Abstract
Snails of the family Lymnaeidae are an essential link in the transmission of zoonotic diseases. Radix peregra is a European freshwater snail and a susceptible intermediate host of Fasciola hepatica, the causing agent of fascioliasis. Essential oils (EOs) extracted from Anethum graveolens (dill), Cuminum cyminum (cumin), Foeniculum vulgare var. vulgare (bitter fennel) and Petroselinum crispum (plain leaf parsley) were characterized by GC and GC–MS. Seven EOs and 11 constituents were first screened through a single-dose bioassay against R. peregra (10 mg L−1 for juveniles and 50 mg L−1 for egg masses and mature snails). EOs from parsley, cumin and bitter fennel (leaves plus stems) were highly active towards eggs and adults at 50 mg L−1. Subsequently, dose and time–lethality bioassays were performed against adults to determine lethal parameters (LC50;90 and LT50;90). Estimated 48 h LC50s varied from 13.7 to 46.5 mg L−1, with P. crispum fruits EO exhibiting the most significant activity. EOs from cumin fruits and bitter fennel infrutescences, and cuminaldehyde, were the most time-effective treatments when assessed by continuous exposure (LT50 for a 50 mg L−1 dose = 15.1, 19.3 and 19.5 h, respectively). A short-time exposure (8 h) to bitter fennel EOs was effective for the control of adults (LT50 ≤25 h). The present study uncovers the potential of four well-known Apiaceae species as natural sources of biomolluscicides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Key message
-
Plant species of the Apiaceae family remain undervalued with regard to their activity as biomolluscicides.
-
Bitter fennel, parsley, cumin and dill EOs are highly toxic against the freshwater snail Radix peregra, an alternative intermediate host of fascioliasis.
-
Estimated 48 h LC50 of the several EOs and compounds tested range from 13.7 to 46.5 mg L−1.
-
Parsley fruit EO exhibits the most significant dose effectiveness of the assayed EOs.
-
A short-time exposure (8 h) to two bitter fennel EOs was effective for snails’ control (LT50 of 23.0 and 25.7 h) in laboratory conditions.
-
EOs and compounds from the four studied Apiaceae have great potential as biomolluscicides.
Introduction
Snails of the family Lymnaeidae are freshwater pulmonate snails (Gastropoda: Pulmonata) that can inhabit a variety of wet and aquatic habitats, from large lakes to small temporary ponds (Watson and Dallwitz 2005). As intermediate hosts of plant-borne trematodes, lymnaeids species play a key role in the transmission of fascioliasis and others trematodiases of major medical and veterinary relevance. Fascioliasis, which is caused by species of the genus Fasciola L. (Digenea: Fasciolidae), is recognized as a major veterinary problem of vertebrate domestic livestock, causing a decrease in animals’ productivity and considerable economic losses (Correa et al. 2010).
Although the natural and primary intermediate host of Fasciola hepatica L. in Europe is Galba (sin. Lymnaea) truncatula Müller (Bargues et al. 2001), others lymnaeids from European populations have been identified as alternative intermediate hosts of this fasciolid under special natural conditions (Dreyfuss et al. 2000; Bargues et al. 2001). Climate changes and abnormal environmental phenomena may greatly influence host–parasite interactions, and by consequence the epidemiological patterns of fascioliasis and other trematodiases (Mas-Coma et al. 2009). The spreading power of fascioliasis is also related to the remarkable adaptive abilities of fasciolids to colonize new environments and to infect different intermediate and definitive hosts species all over the world (Mas-Coma et al. 2005). For instance, young specimens of Radix (sin. Lymnaea) peregra Müller (Lymnaeidae) were found to be susceptible to F. hepatica infection in laboratory conditions (Sindou et al. 1991; Dreyfuss et al. 1997). The prevalence of this species in some habitats where the G. truncatula is less abundant, and the occurrence of cattle fasciolosis in some of these areas, gives additional evidences of its potential as an alternative or secondary intermediate of the common liver flukes (Relf et al. 2009). In the Azorean island of S. Miguel, the density of R. peregra is effectively increasing in some locations where G. truncatula populations were previously found (Teixeira et al. 2011), including areas with identified cases of fascioliasis (Martins 1991). R. peregra may also display a role in the transmission of the giant liver fluke Fascioloides magna Bassi in cervids and domestic ruminants (Faltynkova et al. 2006), being also a facultative host of F. gigantica in near east, middle east and southern states of old USSR (Bargues et al. 2001).
As all plant-borne trematodes, including flukes, require aquatic intermediate hosts to accomplish part of their life cycle, the elimination of this essential link is the most effective way of preventing transmission of fascioliasis and others trematodiases (WHO 1995; Singh et al. 2010). The drainage of water bodies and/or treatment with synthetic molluscicides (i.e. niclosamide, sodium pentachlorophenate, copper sulphate, etc.) are possible measures stated by the World Health Organization for the control of these intermediate hosts (WHO 1995). Niclosamide is recommended to be used in management programs (WHO 1993); however, its toxicity to non-target organisms (from highly to very highly toxic to freshwater fishes) (USEPA 2013) rises some concerns. In order to mitigate the impact of molluscicides application on aquatic systems and organisms, several efforts have been developed to discover molluscicidal products of natural sources that could present lower risks (Singh et al. 2010). In that context, and considering the long history of uses, effectiveness, low cost and reduced toxicity, plants have been considered as ideal and sustainable sources of bioactive chemicals to control snails’ populations (WHO 1983; Singh et al. 1996). Plants species belonging to the Euphorbiaceae, Asteraceae, Leguminosae, Phytolaccaceae, Apocynaceae and Solanaceae families have been frequently assessed for this purpose (WHO 1983; Singh et al. 2005, 2010). The activity of EO or their constituents towards schistosomiasis and fascioliasis intermediate hosts, and pest snails have been confirmed in several studies (Lahlou and Berrada 2001; Kumar and Singh 2006; Jaiswal and Singh 2009; Mc Donnell et al. 2016). Nevertheless, Apiaceae (Umbelliferae) species remain undervalued with regard to their potential as freshwater snails’ control agents, despite their large spectrum of activities, namely against insect species (Yeom et al. 2012; Evergetis et al. 2013; Kim et al. 2013; Sousa et al. 2013).Thus far, extracts of Trachyspermum ammi (L.) Sprague, Ferula assafoetida L. and Carum carvi L. were formerly tested against the liver flukes’ natural host in Southern Asia, Lymnaea acuminata Lamark (Singh et al. 1997; Kumar and Singh 2006). However, to the extent of our knowledge, scientific reports concerning the potential of Apiaceae EOs for the control of trematode intermediate hosts are by far scarcest.
Hence, we herein propose to evaluate the molluscicidal potential of EOs and compounds from four well-known and worldwide cultivated Apiaceae species [Anethum graveolens L., Cuminum cyminum L., Foeniculum vulgare Mill. and Petroselinum crispum (Mill.) Nym ex A.W. Hill]. For this purpose, we performed screening assays against several developmental stages (eggs, juveniles and adults) of Radix peregra, a facultative intermediate host of trematodes in Europe, including Fasciola hepatica. Furthermore, dose- and time-related activities of EOs/compounds on the adult form of this freshwater snail were also investigated.
Materials and methods
Plant material and extraction of essential oils
Foeniculum vulgare subsp. vulgare var. vulgare (bitter fennel) culture was started from seeds collected from plant of a wild-growing population in north of Portugal (41°36′03.8′′N, 8°26′50.25′′W), and A. graveolens (dill) and P. crispum var. neapolitanum (plain leaf parsley) cultures were established from commercial seeds (packed by N.V. Somers, Co.). F. vulgare var. vulgare was identified based on both morphological and chemical characterizations, and voucher specimens (stems with leaves and inflorescences and fruits) are deposited at the University of Porto (Portugal) herbarium (accession number PO1000MFF). All plants were grown in an open-air experimental field. Fresh stems and leaves were collected from dill plants during the fruit pre-ripening stage and from bitter fennel plants during the vegetative phase. Green infrutescences bearing fully formed fruits were harvested from dill, parsley and bitter fennel plants, after 5, 8 and 14 months of growth, respectively. A total of 150–300 g of the aforementioned fresh samples was separately submitted to a 2-h hydrodistillation with 2–3 L of deionized water in a Clevenger-modified apparatus. EOs were collected and dried over anhydrous sodium sulphate before their storage in brown sealed vials at −20 °C. In addition, certified EOs from P. crispum (parsley) and C. cyminum (cumin) fruits were purchased from Sigma-Aldrich, Co (St. Louis, MO, USA) and included in the study.
Chemical analyses of essential oils
The quantitative and qualitative characterization of EOs was achieved by GC and GC–MS analyses as previously described (Sousa et al. 2013).
Chemicals
The following pure standard compounds that were identified in the EOs were included in bioassays: trans-anethole (99%), (S)-(+)-carvone (96%), cuminaldehyde (98%), estragole (98%), (+)-fenchone (99.5%), γ-terpinene (≥97%), (−)-β-pinene (99%), (−)-α-pinene and (+)-α-pinene (98%) were purchased from Sigma-Aldrich and Fluka (Aldrich chemical Co., St. Louis, MO, USA). The compounds myristicin (≥95%) and apiole (≥82%) were separated from parsley fruit EO by performing successive fractionations through gravity column chromatography as described in Sousa et al. (2013). The compound CuSO4 (pro-analysis grade, purity 99%, purchased from Merck) was included in assessments as a control molluscicide. Solvents (n-hexane, ethyl acetate and ethanol) were of reagent grade (≥99.9%).
Preparation of EOs/compounds emulsions
EOs/compounds were initially diluted in pure ethanol (300 mg mL−1). The dispersion of the oil fraction into a small volume of spring water was achieved using an ultrasonic homogenizer (Sonopuls HD 2200 from BANDELIN) coupled with a 3-mm microtip (amplitude 302 µm/ss; power 25–30%; time 5 s on–off cycles for 2 min) to obtain stock emulsions (33 mg EO per mL−1). Different volumes were pipetted, according to the doses to be tested, and vigorously mixed with spring water making up the final volumes used in bioassays (10 mL for eggs and juveniles and 220 mL for adults). Emulsions were left to stabilize 15 min before use. The spring water used in the preparation of emulsions is from S. Miguel Island (Povoação, Furnas, São Miguel Island, Azores Archipelago, Portugal) and commercialized under the trade name of “Gloria Patri” (water pH at 19 °C:6.95; total mineral content of 213 mg L−1; HCO3 −:81 mg L−1; SiO2:57 mg L−1, Na+:26 mg L−1; Cl−:14 mg L−1; K+:11.4 mg L−1; NO3 −:7.8 mg L−1; SO42−:5.6 mg L−1; Ca2+:4.9 mg L−1; Mg+:4.6 mg L−1).
Activity against Radix peregra
Animals
Radix peregra adult snails (with average shell length of 1.34 ± 0.20 cm) were caught in small freshwater bodies nearby livestock water tanks found in the Povoação locality (Southwest of São Miguel Island, Azores Archipelago, Portugal). Species identification has been confirmed by a malacologist, Professor Frias Martins A., and specimens (accession number DBUA-MT1533) are deposited in the molluscs’ collection of the Biology Department of the University of the Azores (Ponta Delgada, São Miguel, Portugal). Animals were maintained in a polyethylene aquarium (35.7 cm × 23.5 cm × 13.4 cm) containing 3 L of spring water permanently aerated with air pumps and fed with lettuce leaves (Lactuca sativa L.). Snails were acclimatized under laboratory conditions (natural photoperiod and 23 ± 1 °C) for at least 72 h before being used in bioassays. In order to prevent any contamination, the aquarium was cleaned three times a week by removing excrements and dead snails and by renewing the water. Sexually mature snails were kept in the aquarium for reproduction purpose, to obtain egg masses. Freshly laid egg masses (0–48 h old) were observed under the stereoscopic microscope to confirm their viability and ascertain their initial stage of embryonic development (4–8 cells) prior to the ovicidal assays. In addition, several egg masses were maintained under constant artificial aeration in a separated container until hatching. Juvenile snails of 48–72 h old were selected for the molluscicidal assays.
Ovicidal activity
R. peregra egg masses with less than 48 h were exposed to single-dose treatments of EOs/compounds (50 mg L−1 of spring water) to assess their ovicidal activity. Masses containing around 32 (±9.3) viable snail embryos were placed in freshly prepared emulsions (10 mL). Two sets of negative controls were included and performed simultaneously, one with spring water and the other with an ethanol aqueous solution in which the ethanol concentration was equivalent to that used in EOs/compounds’ emulsions tested (0.1 µL mL−1). Each treatment included at least six replicates (six-well plates). Egg masses were incubated under controlled temperature (23 ± 1 °C) and relative humidity (70 ± 5%) and exposed to natural photoperiod. Eggs were daily inspected to monitor their development. The ovidical activity of the treatments was evaluate by calculating the percentage of hatching (snail emerged from the capsule) 15 days later (time for which the hatching in the control groups was ≥95%). Embryos were considered dead whenever cells became disaggregated and/or if they remained unhatched at the end of the experiment.
Single-dose screening assays against juveniles and adults
EOs/compounds’ molluscicidal activity was assessed following the WHO guidelines for the immersion method (WHO 1983). A single-dose screening was performed with juveniles and adults using freshly prepared emulsions at 10 and 50 mg L−1 of spring water, respectively. Groups of ten juveniles were gently placed in six-well plates (ten per wells) with the help of a micropipette and a large tip. After removing the water, 10 mL of emulsions (10 mg L−1) was carefully dispensed in each well. Concerning the immersion of the adults, groups of ten snails were transferred into glass Petri dishes and distributed randomly by disposable plastic beakers containing 220 mL of EOs/compounds emulsions (at 50 mg L−1 of spring water). To prevent adults from falling out or escape, cups were covered with a perforated plastic film or a Petri dish. A positive control with copper sulphate was included in both assays at the same concentration used for EOs/compounds. Again, two sets of negative controls, one with spring water and the other with ethanol aqueous solution (0.02 mL L−1 for juveniles and 0.1 mL L−1 for adults), were performed simultaneously. Four to six replicates of ten snails per EOs/compounds/controls were included in each single-dose assay (40 ≤ n ≤ 60). Snails’ mortality was first determined after 24 h of exposure. The surviving snails were rinsed with spring water to remove emulsion residues, transferred into new cups containing an identical volume of spring water and fed with fresh lettuce leaves. Following a 24-h recovery period, the percentage of dead juveniles and adults was determined once again. The mortality was monitored by observation with a stereo microscope. Juveniles’ death was confirmed by absence of heartbeat, whereas adults were considered dead when the deterioration of the tissues was evident (foot discoloration and body fluid leakage) or if they failed to react when prodded (typical withdrawal movements). Experiments were carried out under natural photoperiod at 23 ± 1 °C and 70 ± 5% of RH.
Dose–response assay against adults (LCs)
Based on the information obtained from the single-dose assay, we established to determine the LC values of the most lethal treatments (whenever adults’ mortality >80%, after the recovery period). Besides, the compound (S)-(+)-carvone was also considered for further evaluations despite the first indication of its lower molluscicidal activity revealed in the preliminary assay. Owing to several reasons (high availability, lower cost and higher solubility in water) this oxygenated monoterpene exhibits great applicability in the context of plant-based biopesticides. Moreover, its bioactive potential has been shown and discussed in our previous work (Sousa et al. 2013).
The selected EOs and compounds were tested in the range of 10–60 mg L−1, following the procedures described in the previous sections and the WHO guidelines. The positive control (CuSO4) was tested at concentrations ranging from 0.1 to 1 mg L−1. To establish a dose–response relationship, four to six replicates of ten snails per concentration and at least four concentrations per EO/compound were used (160 ≤ n ≤ 300). The mortality percentage was recorded at 24 h of exposure and after the 24 h of recovery period to determine the concentrations causing 50 and 90% of lethality (LC50 and LC90).
Time–response assay against adults (LTs)
The evaluation of time-dependent lethality was performed with the most active EOs/compounds and (S)-(+)-carvone following the methodology above described. With this assay we assessed treatments’ lethality as a function of times of exposure, in order to ascertain whether or not treatments’ effectiveness was affected by the duration of exposure and in what extent. The concentration of 50 mg L−1 was chosen based on its proximity to most of the estimated LC90 values determined after the 24-h recovery period. Following the same criteria, the CuSO4 (positive control) was assessed at 0.75 mg L−1.
The time–lethality was assessed by continuous and short-term exposure (8 and 16 h). Adult snails that were exposed to treatments during 8 and 16 h were rinsed with spring water and left to recover in clean beakers containing fresh water and lettuce leaves. Subsequently, the mortality was monitored every 2 h or every hour if acuter effects were noticed. Snails submitted in continuation to treatments were monitored every 2 h, and the mortality percentage recorded up to 90% of death. In each assay, four replicates of ten individuals (n = 40) were used per treatment. For all three experiments, time started counting at the beginning of the exposure.
Statistical analysis
Data obtained in the single-dose assays (hatching and mortality percentages) were transformed by the arcsine function prior to the one-way analysis of variance (one-way ANOVA). Means of treatments were separated at 5% significance level by LSD test, available on IBM SPSS statistic package (version 20.0 for Windows, IBM corp., USA, 2011). Mortality percentages recorded in dose- and time-dependent experiments were corrected with the Abbott’s formula (Abbott 1925) whenever mortality in the negative control exceeded 5%. LC50 and LC90 after 48 h (24 h of exposure more 24 h of recovery) were determined by probit analysis according to Litchfield and Wilcoxon (1949), using IBM SPSS statistic package. The same linear model was applied to time–response data, to determine EOs/compounds LT50 and LT90 (times required to cause 50 and 90% of adults’ mortality) at the dose of 50 mg L−1. Pearson Chi-squared (χ 2) test was used to assess the goodness of fit. A good fit of data to the model was found whenever χ 2 values were lower than χ 2 critical values for n−2 degrees of freedom, at 5% of significance level. Treatments’ bioactivities were considered significantly different whenever the 95% confidence intervals of lethal concentrations or lethal times failed to overlap.
Results
Essential oils composition
The chemical characterization of bitter fennel, dill, parsley and cumin EOs used in the bioassays is presented in Table 1. The total number of compounds with relative content above 0.05% was variable among EOs (9–24), depending on the plant species, as well as on the plant organ. The overall identification achieved for EOs constituents was about 98.5–99.9%.
EOs obtained from different organs of F. vulgare var. vulgare presented a similar qualitative composition, although with substantial differences in their compound contents. Fv-L + S accumulated high contents of several monoterpenes hydrocarbons (pinenes and phellandrenes) and lower contents of phenylpropene derivatives (30%), whereas Fv-I was richer in oxygenated compounds (65% estragole and 16% of fenchone). EOs of A. graveolens followed the same general trend although with some several qualitative differences. A large fraction of Ag-I (67%) was constituted by (S)-carvone, contrarily to Ag-L + S where this compound was not detected. Dill ether (3,9-epoxy-1-p-menthene) was detected in both dill EOs from green freshly collected materials (Ag-I and Ag-L + S), although with different percentages (22% in leaves + stems and 1.3% in infrutescences). Furthermore, α-phellandrene and limonene mixed with β-phellandrene represented, together, a significant part in both dill EOs (70 and 28% in leave + stems and infrutescences, respectively), roughly the double of their corresponding sum in bitter fennel EOs. Some qualitative and quantitative differences were also found in parsley EOs. Both presented phenylpropene derivatives (myristicin and apiole) as major compounds and high contents of pinenes (total of 30 and 44% of the EO). Nevertheless, the amount of the hydrocarbon monoterpenic fraction was superior in Pc-I (total of 72%), while the phenylpropene derivatives was prevailing in Pc-F (59.4%). The cumin fruit EO (Cc-F) was mainly composed by cuminaldehyde (39%) plus several monoterpenes hydrocarbons accounting for 48% of the EO. Despite the difference of their qualitative profiles profile, Cc-F and Pc-F presented similar proportion of oxygenated/non-oxygenated compounds.
Ovicidal and molluscicidal activity
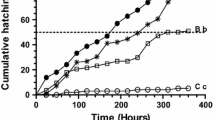
The lethal activities recorded for the seven EOs and eleven volatile compounds tested against R. peregra eggs, juveniles and adult are presented in Fig. 1a–c. In general, EOs from the four Apiaceae species and their major compounds displayed distinct toxicities against the embryonic and post-embryonic stages of R. peregra when tested at unique dosage. The ovicidal activities recorded for treatments were highly variable (Fig. 1a) and significantly distinct (F = 49.378, d.f. = 133, p = 0.000) (Online Resource 1). In particular, Pc-F, (−)-β-pinene, Cc-F, S-(+)-carvone, (−)-α-pinene, myristicin, (+)-α-pinene, cuminaldehyde, Pc-I and Fv-L + S (cited by decreasing order of activity) exhibited the most interesting hatching inhibitory effects (mean hatching values ≤25%). Nonetheless, only the first two treatments showed comparable activity to that of the positive control. Satisfactory or moderate ovicidal activities were attributed to the treatments Ag-I, (+)-fenchone, apiole, trans-anethole, Ag-L + S and estragole, for which the hatching ranged from 37 to 71%.
a–c Lethal activity of Anethum graveolens (Ag), Cuminum cyminum (Cc), Foeniculum vulgare var. vulgare (Fv) and Petroselinum crispum (Pc) essential oils (EOs) and 11 volatile compounds on Radix peregra embryos (a), juveniles (b) and adults (c). a Emergence of juveniles (hatching) after 15 days of exposure to 50 mg L−1 of EOs/pure constituents; b lethality of EOs/compounds against juveniles after 24 h of exposure to 10 mg L−1 followed by a 24-h recovering period (48 h); c lethality of EOs/compounds against adults after 24 h of exposure to 50 mg L−1 following a 24-h recovering period (48 h). Note: The final concentrations of ethanol (negative control) were of 0.02 mL L−1 for assay with juveniles and 0.1 mL L−1 for eggs and adults. The positive control (CuSO4) was tested at the same dose as EOs/compounds (10 mg L−1 against juveniles and 50 mg L−1 against eggs and adults). I infrutescences, L + S leaves plus stems, F fruits
Moreover, the newly emerged juveniles and adult snails showed different susceptibility to EOs and compounds (Fig. 1b, c). At the end of the experiments (48 h), mortalities varied from 3 to 59% (F = 42.624, d.f. = 102, p = 0.000) and 3 to 100% (F = 42.318, d.f. = 97, p = 0.000) for juveniles and adults, respectively (Fig. 1b, c) (Online Resource 1). We have observed moderate to noteworthy increases in lethality for the majority of compounds against juveniles and adults, respectively, during the recovery period (compare 24- and 48-h values in Fig. 1b, c). EOs from dill, parsley, cumin and bitter fennel (L + S), as well as the compound apiole, were the most active treatments against juveniles at 24 h. Cuminaldehyde attained a similar activity against juveniles after the recovery period. Consistently, these treatments were highly effective against adults. In addition, Fv-I and the compounds trans-anethole, estragole, γ-terpinene and (−)-β-pinene exhibited a satisfactory activity against adults (>70%).
Dose–response
The toxicity of seven EOs and six individual compounds against adults’ snails was dose dependent, which allowed the estimation of lethal concentrations within acceptable confidence limits (CLs) (Table 2). In general, most of the data fitted well in the assumptions of the linear model. However, results obtained for the control (CuSO4) and cuminaldehyde were less homogenous (higher H) resulting in a lower goodness of fit. Depending on the treatment, slopes of the log-probit regressions varied from 3.2 (±0.28) to 9.5 (±0.83). For the total period of the experiment (48 h), LC50 and LC90 values of EOs/compounds ranged from 13.7 to 46.5 and 29.2 to 83.1 mg L−1, respectively.
Based on LCs values and the non-overlapping of CLs we have established, with a probability of 95%, that Pc-F was the most toxic treatment (both LCs ‹ 30 mg L−1) 24 h after the interruption of the exposure, while S-(+)-carvone was the least toxic. By comparing the CLs of remaining treatments, we found that Pc-I, Fv-L + S, Ag-I and γ-terpinene were slightly more active than (−)-β-pinene, Ag-L + S, Fv-I, trans-anethole and estragole and significantly more lethal to adult snails than cuminaldehyde and Cc-F (LC50 of 34.7 and 38.8 mg L−1, respectively). Even though, when considering the LC90 parameter, the statistical differences concerning the toxicity of these treatments were less evident.
Furthermore, different slope values of the linear regressions have evidenced the higher sensitiveness of the snail population to small increases in the concentration of Cc-F, cuminaldehyde and estragole (Table 2). Consequently, inferior LC90/50 ratios were obtained for Cc-F, cuminaldehyde and estragole (1.3–1.5) comparatively to remaining ones (1.5–2.5) (Online Resource 3).
Time–response
The toxicity of seven EOs and six volatiles compounds against R. peregra varied in function with time (Online Resource 2) and the duration of exposure (Table 3). The LT50 calculated for the continuous, the 16-h and the 8-h exposure varied from 15.1–34.3, 20.1–32.5 (excluding carvone) and 23.0–134.7 h, respectively, while LT90 varied from 21.6–49.3, 24.6–54.5 and 33.0–271 h, respectively. Moreover, the lethal parameters after 8 h of contact for Ag-I, Ag-L + S, Cc-F, trans-anethole, cuminaldehyde and γ-terpinene could not be estimated within a reasonable time of observation, nor within acceptable confidence limits.
With basis on the determined LTs values (and respective 95% CLs) Ag-I, Cc-F, Fv-I, Fv-L + S, Pc-F, trans-anethole, cuminaldehyde and the positive control showed the acutest time-related activity on 50% of snails when these were continuously exposed (LTs50 below 22 h). With the exception of Pc-I and (S)-(+)-carvone, treatments were active against 90% of individuals within 21.6–38.8 h of exposure (with significant differences in their time effectiveness). Regarding the 16-h exposure assay, Cc-F, Fv-I, Fv-L + S, Ag-I, trans-anethole, cuminaldehyde, as well as the positive control, exhibited a 90% lethal effect within a significantly lower extent of exposure time (total of 24.6–32.1 h from the beginning of the experiment) relatively to the other treatments (37.7–54.5 h). Pc-I and (S)-(+)-carvone were found to be the least active treatment (LT90 of 49 h) when assessed by the 16-h exposure assay. In contrast, when considering the 8-h short-term exposure, only the positive control and both bitter fennel EOs (Fv-I and Fv-L + S) were still exhibiting the most satisfactory time-related activity, causing the death of 50% of snails within 23–25.6 h (total time). In opposition, Ag-I, Ag-L + S and γ-terpinene were considerably less effective through time, with accumulated mortalities inferior to 25% after 120 h of monitoring.
Discussion
In the current work, we found that EOs from parsley and cumin fruits, and the compounds myristicin, (S)-(+)-carvone, cuminaldehyde and pinenes affected eggs hatchability the most (Fig. 1a). Our study also demonstrated that exposure of embryos to several treatments, namely Pc-F, Cc-F, myristicin and (−)-β-pinene, caused extensive death, associated with a disintegrated structure, similarly to what has been described by other authors (dos Santos and Sant’ Ana 2001). Accordingly, we infer that the 50 mg mL−1 of these EOs/compounds displays an effective biocidal activity against snails’ embryos by disrupting embryogenesis. Although there are several aspects that may possibly have great influence on these results, it is most likely that embryos’ susceptibility to extracts/natural compounds is intrinsically related to the physical and chemical properties of each individual constituents, their ability to disperse into the gelatinous matrix of the egg mass and the nature of interactions (between compounds and the tissues/molecular targets). It is recognized that the lipophilic character of fragrance compounds and their affinity towards lipid-rich tissues is mainly responsible for the broad spectrum of biological activities (Lahlou 2004).
The EOs from dill, parsley, cumin and bitter fennel (L + S), as well as the compound cuminaldehyde and apiole, were the most active against both young and adult snails (Fig. 1b, c, respectively). On the other hand, we observed that lethality of treatments over juveniles and adults could differentially increase from the onset to the end of the recovery period (24 and 48 h) (Fig. 1b, c), as mortality rates of newly hatched snails and adults increased 1.2–3.2 times and 1.2–4.8 times, respectively (Online Resource 1). Several reports have shown that snails can be differentially susceptible to chemicals depending on their developmental stages. For instance, Euphorbia splendens and Phytolacca dodecandra are toxic to snails but have a low ovicidal activity at levels that are lethal to adults (McCullough et al. 1980; Schall et al. 1998). On the other hand, young snails of R. peregra and Biomphalaria alexandrina are more susceptible than elder ones to some plant EOs and methanol extracts, respectively (Teixeira et al. 2011; Bakry 2009). However, activity against both adult and egg stages is of upmost importance for an efficient control of host snail populations.
A time-dependent study was previously performed against R. peregra with EOs from plant species of the Macaronesia flora found in Azores (Teixeira et al. 2011). Therefore, we consider that a suitable assessment of molluscicidal activity of a given extract or compound may involve the respective dose-related evaluation associated with a time-dependent effect. Under this perspective, we conclude that Pc-F was the most effective product regarding the dose activity criteria, though its lethality was more lagged in time (Table 3). On the contrary, when compared to Pc-F, Cc-F induced its highest lethality level within short times despite its much lower dose-dependent lethality, as LC50 and LC90 values for Cc-F were 2.8 and 1.8 times greater, respectively, than Pc-F. Apparently, Cc-F impairs vital mechanisms more rapidly than Pc-F, resulting in overall failure of the organism. According to Price and Berry (2008), millimolar concentrations of EOs compounds have a blocking effect on action potentials, besides impairing the response to some neurotransmitters (octopamine, dopamine and acetylcholine). For instance, the compound myristicin, extracted from M. fragrans and herein found in parsley fruits (31.5%), was highly toxic to L. acuminata (LC50 ≤10 mg L−1) and established as a potent inhibitor of acetylcholinesterase (IC = 0.03 mM) and of acid phosphatase (0.07 mM) in (Jaiswal and Singh 2009). Even so, as it is true for most insecticides, the mode of action of a molluscicide should be a multi-component process affecting more than one system, which culminates in the death of the organism (McCullough et al. 1980; WHO 1983).
In general, EOs are recognized as non-persistent plant products owing to the low molecular weight (high volatility) and biodegradability of their constituents (Regnault-Roger et al. 2012). Changes of chemical profile over time are one of the many aspects that might have a severe impact on their biological effects and the overall effectiveness (Isman and Miresmailli 2010). Like any natural or synthetic compound, reliable results under field conditions might be compromised by the action of the biota. For example, extracts of P. dodecandra (Phytolaccaceae) and its active compound (oleanolic acid glucoside) are rapidly biodegraded with a consequent loss of the molluscicidal potency after a few days (McCullough et al. 1980). Likewise, depletion of some molluscicides (like niclosamide and copper-based compounds) takes place soon after their application into water (Muir and Yarechewski 1982; Francis-Floyd et al. 1997). Even though this could represent an advantage regarding environmental pollution, such rapid degradation may be a constraint for the application of molluscicides from natural origins. Eventually the use of controlled delivery device would be a good strategy to improve their solubility, ensure their stability and the slow release of these products. In addition, products application should also be examined regarding the potential development of heritable resistances under field conditions, especially if there is evidence of a large percentage of survival. Therefore, in this study we questioned whether there were possible differences in the recovering dynamics of R. peregra snails and opted to explore eventual loss of effectiveness when the exposure was shorter. In most cases (except for carvone and Pc-I) we confirmed that a 16-h exposure at a concentration of 50 mg L−1 might be sufficient to reduce 90% of R. peregra adult population (Table 3). Nevertheless, we verified that survival percentages following the 8-h exposure test were undesirably high for the majority of EOs/compounds (respective estimated LTs were moderately to considerably superior and statistically distinct to their counterparts determined for the other exposure times assays). Under these conditions (after 8 h of contact), only the bitter fennel EOs characterized herein (rich in estragole, fenchone and α-pinene and α- and β-phellandrenes) maintained their effectiveness at the tested concentration (Table 3). Indeed, a 8-h period of exposure to dill EOs, trans-anethole, cuminaldehyde or γ-terpinene (at 50 mg L−1) was not enough to cause acute toxicity and high mortality percentage in adults. In what concerns dill EOs, trans-anethole, cuminaldehyde and γ-terpinene our findings suggest that a 8-h period of contact was not enough to cause an irreversible state of toxicity and unrecoverable injuries to snails’ organism. We suppose that under the tested circumstances most individuals exposed to these natural plant products for 8 h were able to recover when transferred to clear water. These findings are consistent with those of Price and Berry (2008) who reported the reversible knockdown effects of the volatile compounds citral, geraniol and eugenol on Planorbis corneus after a brief 5-h exposure to acute lethal doses. The uptake and progressive accumulation of the active compound(s) into the snail body and/or their metabolization into more toxic forms by the organism enzymatic system have been suggested as possible events responsible for time-related toxicity (Kumar and Singh 2006; Jaiswal and Singh 2009).
In this work, we noticed that higher dosages elicited acute symptoms (increased mucous secretion and unresponsiveness to stimuli) in the first hours of exposure to EOs and compounds. Those symptoms were followed by swelling of the cephalopedal mass with haemolymph leakage, protrusion of the snail body, discoloration of the tissues and disintegration of the foot epithelium (Online resource 4). Such behavioural responses are in accordance with those described by several authors for pulmonate snails exposed to different plant extracts and constituents (McCullough et al. 1980; Lahlou and Berrada 2001; Price and Berry 2008). On the contrary, we observed that snails exposed to copper salt manifested strong avoiding behaviour by attempting to evade the solution and, in a second phase, an absence of responsiveness. It is worth mentioning that the activity of this copper-based molluscicide against R. peregra (LCs of CuSO4 ‹1 mg L−1) recorded herein was significantly higher than that of EOs and volatiles compounds (LC90 was 45–128 times superior). The LC50 of copper sulphate on R. peregra was very close to that determined against B. glabrata (48 h LC50 = 0.48 mg L−1) (de Oliveira-Filho et al. 2004).
The biocidal effects observed for EOs in each assay were somewhat consistent with the activity determined for the compounds tested. Cc-F and cuminaldehyde activities were coherent in terms of LCs, with parallel dose–mortality rates (same slopes) (Table 2), and ovicidal activity (Fig. 1a). Thus, cuminaldehyde reflected EO activity in both aspects, while γ-terpinene did not. This finding strongly indicates that most of the Cc-F activity is likely attributable to this major compound (cuminaldehyde) found at 39%, but also to β-pinene (12%), rather than to γ-terpinene, which constitute 16% of the cumin EO (Table 1). Nonetheless, we cannot rule out that other compounds (i.e. p-cymene, p-mentha-1,4-dien-7-al, etc.) comprised in the remaining part of the EO (1/3) could play a role in the dose and time effectiveness observed for the cumin fruits EO, by acting synergistically with cuminaldehyde. Interestingly, Pc-F and Pc-I showed comparable toxicity in terms of LCs and dose-dependent increases in snails’ mortality (Table 2), despite the considerable differences in their composition (Table 1). The results obtained in the single-dose assays indicate that the ovicidal activity of Pc-F was influenced by the pinenes and myristicin activities, while apiole seemed to have had a higher impact in the toxicity of parsley EOs against adults than against embryos (Fig. 1a, c). Contrasting results were also obtained for bitter fennel EOs, since Fv-L + S was significantly more active than Fv-I against eggs and juveniles. On the other hand, estragole was effective against adults but almost inactive towards eggs and juveniles (Fig. 1). This could explain, to a certain extent, why both EOs only showed equivalent effectiveness against the adult form regardless of their distinct quantitative profiles. This relation is evidenced by the coherent values of LCs and regression slopes exhibited by Fv-I EO and its main constituent estragole (65%), as shown in Table 2. On the contrary, we have found that the dose- and time-dependent toxicity of Ag-I against adults was not very consistent with the toxicity of (S)-(+)-carvone, which represents the major part of this EO (2/3), whereas both were coherent in terms of ovicidal activity. These results also evidence that R. peregra susceptibility to Ag-I and (S)-(+)-carvone is variable depending on the developmental stage and that toxicity towards adults is more likely to be attributed to other non-tested compounds, which were found in considerable amounts in both dill EOs, or a combination of these (α- and β-phellandrene and limonene accounted for 70 and 28% in leaves plus stems and infrutescences EOs, respectively). Little is known about the molluscicidal activity of phellandrenes, but limonene has been previously reported as a good molluscicide against L. acuminata (Kumar and Singh 2006; Agrahari and Singh 2013).
Conclusion
The present findings evidenced the strong potential of the tested EOs/compounds against the trematodes intermediate host, R. peregra. Interestingly, our results show that R. peregra susceptibility to EOs and compounds is variable and visibly reliant on its developmental stage. The estimated LC50 of the tested EOs (13.7–38.8 mg L−1) was below the threshold value of 40 mg L−1 that distinguishes the plant crude extracts with the highest molluscicidal potential and accordingly eligible to be employed against mollusc populations (WHO 1993, 1995; Singh et al. 1996). One key question in our work was: how long should the exposure to some of the most dose-active treatments last to trigger irreversible acute symptoms? Our results indicate that effectiveness can be achieved within a short time (at least 16 h of exposure for most treatments), which is clearly an appealing aspect of this type of alternative product, considering its high biodegradability and volatile nature. Moreover, the ovicidal effect recorded for EOs at levels that are lethal to adult snails is of great advantage. Thus, we suggest that the application of the studied EOs as natural molluscicidal agents could be a profitable natural alternative considering their significant bioactivity in a time-effective mode, the EO productivity of these plant species and the availability of these products in the market. For some EOs, the bioactivity recorded was related to the activity of the compounds included in the study. Therefore, we could consider the use of these constituents in artificial mixture in combination with other compounds to potentiate their activity. As a perspective for future work, and being aware of the influenced of several factors on the efficacy of EOs, we shall consider to perform some assays concerning the physical chemical stability of these products. Further studies, making use of controlled release systems, which are already been applied in some commercialized pesticides, are also under consideration.
Author Contributions
MF-F (coordinator), AC and JR provided the reagents and tools. RMS and JR designed and performed the experiments and data analysis. The manuscript was drafted by RMS. All authors reviewed and approved the manuscript.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Agrahari P, Singh DK (2013) Influence of abiotic factors on the molluscicidal activity of a bait containing limonene targeted at the pest snail Lymnaea acuminata. Int J Pest Manag 59(3):217–223
Bakry FA (2009) Use of some plant extracts to control Biomphalaria alexandrina snails with emphasis on some biological effects. Pestic Biochem Physiol 95:159–165
Bargues MD, Vigo M, Horak P, Dvorak J, Patzner RA, Pointier JP, Jackiewicz M, Meier-Brook C, Mas-Coma S (2001) European Lymnaeidae (Mollusca: Gastropoda), intermediate hosts of trematodiases, based on nuclear ribosomal DNA ITS-2 sequences. Infect Genet Evol 1:85–107
Correa AC, Escobar JS, Durand P, Renaud F, David P, Jarne P, Hurtrez-Boussès S (2010) Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of Fascioliasis. BMC Evol Biol 10(1):381
de Oliveira-Filho EC, Lopes RM, Paumgartten FJR (2004) Comparative study on the susceptibility of freshwater species to copper-based pesticides. Chemosphere 56:369–374
dos Santos AF, Sant’ana AEG (2001) Molluscicidal properties of some species of Annona. Phytomedicine 8:115–120
Dreyfuss G, Abrous M, Rondelaud D (1997) Fasciola hepatica Linné: la charge rédienne et les émissions cercariennes chez les juvéniles de Lymnaea peregra peregra Müller. Rev Med Vet 148(7):609–612
Dreyfuss G, Vignoles P, Rondelaud D (2000) Variability of Fasciola hepatica infection in Lymnaea ovata in relation to snail population and snail age. Parasitol Res 86:69–73
Evergetis E, Michaelakis A, Haroutounian SA (2013) Exploitation of Apiaceae family essential oils as potent biopesticides and rich source of phellandrenes. Ind Crop Prod 41:365–370
Faltynkova A, Horackova E, Hirtova L, Novobilsky A, Modry D, Scholz T (2006) Is Radix peregra a new intermediate host of Fascioloides magna (Trematoda) in Europe? Field and experimental evidence. Acta Parasitol 51:87–90
Francis-Floyd R, Gildea J, Reed P, Klinger R (1997) Use of bayluscide (Bayer 73) for snail control in fish ponds. J Aquat Anim Health 9(1):41–48
Isman MB, Miresmailli S (2010) Plant essential oils as repellents and deterrents to agricultural pests. In: Coats J, Paluch G (Eds.), Recent developments in invertebrate and vertebrate repellents. American Chemical Society Symposium Series 1090, pp 67–77
Jaiswal P, Singh DK (2009) Molluscicidal activity of nutmeg and mace (Myristica Fragrans Houtt.) against the vector snail Lymnaea acuminata. J Herbs Spices Med Plants 15:177–186
Kim S-W, Kang J, Park I-K (2013) Fumigant toxicity of Apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. J Asia Pac Entomol 16:443–448
Kumar P, Singh DK (2006) Molluscicidal activity of Ferula asafoetida, Syzygium aromaticum and Carum carvi and their active components against the snail Lymnaea acuminata. Chemosphere 63(9):1568–1574
Lahlou M (2004) Essential oils and fragrance compounds: bioactivity and mechanisms of action. Flavour Frag J 19(2):159–165
Lahlou M, Berrada R (2001) Potential of essential oils in schistosomiasis control in Morocco. Int J Aromather 11(2):87–96
Litchfield JT Jr, Wilcoxon R (1949) A simplified method of evaluating dose–effect experiments. J Pharm Exp Ther 96:99–113
Martins AMF (1991) Distribuicão dos moluscos de água doce em São Miguel e na Terceira. Açoreana 7:257–276
Mas-Coma S, Bargues MD, Valero MA (2005) Fascioliasis and other plant-borne trematode zoonose. Int Parasitol 35:1255–1278
Mas-Coma S, Valero MA, Bargues MD (2009) Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet Parasitol 16(3–4):264–280
Mc Donnell R, Yoo J, Patel K, Rios L, Hollingsworth R, Millar J, Paine T (2016) Can essential oils be used as novel drench treatments for the eggs and juveniles of the pest snail Cornu aspersum in potted plants? J Pest Sci 89(2):549–555
McCullough FS, Gayral P, Duncan J, Christie JD (1980) Molluscicides in schistosomiasis control. Bull World Health Organ 58(5):681–689
Muir DCG, Yarechewski AI (1982) Degradation of niclosamide (Bayer 73) in water and sediment samples. Int J Environ Anal Chem 6:1–14
Price DN, Berry MS (2008) Neurophysiological effects of naturally occurring defensive compounds on the freshwater snail Planorbis corneus: comparison with effects in insects. J Chem Ecol 34:994–1004
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424
Relf V, Good B, McCarthy E, de Waal T (2009) Evidence of Fasciola hepatica infection in Radix peregra and a mollusc of the family Succineidae in Ireland. Vet Parasitol 163:152–155
Schall VT, de Vasconcellos MC, Souza CP, Baptista DF (1998) The molluscicidal activity of Crown of Christ (Euphorbia splendens var. hislopii) latex on snails acting as intermediate hosts of Schistosoma mansoni and Schistosoma haematobium. Am J Trop Med Hyg 58(1):7–10
Sindou P, Cabaret J, Rondelaud D (1991) Survival of snails and characteristic lesions of Fasciola hepatica infection in four European species of Lymnaea. Vet Parasitol 40(1):47–58
Singh A, Singh DK, Mishra TN, Agarwal RA (1996) Molluscicides of plant origin. Biol Agric Hortic 13:205–252
Singh S, Singh VK, Singh DK (1997) Molluscicidal activity of some common spice plants. Biol Agric Hortic 14:237–249
Singh A, Singh SK, Yadav RP, Srivastava VK, Singh D, Tiwari S (2005) Eco-friendly molluscicides, piscicides and insecticides from common plants. In: Livingston JV (ed) Trends in agriculture and soil pollution research. Nova Science Publishers Inc., New York, pp 205–230
Singh SK, Yadav RP, Singh A (2010) Molluscicides from some common medicinal plants of eastern Uttar Pradesh, India. J Appl Toxicol 30:1–7
Sousa RMOF, Rosa JS, Oliveira L, Cunha A, Fernandes-Ferreira M (2013) Activities of Apiaceae essential oils against armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). J Agric Food Chem 61:7661–7672
Sousa RMOF, Rosa JS, Silva CA, Almeida MTM, Novo MT, Cunha A, Fernandes-Ferreira M (2015) Larvicidal, molluscicidal and nematicidal activities of essential oils and compounds from Foeniculum vulgare. J Pest Sci 88(2):413–426
Teixeira T, Rosa JS, Rainha N, Baptista J, Rodrigues A (2011) Assessment of molluscicidal activity of essential oils from five Azorean plants against Radix peregra (Müller, 1774). Chemosphere 87:1–6
USEPA (2013) Problem formulation for the environmental fate and ecological risk, endangered species and human health drinking water assessments in support of the registration review of TMF and niclosamide. Office of Chemical Safety and Pollution Prevention, Washington, DC, pp 1–68
Watson L, Dallwitz MJ (2005) [Online] The families of British non-marine molluscs (slugs, snails and mussels). URL:http://delta-intkey.com/britmo/www/lymnaeid.htm. Aassessed 24 Feb 2016
World Health Organization (1983) Reports of the scientific working group on plant molluscicides and guidelines for evaluation of plant molluscicides. World Health Organ. TDR/SCH-SWG (4)/83.3, p 11
World Health Organization (1993) Tropical disease research. Bull World Health Organ 830:1–86
World Health Organization (1995) Control of foodborne trematode infections. WHO Tech Rep Ser 849:1–157
Yeom HJ, Kang JS, Kim GH, Park IK (2012) Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). J Agric Food Chem 60:7194–7203
Acknowledgements
This work was supported by national funds (FCT—Portuguese Foundation for Science and Technology) under the project UID/AGR/04033/2013. RM Sousa was financially supported by the FCT through a PhD grant SFRH/BD/66041/2009. Authors are grateful to S. Chaves for the English language revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
Not applicable.
Additional information
Communicated by M. B. Isman.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sousa, R.M.O.F., Rosa, J.S., Cunha, A.C. et al. Molluscicidal activity of four Apiaceae essential oils against the freshwater snail Radix peregra . J Pest Sci 90, 971–984 (2017). https://doi.org/10.1007/s10340-017-0842-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0842-3