Abstract
Industrial roundwood is an important commodity with global trade of 125 million m3 in 2016. Fumigation is the main phytosanitary treatment for bulk wood commodities. Joule heating is a potential alternative phytosanitary treatment for export Pinus radiata D. Don (Pinaceae) logs, but its effectiveness against insects has not yet been confirmed experimentally. To define treatment parameters for Joule heating, we quantified the thermal tolerance of two forest insects, Hylurgus ligniperda (Fabricius) (Scolytinae) and Arhopalus ferus (Mulsant) (Cerambycidae) that are potentially present on P. radiata logs. Heat was applied using dry bath heat blocks to life stages present when phytosanitary treatments are applied. Arhopalus ferus eggs were the most heat-tolerant life stage with decreasing tolerance at 30 min of H. ligniperda adults > H. ligniperda larvae > A. ferus adults, > A. ferus larvae, H. ligniperda eggs, and H. ligniperda pupae. Additional testing of A. ferus eggs (in dry and humidified environments) determined that an upper LT99.99 with 95% confidence of 55.6 °C applied for 30-min controlled A. ferus and hence all other life stages. This is within the range of published studies of other bark- and wood-boring insects, which are reviewed. The LT99.99 and wood-borer biology are discussed in the context of Joule heating as a potential phytosanitary treatment for bulk wood exports. Joule heating of 32 test logs (3.3 m long) demonstrated temperatures exceeding 60 °C for at least 60 min. Ten logs infested with H. ligniperda were Joule-heated to the same profile, resulting in 100% mortality of adults and larvae. Our results are consistent with current ISPM 15 treatment parameters and show that Joule heating is an effective, non-chemical, alternative option to fumigation for quarantine pest control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Increasing international trade of wood products brings concurrent risks that forest insects are moved between countries, establish and have environmental and/or economic impacts
-
Chemical fumigants are the dominant phytosanitary treatment applied to the global movement of 125 million m3 of industrial roundwood traded in 2016
-
Here, we provide robust quantitative data to support heat applied at 55.6 °C applied for 30 min via Joule heating as an alternative, non-chemical, phytosanitary treatment for raw logs
Introduction
International trade of wood products is a pathway for the movement of wood- and bark-boring and hitchhiker insect species (Aukema et al. 2010; Brockerhoff et al. 2006; Eyre and Haack 2017). Although most exotic species have relatively minor impacts (economic and environmental), some establishments have resulted in substantial economic and environmental impacts (Aukema et al. 2010; Brockerhoff et al. 2010). To reduce the international spread of plant pests and diseases, it is important that effective phytosanitary treatments are available to reduce the rates of transport and establishment of potential quarantine pests.
Globally 125 million m3 of industrial roundwood was traded in 2016 (FAO 2016). Although global statistics of fumigant use are not available, data for some countries can provide an indication of the extent of fumigant use. For example, New Zealand applied phytosanitary measures to 80% of the 15.4 million m3 of logs exported in the year to December 2015. This comprised 63% phosphine, 29% methyl bromide and 8% by debarking (MPI 2016). Methyl bromide fumigation is currently the most important phytosanitary treatment of wood exports worldwide and is permitted for quarantine and pre-shipment purposes. However, the International Plant Protection Convention (IPPC) has recommended that countries replace or reduce methyl bromide as a phytosanitary treatment due to its ozone-depleting properties (IPPC 2008).
Heat treatment is widely used to treat wood packaging and is the dominant treatment used to meet the International Standard for Phytosanitary Measures No. 15 (ISPM 15, IPPC 2016a). ISPM 15 specifies that wood must be appropriately debarked and treated by either heat (56 °C for a minimum of 30 min to the core of treated wood) or methyl bromide as per approved rates. Recently, the standard has been amended to allow the application of heat via dielectric methods (minimum temperature of 60 °C for 1 continuous minute throughout the entire profile of the wood (including its surface) (Haack et al. 2014; IPPC 2016a). The use of heat as an approved ISPM 15 treatment was developed in response to the threat from the pinewood nematode (Bursaphelenchus xylophilus (Steiner & Buhrer) Nickle), the causal agent of pine wilt disease; ISPM 15 treatments are thought to be highly effective at controlling numerous other key forest pests that can be found in wood packaging material (Hoover et al. 2010; Mushrow et al. 2004; Myers and Bailey 2011; Uzunovic et al. 2013). However, other studies suggest that 56 °C for 30 min may not be completely effective against some forest pests, e.g. Agrilus planipennis Obenberger (emerald ash borer—EAB, McCullough et al. 2007; Myers et al. 2009; Nzokou et al. 2008), but this may be dependent on heating conditions. The EAB has been the subject of the most published studies regarding heat tolerance of Coleoptera infesting wood (Table 1). Variation in the reported lethal temperature-by-time combination could, in part, be due to methodological differences and, potentially, inadequate heating as temperatures are not always measured throughout the treated material (Haack et al. 2014). For example, Nzokou et al. (2008) suggest that uneven heat distribution may have been a factor in the survival of EAB in their microwave heating trials.
To our knowledge, heat is not used as a treatment for bulk log exports. The low-value, large-volume commodity status of export logs creates barriers to the adoption of traditional heat technology for phytosanitary purposes. Cost of treatment compared to existing options (e.g. chemical fumigation) and the ability to effectively treat sufficient volumes within prescribed time periods are key constraints. Traditionally, heat is used for bulk wood to dry sawn timber in kilns. Heating logs in kilns can be achieved; however, the process is slow and not cost-effective. If kiln temperatures only reach a sub-lethal level, there is the opportunity for organisms to express heat shock proteins that could increase thermal tolerance (Sobek et al. 2011). Our experiments were conducted under the operational context of Joule heating, which heats logs by passing an electrical current through them. Electrodes are placed on either end of the log, and a constant power AC (alternating current) excitation is applied. Freshly harvested wood has high ohmic resistance, relative to metallic conductors, resulting in the conversion of almost all the electrical energy supplied to heat in the wood. This new approach has been shown to quickly and effectively heat logs in a matter of minutes not hours (Heffernan 2013). Consequently, we use protocols with rapid ramp times and short treatment durations compared to other studies. Our results permit an assessment of the efficacy and efficiency of Joule heating as an alternative phytosanitary treatment. Furthermore, a precise understanding of insect tolerance to heat and the location of life stages within the log (beneath bark versus in wood closer to the core) allows the optimization of treatment schedules to ensure treatments are effective whilst avoiding the application of energy beyond what is necessary to ensure phytosanitary compliance.
We tested the thermal tolerance of two regulated quarantine insect species that have the potential to be present on P. radiata logs exported from New Zealand (Pawson et al. 2014). These species are Hylurgus ligniperda (Fabricius) (Coleoptera, Curculionidae, Scolytinae), a native of the Palearctic that disperses readily and has established in at least eight countries, including New Zealand (Brockerhoff et al. 2006), and Arhopalus ferus (Mulsant) (Coleoptera: Cerambycidae), an exotic longhorn beetle from Eurasia (Brockerhoff and Hosking 2001). Accurately quantifying the thermal mortality relationship of these two species is necessary to specify the minimum heat treatment required to ensure New Zealand log exports are free of these regulated pests.
Methods
Collection of insects
Adult H. ligniperda were field collected using alpha-Pinene and ethanol-baited black panel traps (Meurisse and Pawson 2017) placed amongst recent clearfells in P. radiata plantation forests (Bottle Lake (− 43°27′12″ S, 172°41′51″ E) and West Melton Forests (− 43°27′56″ S, 172°22′49″ E) in Canterbury, and Kaingaroa Forest (− 38°39′55″ S, 176°27′26″ E) in Rotorua, New Zealand). Adults were either heat treated within 48 h of collection (held at ambient laboratory temperatures of ~ 20 °C in humidified containers on damp paper towel), or used to infest freshly felled P. radiata logs (1.5 m cut lengths, approximate diameter 0.25 m, with paraffin-waxed ends) to breed additional life stages. Cohorts of eggs, larvae, and pupae of H. ligniperda were then extracted at different times from infested log material for subsequent heat treatment.
Adult A. ferus were collected by hand from timber processing sites in Canterbury, New Zealand, where they are attracted by volatiles emitted from drying kilns and the site lighting at night (Pawson et al. 2009). Eggs were collected by placing adult A. ferus in plastic containers with wax paper as an egg-laying substrate. Containers were held in a refrigerated incubator (MIR-153, Sanyo Electric Co. Ltd, Japan) at 20 ± 0.5 °C with no humidity control. Fresh eggs were removed daily and either used for testing or discarded. Larvae for testing were collected from beneath the bark of fire-damaged logs infested with A. ferus from a planted forest in West Melton, Canterbury (− 43°28′8″ S, 172°20′34″ E), New Zealand.
Adult H. ligniperda were placed naked (not covered in wood or diet) inside dry 1.5-ml micro-centrifuge tubes for treatment. Adult A. ferus were placed naked inside dry 5-ml micro-centrifuge tubes. Larvae of H. ligniperda and A. ferus were extracted from logs and weighed individually before being placed naked inside dry 1.5-ml micro-centrifuge tubes. Mass (measured using a Sartorius BP210A, Göttingen, Germany) was used as a proxy for larval age (as instar head widths have not been characterized for these species), as this may influence thermal tolerance. Eggs of both species were placed in 500-µl micro-centrifuge tubes that were placed inside water-filled 1.5-ml micro-centrifuge tubes. The outer water-filled tube was to overcome any insulating air gaps. All life stages were treated in the micro-centrifuge tubes. We excluded the pupal stage of A. ferus from our efficacy testing as larval development time precludes the presence of the pupal stage in logs within the time between harvest, arrival at the port, loading onto ships (typically less than 56 days from harvest to loading, Don Hammond, Hammond Resource Management, Rotorua, New Zealand, personal communication), and travel time to destination from New Zealand (about 17–22 days, Mark Self, Genera Ltd., Tauranga, New Zealand, personal communication). Our rationale is based on Hosking and Bain (1977) who studied the biology and life cycle of A. ferus over 3 years in New Zealand and found that the insect had a 1–2-year life cycle (primarily due to the long period of larval development). In more recent developmental trials conducted using artificial media in the laboratory, the larval stage of A. ferus was found to typically take 216 days (males) to 227 days (females) before pupation occurred (Van Epenhijusen et al. 2012). Hence, we excluded the pupal stage of A. ferus from our efficacy testing as larval development time makes it extremely unlikely that pupae will be present on freshly harvested logs at the time phytosanitary treatments are applied. Although A. ferus may lay eggs on physically damaged and dying trees, e.g. fire scorched (Brockerhoff and Hosking 2001), these are predominantly excluded from the export pathway (Ministry for Primary Industries, Plant Export Group, personal communication).
Application of treatments
A two-stage testing process was undertaken as per the recommendation of ISPM 28 for the collection of efficacy data under controlled conditions (IPPC 2016b). Phase 1 tested all relevant life stages of each species to identify the species and life stage that was most tolerant of heat. Phase 2 consisted of a series of confirmatory tests on the most tolerant species and life stage identified in phase 1. Phase 1 tests were conducted by placing frames containing multiple individual treatment tubes into the metal heat blocks of a calibrated dry temperature bath (Benchmark Scientific Inc., Edison, NJ, USA. Model: BSH1004, and DAIHAN LabTech, Washington, USA. Model: HB-96D). Frames allowed groups of tubes to be inserted and removed simultaneously. Heat blocks were preheated for at least 1 h prior to treatment to ensure the required temperature was stabilized. One replicate of control individuals was used each time a treatment was applied. Each control replicate had the same number of pseudo-replicates as those used for the treatments. We define pseudo-replicates as the number of individuals tested as part of a single true replicate, i.e. the application of one test run of a particular temperature-by-time treatment combination. Only data with a control survival of greater than 70% were retained for analysis; otherwise, these treatments were repeated.
A single temperature was applied to each individual test subject. All species and life stages were exposed to the given temperature for periods of 5, 15, and 30 min. For A. ferus adults, there was an additional 2-min adjustment period between vials being placed in the heat block and when the official treatment time began, to allow the target temperature in the larger 5-ml tubes to be reached. Where sufficient test individuals were available, we treated four replicates of 16 individuals per temperature-by-time combination. Initial treatments began at 40 °C, and additional treatments of higher temperatures were tested until a temperature of 4 °C beyond the first temperature that achieved 100% mortality had been tested. If insufficient individuals were available to undertake the complete range of temperatures (this applied to H. ligniperda eggs and pupae), then the initial start temperature for that life stage was based on mortality observed from other life stages. Subsequent treatments were applied at higher or lower temperatures depending on the results of the first temperature tested, i.e. if treatment was effective, then the subsequent test was at a lower temperature and vice versa. In addition, the desired number of pseudo-replicates (16) was reduced for some treatments where individuals were difficult to obtain. The number of individuals of each species by life stage, by temperature, by time treatment for phase 1 testing is provided in Supplementary Table S1.
We used platinum resistance detectors (PT100 (1/3rd DIN Type, DM-304), Lab Facility, Bognor Regis, UK) connected to a USB PT-104 four-channel, high-resolution temperature converter (PICO Technology, UK) to quantify the temperature experienced in a treatment vial. We chose to calibrate our treatment protocol with a platinum resistance detector as they have greater stability, accuracy, and repeatability compared to thermocouples (Jones 2010). Tests were undertaken with the PT100 in plastic tubes containing test subjects and in separate plastic tubes for comparison. The total potential error of the PT100 sensors is a combination of several errors; at 60 °C (manufacturer data were not available to calculate total error at 55 °C), the errors were: PT-100 sensor (± 0.2 °C), PT-104 logger (± 0.037 °C, includes accuracy, linearity, and root-mean-square noise errors), self-heating (+ 0.062 °C) and stability (± 0.16 °C). Thus, total error of the PT-100 sensor system was calculated at + 0.459 and − 0.335 °C. The asymmetric error is a result of the self-heating error that only has a positive error effect. As a result of calibration testing during phase 1, we were aware of minor differences between the set temperature of the heat block and the actual temperature insects were exposed to. However, there was no evidence that these differences were not applied evenly across all treatments, as such no bias between species and life stages were introduced. Results in Figs. 1, 2, 3, 4 and 5 present the corrected temperature that accounts for this difference.
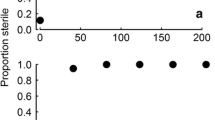
Egg mortality of A. ferus (left panels) and H. ligniperda (right panels) in response to temperature at different exposure times. Grey-shaded areas indicate the confidence interval of the model fit. LT99 = lethal temperature for the indicated mortality levels ± 95% confidence limit. A small amount of horizontal jitter (random noise) was added to the values on the x-axis to separate overlapping points
Larval mortality of A. ferus (left panels) and H. ligniperda (right panels) in response to temperature at different exposure times. Grey-shaded areas indicate the confidence interval of the model fit. LT99 = lethal temperature for the indicated mortality levels ± 95% confidence limit. A small amount of horizontal jitter (random noise) was added to the values on the x-axis to separate overlapping points
Mortality of H. ligniperda pupae in response to temperature at different exposure times. Grey-shaded areas indicate the confidence interval of the model fit. LT99 = lethal temperature for the indicated mortality levels ± 95% confidence limit. A small amount of horizontal jitter (random noise) was added to the values on the x-axis to separate overlapping points
Adult mortality of A. ferus (left panels) and H. ligniperda (right panels) in response to temperature at different exposure times. Grey-shaded areas indicate the confidence interval of the model fit. LT99 = lethal temperature for the indicated mortality levels ± 95% confidence limit. A small amount of horizontal jitter (random noise) was added to the values on the x-axis to separate overlapping points
Additional phase 2 mortality testing of A. ferus eggs in response to a 30-min exposure time. Grey-shaded areas indicate the confidence interval of the model fit. LTxx.xx = lethal temperature for the indicated mortality levels ± 95% confidence limit. Temperature on the x-axis is the stable test temperature per replicate as read in real time with the PT100 sensor that has an error of + 0.453 and − 0.329 °C. A small amount of horizontal jitter (random noise) was added to the values on the x-axis to separate overlapping points
Phase 2 testing focused on A. ferus eggs as these were the most tolerant of heating in phase 1. Eggs were tested at a range of temperatures to provide greater accuracy of the temperature—mortality relationship for this life stage. An additional 1520 individual eggs were treated at heat block set-point temperatures ranging from 46 to 54 °C (Supp. Table S1). To measure the temperature that phase 2 treatments were exposed to, each replicate was accompanied by two PT100 sensors in slots immediately adjacent to each treatment vial in the heat block. Analyses of phase 2 A. ferus egg mortality data used the final temperature recorded by the PT100s after 30 min of treatment as the temperature received by the treatment individuals. Further testing of two replicates of ten individuals was completed using a humidified treatment vial to test the potential impact of humidity on the phase 2 treatment protocol. Humidity was raised by placing a small piece of moist cotton wool in the base of the tube; eggs were then placed on a small piece of aluminium foil that was lowered to the surface of the cotton wool.
All treated and control adults, larvae, and pupae (dead and alive) from a given replicate were placed into recovery chambers prior to an assessment of mortality after 24 h. Recovery chambers were used as individuals that initially look dead are known to potentially recover from heat treatment with time (Mushrow et al. 2004). A single container was used for each treatment replicate and life stage combination. Recovery chambers consisted of a 2-l plastic container with a ~ 100-cm2 nylon mesh window (1 mm mesh) that contained one 15-cm-diameter by 5-cm stem disc of fresh P. radiata and moist filter paper. Twenty-four hours after treatment, adults, larvae and pupae were subjected to a ‘prod test’ with an angled spatula (micro-tool slide set, Australian Entomological Supplies, Coorabell, New South Wales, Australia) and those which moved were recorded as alive. In the case of adult H. ligniperda, those not visible because they had burrowed into the disc of fresh wood were also considered to be alive, as only vigorous beetles are capable of burrowing into bark. Egg mortality was assessed by incubating treated and control individuals in ventilated 0.5-ml Eppendorf tubes housed in a petri dish with moist filter paper at 20 °C to assess for successful egg hatching over 2 weeks post-treatment.
An opportunity existed to undertake a preliminary trial of the effectiveness of Joule heating on the survival of H. ligniperda (adults and larvae) in P. radiata logs. This opportunity coincided with testing by research engineers developing Joule heating as a novel alternative heat treatment. As part of a test batch of 42 logs, in which extensive measurements of temperature distribution were taken after Joule heating, 10 P. radiata logs (3.3 m in length) were infested with adult H. ligniperda by drilling a 10-mm diameter hole through the bark to the cambium–sapwood interface. Groups of ten adult beetles were placed in a 5-ml plastic specimen tube that was then inserted into the hole. Beetles then colonized the logs in three known places per log. Logs were left for a period of 22–60 days to develop gallery structures and larvae. Logs were then treated with three electrical excitations of Joule heating, interspersed with 10-min relaxation periods, with the aim of increasing temperatures by 50–60 °C above ambient. Bark was subsequently stripped from treated logs at least 24 h after testing, with adult and larval individuals subjected to a prod test to evaluate live/dead status. As this was an exploratory test, no formal statistical analyses were done on this small data set.
Statistical analysis
Generalized linear models with binomial errors and logit link were applied to analyse the thermal tolerance data using R version 3.3.3 (R Development Core Team 2017). For each life stage and exposure time, the proportion of dead organisms served as the response variable which was modelled as a function of temperature. In most of the models, the dispersion parameter ϕ was substantially greater than one indicating over-dispersion which was accounted for using the quasi-binomial ‘family’. In the analysis of larval data, we tested mass as a proxy for larval age as a model covariate. Larval mass produced no significant effects, except for two weak interactions with temperature, one each in the 5- and 15-min heat treatments (A. ferus at 5-min exposure time, H. ligniperda at 15-min exposure time). As judged by 3-D plots, the weak mass × temperature interactions had little biological relevance. Together with the fact that such short exposure times are not realistic operating conditions, this led us to run the final larval models with temperature as sole predictor variable. Temperatures lethal to 99% (LT99, phase 1) and 99.99% (LT99.99, phase 2) of the samples, respectively, and the associated 95% confidence interval were estimated from the models using the ‘dose.p’ function (R-package MASS).
Results
Phase 1 testing
Tolerance of eggs
The observed minimum temperature that caused 100% mortality of test subjects was 45.8 °C (H. ligniperda) and 49.4 °C (A. ferus) applied for 30 min. Mortality-by-temperature models showed a strong sigmoidal relationship at each exposure period; however, the initial lag phase was not observed for H. ligniperda eggs at 5 and 15 min (Fig. 2). The maximum lethal temperature for eggs occurred in the 5-min exposure time, with a lower but consistent LT99 in 15- and 30-min treatments.
Based on the model fits (Fig. 1), the minimum temperature required to achieve 99% egg mortality of A. ferus species with a statistical confidence of 95% was calculated as 56.7 °C applied for 30 min.
Tolerance of larvae
The observed minimum temperature that caused 100% mortality of test subjects was 47.6 °C (H. ligniperda) and 44.8 °C (A. ferus) applied for 30 min. Mortality-by-temperature models fitted for the larvae of H. ligniperda and A. ferus showed strong sigmoidal relationships at each exposure period (Fig. 2). For A. ferus larvae, the lethal temperature to achieve a predicted mortality ≥ 99% ranged from 47.6 to 53.2 °C, depending on the exposure period. Hylurgus ligniperda larvae were more tolerant of higher temperatures than A. ferus at all exposure periods. Lethal temperatures for H. ligniperda larvae showed larger variation than for A. ferus and ranged from 48.1 to 52.7 °C depending on the exposure period. In both species, the highest lethal temperature estimates were associated with the LT99 at 5-min exposure time (Fig. 2).
Model fits (Fig. 2) show that the minimum temperature required to achieve 99% larval mortality for either species was 50.7 °C applied for 30 min with a statistical confidence of 95%.
Tolerance of pupae
The observed minimum temperature that caused 100% mortality of H. ligniperda pupae was 42.2 °C applied for 30 min. Temperature-by-mortality models fitted for the pupae of H. ligniperda show a sigmoidal relationships at each exposure period tested (Fig. 3). Similar to the other life stages, the shortest exposure time was associated with the highest lethal temperatures. Hylurgus ligniperda pupae were the most temperature-sensitive life stage tested. At 15- and 30-min exposure time, 42.6 and 43.7 °C were sufficient to kill 99% of the pupae. Greater sampling was done across a range of temperatures at the 30-min treatment, and we suggest using this treatment combination as the most conservative estimate of thermal tolerance. Based on the model fits (Fig. 3), the minimum temperature that achieves 99% pupal mortality of H. ligniperda was 44.9 °C applied for 30 min (95% confidence).
Tolerance of adults
The observed minimum temperature that caused 100% mortality of test subjects was 44.0 °C (H. ligniperda) and 45.8 °C (A. ferus) applied for 30 min. Temperature-by-mortality models fitted for adult H. ligniperda and A. ferus showed strong sigmoidal relationships at each exposure period. Adult beetles showed greater tolerance of high temperatures than larvae. Although a single A. ferus adult was observed surviving at a higher temperature, the mortality model showed that H. ligniperda adults could withstand higher temperatures than A. ferus (Fig. 4), which is consistent with the larval mortality relationships (Fig. 2). The maximal lethal temperatures occurred after a 5-min exposure time, e.g. the LT99 was 49.8 and 54.3 °C for A. ferus and H. ligniperda, respectively. With increasing exposure time, the LT99 declined by 3 °C for A. ferus and 5.9 °C for H. ligniperda (Fig. 4). Based on the model fits (Fig. 4), the minimum temperature required to achieve 99% adult mortality of either species with a statistical confidence of 95% was calculated as 50.6 °C applied for 30 min to control H. ligniperda.
Phase 2 testing
Arhopalus ferus eggs were the most heat-tolerant life stage, requiring an additional 4.2 °C above the LT99 mortality for H. ligniperda larvae (53.5 °C). Detailed testing of A. ferus eggs for an exposure period of 30 min produced a strong sigmoidal relationship (Fig. 5). Based on the model fits (Fig. 5), the minimum effective temperature required to achieve 99.99% mortality of A. ferus eggs (and hence all life stages tested) with a statistical confidence of 95% was 55.1 °C plus the total potential error of the PT100 sensor (as discussed below). Additional testing of two replicates of 10 individual A. ferus eggs with humidified treatment containers showed 100% control mortality and 80% control survival in each replicate. The heat block in the dry temperature bath was set to a point that would most closely match the LD99.99 (plus the error of the PT sensors), and the heating profile as measured by four PT100 s placed in adjacent tubes during testing is shown (Fig. 6). All adult and larval individuals within the 10 logs treated by Joule heating were killed by the applied treatments.
Discussion
Logistic temperature mortality curves were obtained for each life stage of both H. ligniperda and A. ferus. No eggs, larvae, pupae, or adult individuals of either species that were assessed survived treatment at a set-point temperature of 49.4 °C or more, applied for 30 min. The minimum effective temperature required to achieve 99.99% mortality of the most tolerant species and life stage (phase 2 testing of A. ferus eggs) with a statistical confidence of 95% was 55.1 °C. However, to ensure that a proposed temperature-by-time phytosanitary treatment is effective, we must add the potential total error of the instrumentation used to measure the temperature applied inside the treatment containers during phase 2 testing. The total potential upper bound error in our LT99.99 estimate of the effective heat treatment applied for 30 min is 0.459 °C (upper validity error of the PT sensors) + 1.15 °C (reliability error from natural variation (i.e. 95% confidence interval)) + any source of measurement error. Measurement error attributable to a researcher’s assessment of the live/dead status of individual insects after treatment is difficult to assess. Although measurement error was not specifically quantified in this study, i.e. the chance that an insect appearing dead during the prod test would in fact be alive, we took three steps to reduce potential sources of error. Firstly, we used metal frames to quickly transfer all treatment vials into the heating block to reduce individual variation in heating times. Secondly, we assessed all larval, pupal, and adult individuals 24 h after treatment, as heat-treated individuals may appear dead but later recover from the thermal shock (Mushrow et al. 2004). Thirdly, eggs were reared after treatment to determine survival by hatching, and we discarded any treatments where control survival was less than 70%. Hence, measurement error of egg hatch is unlikely to be a significant source of error as egg hatch status is not a subjective distinction. Combining the total potential error and our model fit of the LT99.99, we conclude that a minimum temperature of 55.56 °C applied for 30 min is required to achieve 99.99% mortality with a statistical confidence of 95% for all life stages of both species. Our effective treatment estimate is slightly less than the 56 °C for 30 min required to meet the criteria of ISPM15 with the use of conventional (i.e. non-dielectric) heating technology (IPPC 2016a).
Comparison with existing published heat treatment data
A review of the literature identified 13 direct heat treatment studies of bark- and wood-boring insects, either in wood or as naked insects (Table 1). Additional studies of bark- and wood-boring insects consider the effects of dielectric (microwave) heating (Kim and Suh 2014; Payette et al. 2015; Suh 2014), but these are beyond the scope of a comparison with results presented here as they rely on short-duration treatments of higher temperatures. Most species are represented by single studies; however, two invasive species that have established in the USA are represented by four (Agrilus planipennis) and three (Pityophthorus juglandis) independent studies. In many cases, no statistical basis, e.g. lethal temperature estimates (LTxx) or probit, is presented making comparisons between studies difficult. This diversity in treatment-by-time combinations and the lack of statistical modelling make it difficult to confirm the universality of a heat treatment schedule as direct comparisons between studies are challenging. Our results were consistent with many studies that have shown that the ISPM 15 requirements are adequate to control infested material or naked insects [Table 1, and discussion by Haack et al. (2014)]. However, some studies were not effective, e.g. Myers et al. (2009) and Nzokou et al. (2008) reporting effective temperatures to control A. planipennis of 63 and 65 °C, respectively, for 30 min. Although the minimum effective temperature tested by McCullough et al. (2007) to control A. planipennis was 60 °C, they only tested 20- and 120-min exposures with effective control only shown at the 120-min exposure. Hence, it is unclear from their study whether 56 °C for 30 min would be effective. Myers and Bailey (2011) also report a treatment at 58 °C for 30 min to effectively control Anoplophora glabripennis. A commonality between studies that did not show effective treatment at 56 °C was the use of infested wood material, i.e. log sections, firewood or chips. Myers et al. (2009) comment that non-uniform wood pieces, e.g. firewood, may have cold spots within them during treatment that might provide refugia. Hence, care is required when interpreting studies of infested log material as opposed to naked insects as the confounding effects of whether an insect was actually exposed to a particular temperature are potentially problematic.
Cost-effectiveness of heat as a potential phytosanitary treatment for export logs
New Zealand exported 17.4 M m3 of logs (predominantly Pinus radiata) in the year ending December 2016 (MPI 2018). The cost of implementing heat as an alternative phytosanitary treatment to existing chemical fumigants (e.g. methyl bromide) will be a function of the efficiency of the heating method, cost of heat energy (steam, electricity, etc.), and the initial capital costs of infrastructure. Setting aside the efficiency and initial capital costs, it is worth considering the target insects’ biology and how their log colonization processes influence the energy requirements for successful disinfestation of export logs. Bark- and wood-boring beetles are not evenly distributed within a log. Bark beetles colonize and their offspring subsequently feed in the phloem layer creating galleries at the interface between the bark and the sapwood (Sauvard 2004). Thus, effective heat treatments must only penetrate to the sapwood surface to control phloem-feeding bark beetle infestations. By contrast, wood borers and ambrosia beetles will often penetrate sapwood and at times the heartwood; however, their development during the typically short time between the harvest of healthy trees and application of phytosanitary treatments prior to export is limited, and entry deep into the wood unlikely. Knowledge of the potential boring depth of quarantine pests on export logs allows an estimate of the effective penetration depth required for heat, or any other, phytosanitary treatment. Romo et al. (In press) estimated that a phytosanitary treatment must penetrate at least 6.9 cm into the sapwood to effectively control A. ferus under a (21/12 °C: day/night) temperature treatment after 20 weeks of development. The temperature chosen reflects the 30-year annual summer normal in the warmest P. radiata-growing regions of New Zealand. The outer 6.9-cm annulus represents 73 and 55% of the total volume for average K & A grade P. radiata export logs, respectively (Ellis 2011). Hence, by integrating knowledge of pest biology it is possible to significantly reduce the overall energy required to use heat as an effective phytosanitary treatment, if you can apply it to the zone of infestation and not the entire log.
We can estimate the broad costs of treating log exports with heat, by assuming an average basic density of 439 kg/m3 (Palmer et al. 2013), an average green density of 1100 kg/m3 (Cown 1999), and a mean post-harvest moisture content of freshly harvested P. radiata of 151%. On this basis, excess heat treatment beyond the depth required to effectively kill insect species present on logs requires a minimum of 0.934 kWh/°C for each cubic metre of wood heated (using Pang et al.’s (1995) specific heat capacity of P. radiata). This represents an additional minimum cost of NZD $0.088 per m3 for every degree of temperature rise, assuming electrical heating with a $0.094/kWh average spot electricity price for a large industrial process user (MBIE 2015) and a hypothetical 100% efficiency in the conversion of electrical energy to heat in the log.
The actual cost will vary as 100% efficiency is unachievable in practice and the moisture content (and hence specific heat capacity) of the sapwood varies.
Joule heating is the most efficient way to rapidly heat logs (Perré 2004). With Joule heating, the majority of the heat is generated in the most electrically conductive portions of the timber. In Pinus radiata logs, the heartwood barely heats during Joule heating, due to its much lower electrical conductivity in comparison with the sapwood (Nursultanov et al. 2017). The heartwood also has a much lower specific heat capacity than the sapwood. Hence, the ratio of sapwood to heartwood, which is highly variable, has a significant effect on the energy requirement to raise either the whole log, or the sapwood portion of it, by a given temperature.
Early attempts at Joule heating of logs (Fleischer and Downs 1953) were ill-controlled as well as somewhat ad hoc and inconclusive. In fact, it has been found that electro-thermal computer modelling of the process, validated by laboratory testing, is the only way to fully understand the generation and diffusion of heat throughout the log, including energy transfers between the log and surrounding atmosphere (Nursultanov 2018).
Results from a 1-dimensional electro-thermal model (described in International (PCT) patent application PCT/NZ2018/050029), verified with a 3-dimensional computational fluid dynamics model in commercial package ANSYS-CFX, were used to determine the Joule heating regime employed on real P. radiata logs to achieve an average sapwood temperature of 75 °C (Nursultanov 2018). The model determined the required energy input and calculated the profile of the temperature rise along the log’s radius from pith to bark, during excitation periods (100 kW power applied) and relaxation (no power applied). It also calculated the electrical resistance of the log, providing real-time feedback to the constant power controller.
Laboratory testing on a sample of forty-two 3.3-m-long logs from two forest provenances (inland and coastal Canterbury), varying in volume from about 0.25 to 0.45 m3, with heartwood volumes ranging from about 10% to 35% of total volume, averaged 38 kWh per cubic metre (introduced at a constant power of 100 kW in three excitations lasting about 3 min each) for an average sapwood temperature rise of 50–60 °C (depending on initial log temperature), 30 min after the final excitation. Ten of the test logs were infested with H. ligniperda (adult and larvae) as a preliminary test of Joule heating and the efficacy of the laboratory LD99.99 estimates. All test subjects were killed during the heating process.
If correctly implemented and controlled, Joule heating as a technology is advantageous as the outer parts of the log (that are the most likely zone of infestation) can be made to heat first as the electrical conductivity of the sapwood is much higher than that of the heartwood and increases with temperature (Nursultanov et al. 2017). Thus, accurately estimating the required lethal temperature and what parts of a log must reach that temperature, and for what duration, area critical determinant of the cost-effectiveness of heat as a phytosanitary treatment for bulk commodities, such as export logs. Using the developed electro-thermal models, the process can be optimized for minimum energy use.
Summary
Our study of naked insects shows that 55.56 °C applied for 30 min is an effective phytosanitary treatment (LT99.99 with 95% confidence) for A. ferus and H. ligniperda. This estimate is within the range of other studies of bark- and wood-boring beetles. To increase the cost-effectiveness of heat for the treatment of bulk commodities, like logs, it is important to target heat treatment at the zones within a log that are potentially infested by quarantine pests. This can be done, to an extent somewhat determined by log species and heart/sapwood composition, extremely rapidly using Joule heating according to a computational electro-thermal model.
Author contribution statement
SP, EB, WH conceived and designed the research. JK and BO conducted the experiments, MB analysed the data. SP wrote the manuscript with contributions and approval from all authors.
References
Aukema JE, McCullough DG, Holle BV, Liebhold AM, Britton K, Frankel SJ (2010) Historical accumulation of nonindigenous forest pests in the continental United States. Bioscience 60:886–897
Brockerhoff EG, Hosking GP (2001) Arhopalus tristis (F.) (Coleoptera: Cerambycidae) (= Arhopalus ferus (Mulsant)): burnt pine longhorn beetle. For Timber Insects N Z 27(Revised):1–8
Brockerhoff EG, Bain J, Kimberley M, Knížek M (2006) Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Can J For Res 36:289–298. https://doi.org/10.1139/x05-250
Brockerhoff EG, Barratt BIP, Beggs JR, Fagan LL, Kay MK, Phillips CB, Vink CJ (2010) Impacts of exotic invertebrates on New Zealand’s indigenous species and ecosystems. N Z J Ecol 34:158–174
Cown DJ (1999) New Zealand pine and douglas-fir: suitability for processing. Rotorua New Zealand: Forest Research Bulletin 216. Forest Research
Dentener PR, Lewthwaite SE, Rogers DJ, Meier X, Whiting DC, McDonald RM (2001) Heat treatments for control of huhu beetle (Prionoplus reticularis larvae in logs). N Z J For Sci 31:273–286
Ellis JC (2011) A log volume formula for exporters. N Z J For 56:20–26
Eyre D, Haack RA (2017) Invasive cerambycid pests and biosecurity measures. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC Press, Boca Raton, pp 563–607
FAO (2016) Global forest products facts and figures (2016). http://www.fao.org/3/I7034EN/i7034en.pdf
Fleischer HO, Downs LE (1953) Heating veneer logs electrically. Technical report, United States Department of Agriculture, Forest Service, Forest Products Laboratory, Madison, WI
Goebel PC, Bumgardner MS, Herms DA, Sabula A (2010) Failure to phytosanitize ash firewood infested with emerald ash borer in a small dry kiln using ISPM-15 standards. J Econ Entomol 103:597–602
Haack RA et al (2014) Effectiveness of the international phytosanitary standard ISPM No. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS ONE 9:e96611. https://doi.org/10.1371/journal.pone.0096611
Hansen LS, Jensen KMV (1996) Upper lethal temperature limits of the common furniture beetle Anobium punctatum (Coleoptera: Anobiidae). Int Biodeterior Biodegrad 37:225–232
Heffernan B (2013) Practical application of joule heating to the sterilization of plantation grown Pinus radiata logs. Presentation to the Electricity Engineers’ Association of New Zealand, 19-21 June 2013, Auckland, New Zealand. http://www.epecentreacnz/docs/research/EEA%20Log%20Joule%20Heating%20Paper%20revF.pdf
Hoover K, Uzunovic A, Gething B, Dale A, Leung K, Ostiguy N, Janowiak JJ (2010) Lethal temperature for pinewood nematode, Bursaphelenchus xylophilus, in infested wood using microwave energy. J Nematol 42:101–110
Hosking GP, Bain J (1977) Arhopalus ferus (Coleoptera: Cerambycidae); its biology in New Zealand. N Z J For Sci 7:3–15
IPPC (2008) IPPC recommendation: replacement or reduction of methyl bromide as a phytosanitary measure. Appendix 6. Report of the Third Session of the Commission on Phytosanitary Measures, Rome, 7–11 Apr 2008, p 10
IPPC (2016a) ISPM 15: regulation of wood packaging material in international trade. 2013-04 CPM-8 adopted revised Annex 1 to ISPM 15 with consequential changes to Annex 2. Rome, IPPC, FAO. Publication history: Last modified June 2016
IPPC (2016b) ISPM 28: phytosanitary treatments for regulated pests. 2007-03 CPM-2 adopted standard. Adopted 2007; published 2016:11
Jones DP (2010) Biomedical sensors. Momentum Press, New York
Kim J, Suh SJ (2014) Lethal temperature against Japanese pine sawyer, Monochamus alternatus, in infested wood using microwave energy. J Agric Life Sci 48:33–40
Luna EK, Sitz RA, Cranshaw WS, Tisserat NA (2013) The effect of temperature on survival of Pityophthorus juglandis (Coleoptera: Curculionidae). Environ Entomol 42:1085–1091. https://doi.org/10.1603/EN13151
Mackes K, Costanzo T, Coleman R, Eckhoff M, Vaughan D (2016) Protocol for heat treating black walnut wood infested with walnut twig beetle. For Prod J 66:274–279. https://doi.org/10.13073/fpj-d-14-00082
Mayfield AE III, Fraedrich SW, Taylor A, Merten P, Myers SW (2014) Efficacy of heat treatment for the thousand cankers disease vector and pathogen in small black walnut logs. J Econ Entomol 107:174–184
MBIE (2015) Nominal annual average fuel prices. New Zealand Ministry for Business, Innovation, and Employment. http://www.med.govt.nz/sectors-industries/energy/energy-modelling/data/prices. Accessed 18 Aug 2015
McCullough DG et al (2007) Effects of chipping, grinding, and heat on survival of emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), in chips. J Econ Entomol 100:1304–1315
Meurisse N, Pawson S (2017) Quantifying dispersal of a non-aggressive saprophytic bark beetle. PLoS ONE. https://doi.org/10.1371/journal.pone.0174111
MPI (2016) Ephyto: electronic phytosanitary system. https://epcsp.maf.govt.nz/MAF.PhytoECert.Web/Home/Login?ReturnUrl=%2fMAF.PhytoECert.Web%2f. Accessed Nov 2016
MPI (2018) Forestry statistics: quarterly production and trade information, Ministry for Primary Industries, Wellington. https://www.mpi.govt.nz/news-and-resources/statistics-and-forecasting/forestry/. Accessed 8 Feb 2018
Mushrow L, Morrison A, Sweeney J, Quiring D (2004) Heat as a phytosanitary treatment for the brown spruce longhorn beetle. For Chron 80:224–228
Myers SW, Bailey SM (2011) Evaluation of a heat treatment schedule for the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). For Prod J 61:46–49
Myers SW, Fraser I, Mastro VC (2009) Evaluation of heat treatment schedules for emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 102:2048–2055
Nursultanov N (2018) Joule heating of green Pinus radiata logs for phytosanitary purposes: an in-depth investigation by experimentation and computational modelling. Chemical and Process Engineering Department, College of Engineering, University of Canterbury, New Zealand
Nursultanov N, Altaner C, Heffernan WJB (2017) Effect of temperature on electrical conductivity of green sapwood of Pinus radiata (radiata pine). Wood Sci Technol 51:795–809. https://doi.org/10.1007/s00226-017-0917-6
Nzokou P, Tourtellot S, Kamdem DP (2008) Kiln and microwave heat treatment of logs infested by the emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae). For Prod J 58:68–72
Palmer DJ, Kimberley MO, Cown DJ, McKinley RB (2013) Assessing prediction accuracy in a regression kriging surface of Pinus radiata outerwood density across New Zealand. For Ecol Manag 308:9–16. https://doi.org/10.1016/j.foreco.2013.07.024
Pang S, Keey RB, Langrish TAG (1995) Modelling the temperature profiles within boards during the high-temperature drying of Pinus radiata timber: the influence of airflow reversals. Int J Heat Mass Transf 38:189–205
Pawson SM, Watt MS, Brockerhoff EG (2009) Using differential responses to light spectra as a monitoring and control tool for Arhopalus ferus (Coleoptera Cerambycidae) and other exotic wood-boring pests. J Econ Entomol 102:79–85. https://doi.org/10.1603/029.102.0112
Pawson SM, Williams N, Gear I, Armstrong JW (2014) Reducing biosecurity business risks for logs and timber. N Z J For 59:36–42
Payette M, Work TT, Drouin P, Koubaa A (2015) Efficacy of microwave irradiation for phytosanitation of wood packing materials. Ind Crops Prod 69:187–196. https://doi.org/10.1016/j.indcrop.2015.01.030
Perré P (2004) Electrical heating of green logs using Joule’s effect: a comprehensive computational model used to find a suitable electrode design. Wood Sci Technol 38:429–449
Sauvard D (2004) General biology of bark beetles. In: Lieutier F, Day KR, Battisti A, Grégoire JC, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis. Kluwer, Dordrecht, pp 63–88
Sobek S, Rajamohan A, Dillon D, Cumming RC, Sinclair BJ (2011) High temperature tolerance and thermal plasticity in emerald ash borer Agrilus planipennis. Agric For Entomol 13:333–340
Suh SJ (2014) Lethal temperature for the black timber bark beetle, Xylosandrus germanus (Coleoptera: Scolytidae) in infested wood using microwave energy. Curr Res Agric Life Sci 32:131–134
R Development Core Team (2017) R: a language and environment for statistical computing. Version 3.3.3. R Foundation for Statistical Computing, Vienna, Austria. ISBN:3-900051-07-0. http://www.R-project.org/
Uzunovic A, Gething B, Coelho A, Dale A, Janowiak JJ, Mack R, Hoover K (2013) Lethal temperature for pinewood nematode, Bursaphelenchus xylophilus, in infested wood using radio frequency (RF) energy. J Wood Sci 59:160–170
Van Epenhijusen CW, Sommerfield KG, Hedderley D (2012) Rearing and storing Arhopalus ferus life stages in the laboratory for experimental purposes. N Z J For Sci 42:15–23
Wang Y et al (2003) Preliminary study on efficacy of heat treatment against Anoplopora nobilis in poplar wood. Plant Quar 17:326–329
Acknowledgements
The authors thank John Ellis of C3 Ltd. for providing additional log metric data to estimate the cost of applying heat as a phytosanitary treatment for New Zealand log exports. This work was funded by the Stakeholders in Methyl Bromide Reduction and the New Zealand Ministry for Business, Innovation and Employment via Scion’s core Funding (Contract C04X1104) through the Better Border Biosecurity Collaboration (www.b3nz.org).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Pawson and Heffernan have received research grants from Stakeholders in Methyl Bromide Reduction.
Ethical approval
All applicable New Zealand guidelines for the care and use of animals were followed.
Additional information
Communicated by J. D. Sweeney.
Special Issue on Invasive Pests of Forests and Urban Trees.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Number of individuals and replicates treated in each of phase 1 and 2 testing and the PT100 temperatures recorded per replicate in phase 2. Refer to excel file (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Pawson, S.M., Bader, M.KF., Brockerhoff, E.G. et al. Quantifying the thermal tolerance of wood borers and bark beetles for the development of Joule heating as a novel phytosanitary treatment of pine logs. J Pest Sci 92, 157–171 (2019). https://doi.org/10.1007/s10340-018-1015-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-018-1015-8