-soluble solids content was measured with a hand-held refractometer. Titratable acidity content was analyzed according to the method stated by Karaçalı (2010) and the results were calculated as % malic acid.
Analysis of Total Phenolic Content
The total phenolic content of the fruits was determined according to the Folin-Ciocalteu method described by Singleton and Rossi (1965). For this purpose, 1 g of myrtle fruit flesh was taken and completely crushed in 4 ml of ethanol. After the samples were kept in a hot water bath for 10 min, they were centrifuged at 8000 rpm for 15 min. Then, 4 ml of 80% ethanol was added to the filtered samples and after they were kept in a hot water bath for 10 min again, the samples were made up to 20 ml with 80% ethanol. A total of 0.5 ml of the extract was taken and 2.5 ml of Folin-Ciocaltaeu (0.2 M) solution (Sigma-Aldrich, Steinheim, Germany) and 1 ml of distilled water were added to this. After the samples were kept for 10 min, 1 ml of sodium carbonate (0.7 M) was added and incubated in the dark for 2 h. Absorbance values were read in a spectrophotometer (T70 Plus Dual Beam/Arlington, USA) set at a wavelength of 760 nm. Results are expressed as mg GAE 100 g−1 of fresh fruit. In order to determine the standard calibration curve, 0, 20, 40, 60 and 80 mg/l gallic acid (Merck, Darmstadt, Germany) solution was prepared and obtained by reading the absorbance values in the spectrophotometer with the application of the same method.
High Pressure Liquid Chromatography Analysis of Phenolic Compounds
Phenolic compound analysis were carried out using Shimadzu brand high pressure liquid chromatography (HPLC) device (Shimadzu Inc., Kyoto, Japan) by the modified method of Caponio et al. (1999). For this purpose, 5 g of myrtle fruit was weighed and 10 ml of methanol was added. It was mixed at room temperature for about 18 h at 100 rpm in a shaker. After the samples were filtered with coarse filter paper, they were again passed through a filter with a pore diameter of 0.45 µm (Vivascience AG, Hannover, Germany). Then, 20 µl of sample was injected into the HPLC device equipped with a DAD detector (λmax = 278) and on a column of Agilent Eclipse XDB-C18 (250 × 4.60 mm, 5 µm) operated at 30 °C. Detection and quantification were performed with a SIL-10AD vp auto sampler, a SCL-10 Avp system controller, a DGU-14A degasser, a LC-10AD vp pump and a CTO-10Avp column oven. The elution solvents were 3% acetic acid (A) and methanol (B). The flow rate was 0.8 ml/min. The gradient program used in the HPLC device is given in Table 1. Peak identification was performed according to the standards (gallic acid, chlorogenic acid, resveratrol, myricetin and quercetin) purchased from Sigma-Aldrich Chemical Co (Steinheim, Germany). The amounts of phenolics were evaluated according to their peak areas. Concentrations were expressed as µg g−1 FW.
High Pressure Liquid Chromatography Analysis of Tannic Acid
Tannic acid analysis were carried out using a Shimadzu HPLC device by modifying of the methods described in Caponio et al. (1999) and Verzele and Delahaye (1983). For this purpose, 5 g of myrtle fruits were weighed and crushed. Then, 20 ml of methanol was added to this and shaken in a magnetic shaker for 1 h. After the samples were filtered with coarse filter paper, they were again passed through a filter with a pore diameter of 0.45 µm (Vivascience AG, Hannover, Germany). A total of 20 µl of the filtered sample was injected into the HPLC device equipped with a DAD detector (280 nm) and on a column of Luna Silica (2) (250 × 4.60 mm, 5 µm) operated at 30 °C. Detection and quantification were performed with an SCL-10 Avp system controller, a DGU-14A degasser, a LC-10AD vp pump and a CTO-10ACvp column oven. The elution solvents were 0.2% phosphoric acid in distilled water (A) and 0.04% phosphoric acid in acetonitrile (B). The flow rate was 1.2 ml/min. The gradient program used in the HPLC device is given in Table 2. Peak identification was performed according to the standard of tannic acid purchased from the Sigma-Aldrich Chemical Co (Steinheim, Germany). The amounts of tannic acid were evaluated according to the peak area. Concentrations were expressed as µg g−1 FW.
High Pressure Liquid Chromatography Analysis of Organic Acids
Organic acid analysis were performed using a Shimadzu HPLC device by modifying the methods described in Alhendawi et al. (1997) and Krapez et al. (2001). Before the analysis measurements were made, the Supelco C18 solid phase cartridge was first conditioned with 3 ml of methanol and then washed with 10 ml of distilled water. After weighing 10 g fruit samples, 20 ml of H3PO4 (2%) was added. The samples were shaken for 5 min in a magnetic shaker and filtered with coarse filter paper. Then, 1 ml of the filtrate was taken and 1 ml of extraction solution was added to this. As the extraction solution, 0.01 M KH2PO4 (pH, 8) was used. A total of 1 ml of this solution was passed through the cartridge and the solution was taken into a tube. Then, after the cartridge was washed with 1 ml of extraction solution again, the solutions were combined and 20 µl of sample was injected into the HPLC device equipped with a SPD-10Avp UV-VIS detector (210 nm) and on a Prodigy ODS‑2 column (250 × 4.6 mm, 5 µm) operated at 30 °C. Analysis of organic acids was performed with a SIL-20AC prominence auto sampler, an LC-20AT prominence system controller, a DGU-20A5 prominence degasser, an LC-20AT prominence pump and a CTO-10ASvp column oven. The elution solvents was phosphoric acid (pH: 2.2). The flow rate was 0.8 ml/min. Peak identification was performed according to the standards (tartaric acid, ascorbic acid, malic acid, citric acid and succinic acid) purchased from Sigma-Aldrich Chemical Co. The amounts of organic acid were evaluated according to their peak areas. Concentrations were expressed as µg g−1 FW.
Experimental Design and Data Analysis
The experiment was planned according to the randomized plot design with three replications. The obtained data were subjected to analysis of variance using the Minitab 17 software program (Minitab Inc.) and the differences between the means were determined at the 5% error level with the TUKEY comparison test and given with different letters.
Results and Discussion
Determination of Physical Properties
In the study, fruit weight, fruit width and fruit length values of myrtle fruits increased statistically significantly with the progress of maturity (p < 0.05). It was observed that the increase in fruit length was higher than the increase in fruit width in the last 4‑week period. In our study, fruit width increased by 14% and fruit length increased by 19% in the last 2 months of maturity. This difference also affected the fruit shape index value. Fruit shape index values, which is a value dependent on fruit width and fruit length parameters, showed similar results with all other parameters as expected. The results obtained were similar to the study of Fadda and Mulas (2010). Between October 3 and November 29, fruit weight increased by approximately 67%. Similar to our results, Aidi-Wannes et al. (2019) reported that fruit width and length increased by 5–19% and 16–28% respectively, in the last 2 months of maturity based on genotypes. While a significant increase was detected in a* value with maturity according to fruit skin color, b* value decreased. There was no statistical difference in the L* value, which expresses the brightness (Table 3). It is known that there is a positive relationship between a* value and anthocyanin content. As a matter of fact, it is reported that anthocyanin content increases in parallel with maturity (Fadda and Mulas 2010).
Titratable Acidity, Total Soluble Solids and PH of Fruit Juice
The highest pH value (5.51) in fruit juice was determined in the full maturity period (29th November). Accordingly, the titratable acidity content also decreased as maturity progressed. As expected, the content of total soluble solids of fruit juices increased regularly as maturity progressed, and this increase was statistically significant (Table 3). The total soluble solids of the fruits harvested at full maturity was found to be 17.10%. Similar to our findings, Kui et al. (2020) reported that as the maturity of myrtle progressed, the content of total soluble solids increased, while titratable acidity decreased. It is also stated that the titratable acid content of myrtle fruits, which was 0.53% after 30 days of full bloom, decreased to 0.16% after 210 days after full bloom (Fadda and Mulas 2010). Additionally, it was reported by Mezni et al. (2019) that the total soluble solids and titratable acidity contents vary significantly according to the cultivars. Researchers have reported that the content of total soluble solid varied between 4 and 10%, and the titratable acid content varied between 0.3 and 0.7%, depending on the genotype and applications.
Organic Acid Contents
It was determined that citric and malic acid ratios increased in parallel with the progress of maturity in fruits, while tartaric acid decreased significantly. While the malic acid content of the fruits was 790.5 µg g−1 in the pre-maturity period (3rd October), this value increased to 1037.6 µg g−1 in the full maturity period (29th November). It was determined that the citric acid content of the fruits increased by approximately 60% during the full maturity period (29th November) (Table 4). It was determined that malic and citric acid contents increased with maturity. However, the total organic acid ratio decreased with maturity. In support of our study, Güçlü et al. (2022) found that malic acid content in medlar fruit first decreased with maturity and then increased again. Researchers reported that the tartaric acid content of medlar fruits also decreased with maturity, similar to our results. Contrary to our results, it was determined that the citric acid content of passiflora and medlar fruits decreased with maturity (Zhang et al. 2021; Güçlü et al. 2022). The organic acid contents differ according to the species. As a matter of fact, it has been reported that the malate content increases with maturity in apple, banana, grape, kiwifruit, lime, peach, plum and strawberry. It was stated that citrate increased with maturity in guava, lemon, lime, orange, plum, strawberry and pineapple, and decreased in papaya and peach (Batista-Silva et al. 2018). It has been reported that the decrease in organic acid content in many fruits is due to the increase in fruit size rather than organic acid metabolism. In addition, it has been reported that changes in organic acid content in fruit species are significantly affected by environmental conditions such as light, temperature, and cultural practices (Famiani et al. 2015).
Total and Individual Phenolics
Total phenolic content of myrtle juice was found to be higher in immature fruit than in mature fruit (Table 5). In the study, the highest phenolic content (1408 mg 100 g−1) was detected in immature fruits (3rd October) and decreased with maturity. Aidi-Wannes and Marzouk (2013) found the total phenolic content of myrtle fruit to be 1370 mg 100 g−1, similar to our study. Some researchers also stated that the total phenolic content appears at high levels after fruit set and decreases as development progresses (Vvedenskaya and Vorsa 2004; Renard et al. 2007). It was also stated by Aidi-Wannes et al. (2019) that the total polyphenol in myrtle fruits increased until the ripening period of the fruits and decreased after maturity. It is reported that the total phenolic content of jujube fruits is highest during the white maturity period and decreases with the progress of maturity (Wang et al. 2016).
There were no statistically significant differences in terms of gallic acid content in fruits harvested at different maturity periods. It was determined that there was a statistically significant decrease in quercetin contents with maturity (p < 0.05) (Table 5). Yeğin and Uzun (2015) found the quercetin content of myrtle fruit to be 1.18 mg 100 g−1 (11.8 µg g−1), which supports our findings. It was determined that the myricetin content of the fruits increased until the ripening time and decreased again with the progress of ripening. Yeğin and Uzun (2015) found the myricetin content in myrtle fruits to be approximately two to 20 times higher than our results. Researchers stated that myricetin content in fruits is between 1.99 and 25.36 mg 100 g−1 based on genotypes. It is thought that the variability in the fruits of myrtle genotypes is due to factors such as ecology, climate and maturity level (Fadda and Mulas 2010; Barboni et al. 2010; Medda et al. 2021). As a matter of fact, it has been reported that the phenolic content of myrtle fruits varies based on the cultivars, negatively affected by high temperature, and the anthocyanin content increases with altitude (Medda et al. 2022). In addition, it was stated that the phenolic and flavonoid contents of wild-grown myrtle fruits were higher than the cultured genotypes (Çakmak et al. 2021).
While the highest tannic acid content of 52.23 µg g−1 was determined in fruits harvested in the immature period (3rd October), this value decreased to 24.5 µg g−1 at the beginning of maturity, and tannic acid was not detected in fruits harvested in the following period (29th November). It was observed that the tannic acid content of myrtle fruits decreased statistically significantly with maturity. Similar to our results, Fadda and Mulas (2010) stated that the tannin content of myrtle fruit regularly decreases with the progression of maturity. Researchers stated that while the tannin content was 223.55 mg 100 g−1 in the fruits harvested at 30 days after full bloom, it decreased to 6.84 mg 100 g−1 in the fruits harvested at 210 days after full bloom. On the other hand, it was stated by Aidi-Wannes et al. (2019) that the tannic acid content of myrtle fruits increased until maturity and decreased after maturity. There is a relationship between the amount of tannic acid in the myrtle fruit and the bitterness in the fruit flavor. In this case, it is preferred that the amount of tannic acid in the fruits is low (Mezni et al. 2019). It has been reported that the amount of tannic acid in myrtle fruit may differ based on cultivars (Mezni et al. 2019), ecology and precipitation (Medda et al. 2022).
Conclusions
As a result, it was determined that the biochemical contents of myrtle fruits vary significantly according to the degree of ripening. In this respect, it was determined that while the total soluble solid content of myrtle fruits increased, the titratable acidity decreased with the progression of maturity. The total organic acid and tartaric acid content of the fruits decreased regularly with maturity. However, malic and citric acid contents of fruits increased with maturity. It has been determined that immature fruits are richer in phenolic substance than ripe fruits. In particular, it was determined that the content of total phenolics, quercetin and gallic acid decreased by approximately 46, 38 and 24%, respectively, with maturity. It was determined that the tannic acid content, which gives a bitter taste to the fruits, decreased to a trace amount in the full maturity period.
References
Aidi-Wannes W, Marzouk B (2013) Differences between myrtle fruit parts (Myrtus communis var. italica) in phenolics and antioxidant contents. J Food Biochem 37(5):585–594
Aidi-Wannes W, Tounsi MS, Marzouk B (2019) Morphological and chemical characterization of two Wild tunisian myrtle (Myrtus communis L.) populations. Trends Phytochem Res 3(4):231–242
Alhendawi RA, Römheld V, Kirkby EA, Marschner H (1997) Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum and maize. J Plant Nutr 20(12):1731–1753
Alkaltham MS, Salamatullah AM, Özcan MM, Uslu N, Hayat K, Mohamed Ahmed IA (2021) Influence of different drying methods on antioxidant activity, total phenol, and phenolic compounds of myrtle (Myrtus communis L.) fruits. J Food Process Preserv 45:e15308
Aydın C, Özcan MM (2007) Determination of nutritional and physical properties of myrtle (Myrtus communis L.) fruits growing wild in Turkey. J Food Eng 79:453–458
Babou L, Hadidi L, Grosso C, Zaidi F, Valentão P, Andrade PB (2016) Study of phenolic composition and antioxidant activity of Myrtle leaves and fruits as a function of maturation. Eur Food Res Technol 242(9):1447–1457
Barboni T, Cannac M, Massi L, Perez-Ramirez Y, Chiaramonti N (2010) Variability of polyphenol compounds in Myrtus communis L. (Myrtaceae) berries from Corsica. Molecules 15:7849–7860
Batista-Silva W, Nascimento VL, Medeiros DB, Nunes-Nesi A, Ribeiro DM, Zsögön A, Araújo WL (2018) Modifications in organic acid profiles during fruit development and ripening: correlation or causation? Front Plant Sci 9:1689
Bayar B, Şan B (2017) Physical and biochemical changes in pomegranate (Punica granatum L. cv.‘Hicaznar’) fruits harvested at three maturity stages. Sci Pap B Hortic 61:63–68
Baytop T (1999) Türkiye’de bitkiler ile tedavi geçmişte ve bugün. Nobel Tıp Kitap Evleri, İstanbul
Çakmak M, Bakar B, Özer D, Geckil H, Karatas F, Saydam S (2021) Investigation of some biochemical parameters of wild and cultured Myrtus communis L. fruits subjected to different conservation methods. J Food Meas Charact 15:983–993
Caponio F, Alloggio V, Gomes T (1999) Phenolic compounds of virgin olive oil: Influence of paste preparation techniques. Food Chem 64:203–209
Fadda A, Mulas M (2010) Chemical changes during myrtle (Myrtus communis L.) fruit development and ripening. Sci Hortic 125(3):477–485
Famiani F, Battistelli A, Moscatello S, Cruz-Castillo JG, Walker RP (2015) The organic acids that are accumulated in the flesh of fruits: occurrence, metabolism and factors affecting their contents—a review. Rev Chapingo Ser Hortic 21(2):97–128
Gao QH, Wu CS, Wang M, Xu BN, Du LJ (2012) Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugar, organic acids, alpha-tocopherol, beta-carotene, and phenolic compounds. J Agric Food Chem 60:9642–9648
Güçlü SF, Koyuncu F, Atay E (2022) Organic acid, phenolic acid and flavonoids of medlar during different maturation stages. Akademik Ziraat Dergisi 11(2):207–212
Howell A, Kalt W, Duy JC, Forney CF, McDonald JE (2001) Horticultural factors affecting antioxidant capacity of blueberries and other small fruit. HortTechnology 11(4):523–528
Jamoussi B, Romdhane M, Abderraba A, Hassine BB, Gadri AE (2005) Effect of harvest time on the yield and composition of Tunisian myrtle oils. Flavour Fragr J 20:274–277
Karaçalı İ (2010) Bahçe Ürünlerinin Muhafaza ve Pazarlanması. [Storage and marketing of horticulture products]. Publication, vol 494. Ege University, Faculty of Agriculture, İzmir
Kaya B, Aladağ C (2009) Maki ve garig topluluklarının Türkiye’deki yayılış alanları ve ekolojik özelliklerinin incelenmesi. Selçuk Üniversitesi Sosyal Bilimler Enstitüsü Dergisi 22:67–80
Krapez KM, Abram V, Kac M, Ferjancic S (2001) Determination of organic acids in white wines by RP-HPLC. Food Technol Biotechnol 39(2):93–99
Küçüker E, Öztürk B, Özkan Y, Yıldız K (2015) Yapraktan üre uygulamasının farklı armut (Pyrus communıs L.) çeşitlerinde verim, meyve kalitesi ve bioaktif bileşikler üzerine etkisi. Ömer Halisdemir Üniversitesi Mühendislik Bilimleri Dergisi 4(2):78–86
Kui X, Li M, Yang H, Tadda AS, Sun Y, Ma C, Qiu D (2020) Comparison of physico-chemical characteristics of myrtle at different ripening stages. Folia Hortic 32(2):125–133
Medda S, Fadda A, Dessena L, Mulas M (2021) Quantification of total phenols, tannins, anthocyanins content in Myrtus communis L. and antioxidant activity evaluation in function of plant development stages and altitude of origin Site. Agronomy 11:1059
Medda S, Fadda A, Mulas M (2022) Climate variables of the sites of origin and genotype influence on phenolic compounds accumulation in cultivars of Myrtus communis L. Horticulturae 8:928
Mendes MM, Gazarini LC, Rodrigues ML (2001) Acclimation of Myrtus communis to contrasting mediterranean light environments—effects on structure and chemical composition of foliage and plant water relations. Environ Exp Bot 45(2):165–178
Mezni F, Fkiri S, Stiti B, Khaldi A (2019) Chemical characterization of bluish-black and yellowish-white fruits of Myrtus communis L. Int J Food Sci Nutr 4(4):144–146
Pehluvan M, Güleryüz M (2004) Ahududu ve bögürtlenlerin insan sağlığı açısından önemi. Bahçe 33(1–2):51–57
Proestos C, Boziaris IS, Nychas GJ, Komaitis M (2006) Analysis of flavonoids and phenolic acids in Greek aromatic plants: investigation of their antioxidant capacity and antimicrobial activity. Food Chem 95(4):664–671
Renard CMGC, Dupont N, Guillermin P (2007) Concentration and characteristics of procyanidins and other phenolics in apples during fruit growth. Phytochemistry 68:1128–1138
Rossi M, Giussani E, Morelli R, LoScalzo R, Nani RC, Torreggiani D (2003) Effect of fruit blanching on phenolics and radical scavenging activity of high bush blueberry juice. Food Res Int 36(9–10):999–1005
Şan B, Yildirim AN, Polat M, Yildirim F (2015a) Chemical compositions of Myrtle (Myrtus communis L.) genotypes having bluish-black and yellowish-white fruits. Erwerbs-Obstbau 57(4):203–210
Şan B, Karakurt Y, Dönmez F (2015b) Effects of thidiazuron and activated charcoal on in vitro shoot proliferation and rooting of myrtle (Myrtus communis L.). J Agric Sci 21:177–183
Şan B, Yıldırım F, Yıldırım AN (2016) Bioactive compounds of myrtle plant (Myrtus communis L.). Bahçe 45(Special issue 2):185–193
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Verzele M, Delahaye P (1983) Analysis of tannic acids by high-performance liquid chromatography. J Chromatogr 268:469–476
Vvedenskaya IO, Vorsa N (2004) Flavonoid composition over fruit development and maturation in American cranberry, Vaccinium macrocarpon Ait. Plant Sci 167(5):1043–1054
Wang B, Huang Q, Venkitasamy C, Chai H, Gao H, Cheng N, Cao W, Lv X, Pan Z (2016) Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller) during three edible maturity stages. LWT Food Sci Technol 66:56–62
Yanguia I, Younsia F, Ghalic W, Boussaida M, Messaoud C (2021) Phytochemicals, antioxidant and anti-proliferative activities of Myrtus communis L. genotypes from Tunisia. S Afr J Bot 137:35–45
Yeğin AB, Uzun Hİ (2015) Mersin (Myrtus communis L.) meyvelerinin fenolik bileşik içerikleri. Derim 32(1):81–88
Yildirim F, Şan B, Yildirim AN, Polat M, Ercişli S (2015) Mineral composition of leaves and fruit in some myrtle (Myrtus communis L.) genotypes. Erwerbs-Obstbau 57(3):149–152
Zhang X, Wei X, Ali MM, Rizwan HM, Li B, Li H, Jia K, Yang X, Ma S, Li S, Chen F (2021) Changes in the content of organic acids and expression analysis of citric acid accumulation-related genes during fruit development of yellow (Passiflora edulis f. flavicarpa) and purple (Passiflora edulis f. edulis) passion fruits. Int J Mol Sci 22:5765
Acknowledgements
The authors wish to thank to the Research Project Coordination Unit at the Süleyman Demirel University for their financial support (Project number 4586-YL2-16).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Çelik and B. Şan declare that they have no competing interests.
Ethical standards
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
Additional information
The authors contributed equally to the article.
Rights and permissions
Springer Nature oder sein Lizenzgeber (z.B. eine Gesellschaft oder ein*e andere*r Vertragspartner*in) hält die ausschließlichen Nutzungsrechte an diesem Artikel kraft eines Verlagsvertrags mit dem/den Autor*in(nen) oder anderen Rechteinhaber*in(nen); die Selbstarchivierung der akzeptierten Manuskriptversion dieses Artikels durch Autor*in(nen) unterliegt ausschließlich den Bedingungen dieses Verlagsvertrags und dem geltenden Recht.
About this article
Cite this article
Çelik, B., Şan, B. Determination of Biochemical Contents of Myrtle (Myrtus Communis L.) Fruits at Different Maturity Levels. Erwerbs-Obstbau 65, 2501–2507 (2023). https://doi.org/10.1007/s10341-023-00938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-023-00938-0