Abstract
Emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), is an invasive wood-boring beetle first detected in North America in 2002. Relatively little is known about the factors affecting the mating and oviposition behavior of EAB, even though they could have implications for the management of this species. Consequently, we conducted field surveys in Michigan to observe the diurnal behavior of EAB adults, in addition to examining how their behaviors associated with reproduction varied in relation to the size and condition of host trees. We observed most EAB adults between 11:00 and 15:00, and this pattern was the same for both sexes and for all of the specific behaviors examined (feeding, flying, mating, resting, and walking). Regarding host tree size and crown condition, we consistently found that the most EAB eggs, adults, mating pairs, and ovipositing females were observed on intermediately stressed trees with 40–60 % crown reductions (likely resulting from earlier EAB infestations). Additionally, host tree crown condition appeared to be a more important factor than diameter at breast height. Our results therefore provide support to the hypothesis that host tree crown (stressing) condition influences EAB oviposition behavior. Determining which trees are likely to contain the most EAB eggs should help to guide efforts for releasing and promoting the establishment of parasitoids utilized in biologic control. However, future work should attempt to experimentally test the hypothesis that host tree condition (e.g., crown reduction) drives EAB oviposition behavior, and investigate the fitness implications of these host choices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), is an invasive wood-boring beetle that is responsible for killing millions of ash trees (Fraxinus spp.) in North America after being accidentally introduced from northeastern Asia. First identified in the United States in Michigan in 2002 (Cappaert et al. 2005b; Poland and McCullough 2006), as of October 2013 EAB has spread to a further 20 states and two Canadian provinces (USDA-APHIS 2013). Future treatment and/or removal of affected ash trees is projected to cost more than $10 billion over the next decade (Kovacs et al. 2010), and the loss of ash stands will likely have a myriad of other ecological impacts (Gandhi and Herms 2010; Hausman et al. 2010; Ulyshen et al. 2011; Flower et al. 2013a). Consequently, suppressing populations of EAB will have clear benefits both economically and environmentally. However, we still know relatively little about the reproductive behavior of this species or how host trees affect their mating and oviposition behavior, which could have implications for predicting EAB spread and biologic control.
Understanding the life-cycle of EAB could provide some insight into what factors influence their reproductive behavior. Adult females produce around 100 eggs (Wei et al. 2007; Wang et al. 2010) which are oviposited into crevices in the bark of ash trees in the summer. After hatching, EAB larvae tunnel through the bark and begin feeding in the cambium for one (univoltine) or two (semivoltine) growing seasons (Cappaert et al. 2005a), where they form characteristic serpentine galleries (Bauer et al. 2004; Lyons et al. 2004). Both univoltine and semivoltine life-history strategies require larvae to develop through four instars before folding into a J-shape and overwintering via an obligatory diapause. In spring, larvae pupate and emerge as adults after around one month, where they proceed to feed on ash leaves for two to three weeks before females begin ovipositing. While EAB adult feeding typically does not cause any significant damage to ash trees, with sufficient larval galleries the flow of nutrients within the tree becomes restricted and can result in tree death in 3–4 years (Siegert et al. 2007).
For many insects whose offspring develop at the site of oviposition (as with EAB), host plant selection often can be a complex decision involving many factors, and it can have a considerable impact on egg and larval survival (Jaenike 1978; Thompson 1988; Hanks 1999). Among these different factors influencing oviposition, host size can be particularly important because larger plants might provide more resources for larvae (Hanks et al. 2005). Furthermore, hosts with a larger diameter at breast height (DBH) might confer more protection from some natural enemies (e.g., parasitoids) and increase larval survival (Abell et al. 2012). Alternatively, larger and more mature hosts could possess more effective defenses against larvae and be more attractive to certain predators (e.g., woodpeckers), making them suboptimal for oviposition (Boege and Marquis 2005).
Host plant growing condition can also be an important factor affecting oviposition behavior. For instance, host condition is known to affect oviposition in coleopterans, with stressed hosts apparently making more attractive sites for depositing eggs (Hanks et al. 2005; Timms et al. 2006; Tluczek et al. 2011). Indeed, some of this previous work has focused on EAB in particular and has shown that artificially stressed (girdled) (Tluczek et al. 2011), and naturally stressed (previously infested with EAB) (Timms et al. 2006) ash trees are more susceptible to EAB infestations (i.e., recruiting more EAB larvae). Further, leaves from stressed ash trees might be more attractive for adult females to feed on (Chen and Poland 2009). Thus, there is some evidence to suggest that host size and condition could also affect the distribution and abundance of EAB.
We had three specific objectives for the present study: (1) to observe how the mating, oviposition, and other behaviors displayed by EAB adults varied diurnally, (2) to investigate how the number of EAB eggs per tree varied with tree size and crown condition, and (3) to explore how certain EAB behaviors (mating and oviposition) varied with host size and crown condition. Because EAB females are thought to be phototactic and thermotactic (Lyons et al. 2009) and might be attracted to stressed trees (Timms et al. 2006; Tluczek et al. 2011), we predicted that EAB would most be active during the middle of the day and would more frequently select weakened trees as hosts for mating and oviposition.
Methods
Diurnal behavior
Observations of adult EAB diurnal activities were conducted on two sunny days (22 June and 13 July) in the summer of 2008. Based on previous studies (Brown-Rytlewski and Wilson 2005; Cappaert et al. 2005b) and our own anecdotal evidence, June through July appears to represent the pre-peak to peak oviposition period for EAB in southeastern Michigan. Prior to observations, 10 moderately infested (as determined by examining trunks for the presence of any D-shaped exit holes, epicormic growth, and woodpecker damage) green ash trees (Fraxinus pennsylvanica) (DBH range 10–25 cm), were located along the roadside of Grand River Avenue, East Lansing, Michigan. Beginning from 07:00 on each of the two observation days, the main trunks (along with any epicormic shoots) of each tree within 2 m above the ground were searched every hour by an observer for approximately 2 min, for the presence of EAB adults. EAB adults observed during the 2 min search were numerated and then collected with a sweeping net. Observations for each day ended at 19:00 in the early evening, which was primarily for convenience. We acknowledge that we are likely to have missed some EAB adults that were active outside of our period of observation and/or that were active higher up in the trees, but there is some evidence to suggest that these beetles might be most active during the day and on the lower sections of trees (Wei et al. 2007).
Egg survey
EAB egg surveys were conducted in natural ash stands located at three forest sites in Ingham County near Lansing, Michigan. Two sites were ~5 km from each other in Meridian Township (3–5 km east of Lansing) during the summer (18 June to 16 July) of 2009. Site one (42°43′N/84°25′W) included two contiguous Meridian Township, Michigan, parks (Central and Nancy Moore Parks). Site two (42°41′N/84°22′W) spanned two other Meridian Township parks (Harris Nature Center and Legg Park). The third site (42°34′N/84°36′W) was located in Holt, Michigan (~25 km south of Lansing) in the William M. Burchfield Park, approximately 32 km away from the Meridian Township study sites. Tree species composition and other characteristics of these three forests were previously described in studies by Duan et al. (2010, 2011, 2012a). Briefly, these sites were early to intermediate successional stage secondary-growth forests initially dominated by green ash (F. pennsylvanica). Other tree species present included white ash (F. americana), black ash (F. nigra), maple (Acer spp.), oak (Quercus spp.), black cherry (Prunus serotina), poplar (Populus spp.), black walnut (Juglans nigra), basswood (Tilia americana), and pine (Pinus spp.).
At each site, 20 green ash trees (F. pennsylvanica) (DBH range 7–35 cm) were randomly selected by walking 10 m wide belt transects in each forest. Upon selection for the EAB egg survey, the crown condition (CC) of each selected ash tree was then categorized to five classes according to the method utilized by Smith (2006) and Flower et al. (2013b). Class 1 crowns represent the healthiest ash trees with no or little EAB infestation while Class 5 crowns represent the worst ash trees with >80 % crown reduction mostly due to EAB infestations (mean CC ± SE = 3.02 ± 0.13). Freshly laid EAB eggs (with white to yellowish color) were then located by looking beneath flakes of bark on the trunk of each tree for 30 min (up to 2 m above the ground).
Mating and oviposition behavior
Observations of mating and oviposition behavior in relation to host tree CC were conducted on 40 landscaped ash trees (DBH range 7–37 cm) located in Hana Plaza center and the campus of Lansing Community College (both in East Lansing, Michigan). Thirty-seven of the trees were green ash (F. pennsylvanica) and three were white ash (F. americana). Observations were conducted on 6, 8, 9, 12, and 13 July 2009 when EAB adults were near or at the peak of mating and oviposition activities. At each observation time, the main trunk (up to 2 m above the ground) of each tree was searched by an observer for 5 min for the presence of mating and oviposition activities. The total numbers of mating pairs and/or ovipositing adults from each tree were then recorded and numerated.
Data analysis
All of our statistical analyses were conducted using R 2.15.1 (R Development Core Team 2010). We examined how ash tree CC, DBH, site, and date influenced the number of EAB eggs, total number of adults, mating pairs, and females ovipositing per tree. Data were first tested for normality using the Shapiro–Wilk test (package ‘stats’, function ‘shapiro.test’). None of the data met the assumptions of normality (all p < 0.001), and because they were also overdispersed, we tested their fit to a negative binomial distribution using Chi square tests based on residual deviance and degrees of freedom. The data fit the negative binomial distribution (all p > 0.05) and were then analyzed using generalized linear models (package ‘MASS’, function ‘glm.nb’). We compared fits for models with and without the predictor variable of interest (CC, DBH, site, or date) with Chi square tests based on log-likelihood ratios (package ‘car’, function ‘Anova’). Additionally, to compare pairwise differences in behaviors between all combinations of CC classes, we conducted separate multiple comparisons using log-likelihood ratio tests. Because there is no directly analogous R 2 available for negative binomial models, pseudo-R 2 values were calculated for the relationship between DBH and EAB behaviors using McFadden’s R 2 (package ‘pscl’, function ‘pR2’).
Results
Diurnal behavior
We observed a total of 230 EAB adults on trees, of which 159 were female and 71 were male. With the exception of one female resting on the trunk, all individuals were observed on foliage. The number of observations was highest between 11:00 and 15:00, and this trend was similar for males and females (Fig. 1a). Resting was by far the most frequently observed behavior (78.26 %), followed by flying to or from the trunk or epicormic shoots (10.43 %), feeding (7.39 %), mating (2.17 %), and walking (1.74 %). When examining the timing of these specific behaviors, resting (Fig. 1b), flying (Fig. 1c), and feeding (Fig. 1d) all followed the general pattern of observations, peaking between 11:00 and 15:00.
Egg survey
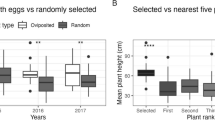
In our egg survey, we recorded a total of 705 EAB eggs (mean number of eggs per tree = 11.75 ± 1.38 SE). The number of eggs on ash trees was significantly affected by CC (χ 2 = 30.83, df = 4, p < 0.001), and trees with intermediately reduced crowns (CC 3) were found to host the highest mean number of eggs (16.44 ± 2.08 SE). No eggs were found on healthy trees (CC 1), which was significantly lower than all other CC classes (Table 1; Fig. 2a). Tree DBH appeared to have no effect on the number of eggs found (χ 2 = 0.21, df = 1, p = 0.646; Fig. 3a) and neither did site (χ 2 = 6.48, df = 3, p = 0.091) or sampling date (χ 2 = 2.58, df = 4, p = 0.630).
Relationships between crown condition (on a scale of 1–5, with 1 being healthy trees and 5 being trees with >80 % crown reduction) and EAB behaviors. Shown are: a the number of eggs laid, b the number of adults, c the number of mating pairs, and d the number of females ovipositing (all ± SE). Different lowercase letters represent crown condition classes that were significantly different from each other (p < 0.05)
Mating and oviposition behavior
We observed the behavior of 443 EAB adults. We found no significant differences in any EAB behaviors between ash species (all p > 0.05) and therefore data were pooled together for subsequent analyses. Both CC (χ 2 = 96.75, df = 4, p < 0.001) and site (χ 2 = 55.29, df = 3, p < 0.001) significantly affected the total number of EAB adults observed on trees. Specifically, the highest mean number of adults (5.95 ± 0.84 SE) was found on trees with considerably reduced crowns (CC 4). There were significantly fewer adults on healthy trees (CC 1) compared to all other trees with reduced crowns (i.e., all other CCs), but there were no significant differences between the number of adults on trees with CC 3 and 4, and CC 2 and 5 (Table 1; Fig. 2b). The total number of adults was not significantly affected by tree DBH (χ 2 = 0.79, df = 1, p = 0.374; Fig. 3b) or sampling date (χ 2 = 9.08, df = 4, p = 0.059).
The number of mating pairs per tree was significantly affected by CC (χ 2 = 55.02, df = 4, p < 0.001), site (χ 2 = 10.37, df = 3, p = 0.016) and sampling date (χ 2 = 14.94, df = 4, p = 0.005), but not tree DBH (χ 2 = 0.06, df = 1, p = 0.801; Fig. 3c). Trees with considerably reduced crowns (CC 4) had the highest mean number of mating pairs (1.42 ± 0.28 SE). There were no significant differences in the number of mating pairs found on trees with CC 1, 2, and 5, or between CC 3 and 4 (Table 1; Fig. 2c).
Oviposition behavior of EAB adults was significantly affected by CC (χ 2 = 44.71, df = 4, p < 0.001), site (χ 2 = 26.56, df = 3, p < 0.001), and sampling date (χ 2 = 9.61, df = 4, p = 0.048). Tree DBH did not affect the number of ovipositing females (χ 2 < 0.01, df = 1, p = 0.987; Fig. 3d). The highest mean number of females ovipositing was observed on trees with considerably reduced crowns (CC 4) (2.31 ± 0.34 SE). There were significantly fewer females ovipositing on trees with CC 1 compared to all other CCs, but we found no significant differences in the number of females ovipositing on trees with CC 2 and 5, or between CC 3 and 4 (Table 1; Fig. 2d).
Discussion
Our results provide support for the hypothesis that host tree growing condition (as measured by crown condition classes) is an important factor that could influence EAB oviposition and mating behavior. Trees with an intermediate to considerable reduction of crown coverage (i.e., CC 3 or 4) were consistently selected by EAB adults as hosts for oviposition and mating. EAB females might select trees in an intermediately stressed condition for oviposition for several reasons. For example, healthier trees could be expected to offer higher resistance, while larvae in the most heavily infested hosts will likely experience greater competition for limited resources (Hanks et al. 2005; Limback et al. 2010). Alternatively the patterns we observed could be explained by EAB females simply aggregating on the host trees from which they emerged, and presently we are not able to distinguish between these two competing hypotheses.
The influence of host condition on larval success likely depends on several other factors, such as competition, parasitism, and predation. For instance, Flaherty et al. (2011) studied larval success of the cerambycid Tetropium fuscum on red spruce (Picea rubens) and found that in the presence of natural enemies larvae had a longer development time, but grew larger and had higher survival on healthy trees (Flaherty et al. 2011). However, when T. fuscum larvae were protected from natural enemies, survival was highest on girdled trees. If applicable to EAB, such results could have implications for biologic control. In addition to several native parasitoids (Duan et al. 2009), three introduced parasitoids (Duan et al. 2010, 2012b) are presently being released to suppress EAB populations and are successfully establishing in some locations (Duan et al. 2013). Determining which trees are likely to contain the most EAB eggs will be particularly useful in guiding efforts for releasing the egg parasitoid Oobius agrili (Zhang et al. 2005; Duan et al. 2011, 2012b).
While we are not aware of any other studies that have quantified EAB host selection using the same methods presented here, our findings regarding host condition are generally consistent with previous work. For example, Timms et al. (2006), McCullough et al. (2009), Tluczek et al. (2011), and Flower et al. (2013b) all found higher numbers of EAB adults or larvae associated with more stressed trees. Furthermore, Ball and Simmons (1986) showed that larvae of another buprestid, the bronze birch borer (Agrilus anxius), were found at higher densities in weakened host trees. Our results indicating a non-significant relationship between DBH and EAB behaviors are also comparable to those of Lyons et al. (2009) and Marshall et al. (2013b). One explanation for the differing influences of tree size and crown condition could be that host selection is scale dependent (Saint-Germain et al. 2007, 2010). Host plant volatiles (associated with tree stress) could be attracting EAB over larger spatial-scales, but random chance (associated with tree size) could be more important at smaller spatial-scales.
We found considerable variation in EAB behaviors among sites, which might reflect differences in their infestation history and tree composition, as well as the bark roughness of trees present among other factors. We did not quantify bark roughness, but EAB females have been shown to prefer ovipositing on trees with rougher bark (Marshall et al. 2013b). There is also evidence for latitudinal variation in EAB body size and egg production (Marshall et al. 2013a), however, it is unlikely that our sites were far enough apart for this to be a significant factor. Additionally, there were significant differences in the numbers of adults and frequencies of behaviors among sampling dates, but these were not entirely unexpected because we anticipated that our observations would occur over the pre-peak and peak activity periods for EAB.
In terms of the diurnal behavior of EAB, we found that most adults were observed in the late morning and early afternoon. These findings are not surprising as EAB females are thought to be phototactic and thermotactic (Lyons et al. 2009), and previous observations have indicated that adults might be most active in the afternoon (Bauer et al. 2004). Similar to prior work, we also found that foliage was utilized as a location for mating behavior (Lelito et al. 2007), and it is possible that aggregating together for mating could increase the chances of multiple mating success for EAB (Rutledge and Keena 2012). These results provide further support for the idea that visual cues are important for EAB feeding and possibly mate location. However, further sampling is required to determine the generality of the patterns we observed and to specifically explore the influence of abiotic factors such as temperature.
While our results suggest that host tree crown condition is an important factor influencing EAB behavior, we acknowledge that our data are correlative and therefore we are not able to isolate cause and effect in these relationships. Consequently, it would be beneficial for future work to explicitly test our findings using manipulative experiments. Perhaps more importantly, future work should also attempt to unify the relationship between oviposition behavior and larval success in EAB. The results from the present study, and potentially those of our suggested future work, may also be used to help parameterize models predicting the future spread of EAB (Prasad et al. 2010; Mercader et al. 2011; Sobek-Swant et al. 2012).
References
Abell KJ, Duan JJ, Bauer L, Lelito JP, Van Driesche RG (2012) The effect of bark thickness on host partitioning between Tetrastichus planipennisi (Hymen: Eulophidae) and Atanycolus spp. (Hymen: Braconidae), two parasitoids of emerald ash borer (Coleop: Buprestidae). Biol Control 63(3):320–325. doi:10.1016/j.biocontrol.2012.08.009
Ball J, Simmons G (1986) The influence of host condition on post first instar development of the bronze birch borer, Agrilus anxius (Coleoptera, Buprestidae). Gt Lakes Entomol 19(2):73–76
Bauer LS, Haack RA, Miller DL, Petrice TR, Liu HP (2004) Emerald ash borer life cycle. In: Mastro V, Reardon R (eds) Proceedings of the emerald ash borer research and technology development meeting, Port Huron, Sept 30–October 1 2003, p 8
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20(8):441–448. doi:10.1016/j.tree.2005.05.001
Brown-Rytlewski DE, Wilson MA (2005) Tracking the emergence of emerald ash borer adults. In: Mastro V, Reardon R (eds) Proceedings of the emerald ash borer research and technology development meeting, Romulus, 5–6 Oct 2004, pp 13–14
Cappaert D, McCullouch DG, Poland TM (2005a) Emerald ash borer life-cycle: a reassessment. In: Mastro V, Reardon R (eds) Proceedings of the emerald ash borer research and technology development meeting, Romulus, 5–6 Oct 2004, pp 19–20
Cappaert D, McCullough DG, Poland TM, Siegert NW (2005b) Emerald ash borer in North America: a research and regulatory challenge. Am Entomol 51(3):152–165
Chen YG, Poland TM (2009) Biotic and abiotic factors affect green ash volatile production and emerald ash borer adult feeding preference. Environ Entomol 38(6):1756–1764
Duan JJ, Fuester RW, Wildonger J, Taylor PB, Barth S, Spichiger SE (2009) Parasitoids attacking the emerald ash borer (Coleoptera: Buprestidae) in western Pennsylvania. Fla Entomol 92(4):588–592. doi:10.1653/024.092.0409
Duan JJ, Ulyshen MD, Bauer LS, Gould J, Van Driesche R (2010) Measuring the impact of biotic factors on populations of immature emerald ash borers (Coleoptera: Buprestidae). Environ Entomol 39(5):1513–1522. doi:10.1603/en10023
Duan JJ, Bauer LS, Ulyshen MD, Gould JR, Van Driesche R (2011) Development of methods for the field evaluation of Oobius agrili (Hymenoptera: Encyrtidae) in North America, a newly introduced egg parasitoid of the emerald ash borer (Coleoptera: Buprestidae). Biol Control 56(2):170–174. doi:10.1016/j.biocontrol.2010.11.009
Duan JJ, Bauer LS, Abell KJ, van Driesche R (2012a) Population responses of hymenopteran parasitoids to the emerald ash borer (Coleoptera: Buprestidae) in recently invaded areas in north central United States. Biocontrol 57(2):199–209. doi:10.1007/s10526-011-9408-0
Duan JJ, Bauer LS, Hansen JA, Abell KJ, Van Driesche R (2012b) An improved method for monitoring parasitism and establishment of Oobius agrili (Hymenoptera: Encyrtidae), an egg parasitoid introduced for biological control of the emerald ash borer (Coleoptera: Buprestidae) in North America. Biol Control 60(3):255–261. doi:10.1016/j.biocontrol.2011.11.007
Duan JJ, Bauer LS, Abell KJ, Lelito J, Van Driesche R (2013) Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 106(3):1145–1154
Flaherty L, Sweeney JD, Pureswaran D, Quiring DT (2011) Influence of host tree condition on the performance of Tetropium fuscum (Coleoptera: Cerambycidae). Environ Entomol 40(5):1200–1209. doi:10.1603/en11114
Flower CE, Knight KS, Gonzalez-Meler MA (2013a) Impacts of the emerald ash borer (Agrilus planipennis Fairmaire) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol Invasions 15(4):931–944. doi:10.1007/s10530-012-0341-7
Flower CE, Knight KS, Rebbeck J, Gonzalez-Meler MA (2013b) The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For Ecol Manag 303:143–147. doi:10.1016/j.foreco.2013.04.017
Gandhi KJK, Herms DA (2010) North American arthropods at risk due to widespread Fraxinus mortality caused by the alien emerald ash borer. Biol Invasions 12(6):1839–1846. doi:10.1007/s10530-009-9594-1
Hanks LM (1999) Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu Rev Entomol 44:483–505. doi:10.1146/annurev.ento.44.1.483
Hanks LM, Paine TD, Millar JG (2005) Influence of the larval environment on performance and adult body size of the wood-boring beetle Phoracantha semipunctata. Entomol Exp Appl 114(1):25–34. doi:10.1111/j.0013-8703.2005.00225.x
Hausman CE, Jaeger JF, Rocha OJ (2010) Impacts of the emerald ash borer (EAB) eradication and tree mortality: potential for a secondary spread of invasive plant species. Biol Invasions 12(7):2013–2023. doi:10.1007/s10530-009-9604-3
Jaenike J (1978) Optimal oviposition behavior in phytophagous insects. Theor Popul Biol 14(3):350–356. doi:10.1016/0040-5809(78)90012-6
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in US communities, 2009–2019. Ecol Econ 69(3):569–578. doi:10.1016/j.ecolecon.2009.09.004
Lelito JP, Fraser I, Mastro VC, Tumlinson JH, Boroczky K, Baker TC (2007) Visually mediated ‘paratrooper copulations’ in the mating behavior of Agrilus planipennis (Coleoptera: Buprestidae), a highly destructive invasive pest of North American ash trees. J Insect Behav 20(6):537–552. doi:10.1007/s10905-007-9097-9
Limback CK, McCullouch DG, Chen YG, Poland TM, Cregg BM (2010) Host vigor and emerald ash borer larvae on green and white ash. In: Lance D, Buck J, Binion D, Mastro V, Reardon R (eds) Proceedings of the emerald ash borer research and technology development meeting, Pittsburgh, 20–21 Oct 2009, pp 45–47
Lyons DB, Jones GC, Wainin-Keizer K (2004) The biology and phenology of the emerald ash borer, Agrilus planipennis. In: Mastro V, Reardon R (eds) Proceedings of the emerald ash borer research and technology development meeting, Port Huron, Sept 30–Oct 1 2003, p 5
Lyons DB, de Groot P, Jones GC, Scharbach R (2009) Host selection by Agrilus planipennis (Coleoptera: Buprestidae): inferences from sticky-band trapping. Can Entomol 141(1):40–52. doi:10.4039/n08-045
Marshall JM, Miller MA, Lelito JP, Storer AJ (2013a) Latitudinal variation in body size of Agrilus planipennis and relationship with fecundity. Agric For Entomol 15(3):294–300. doi:10.1111/afe.12017
Marshall JM, Smith EL, Mech R, Storer AJ (2013b) Estimates of Agrilus planipennis infestation rates and potential survival of ash. Am Midl Nat 169(1):179–193
McCullough DG, Poland TM, Cappaert D (2009) Attraction of the emerald ash borer to ash trees stressed by girdling, herbicide treatment, or wounding. Can J For Res 39(7):1331–1345. doi:10.1139/x09-057
Mercader RJ, Siegert NW, Liebhold AM, McCullough DG (2011) Influence of foraging behavior and host spatial distribution on the localized spread of the emerald ash borer, Agrilus planipennis. Popul Ecol 53(2):271–285. doi:10.1007/s10144-010-0233-6
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J For 104(3):118–124
Prasad AM, Iverson LR, Peters MP, Bossenbroek JM, Matthews SN, Sydnor TD, Schwartz MW (2010) Modeling the invasive emerald ash borer risk of spread using a spatially explicit cellular model. Landsc Ecol 25(3):353–369. doi:10.1007/s10980-009-9434-9
Rutledge CE, Keena MA (2012) Mating frequency and fecundity in the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Ann Entomol Soc Am 105(1):66–72. doi:10.1603/an11037
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.r-project.org
Saint-Germain M, Buddle CM, Drapeau P (2007) Primary attraction and random landing in host-selection by wood-feeding insects: a matter of scale? Agr For Entomol 9(3):227–235. doi:10.1111/j.1461-9563.2007.00337.x
Saint-Germain M, Buddle CM, Drapeau P (2010) Substrate selection by saprophagous wood-borer larvae within highly variable hosts. Entomol Exp Appl 134(3):227–233. doi:10.1111/j.1570-7458.2009.00960.x
Siegert NW, McCullouch DG, Liebhold A, Telewski FW (2007) Resurrected from the ashes: a historical reconstruction of emerald ash borer dynamics through dendrochronological analysis. In: Mastro V, Lance D, Reardon R, Parra G (eds) Proceedings of the emerald ash borer and Asian longhorned beetle research and technology development meeting, Cincinnati, Oct 29–Nov 2 2006, pp 18–19
Smith A (2006) Effects of community structure on forest susceptibility and response to the emerald ash borer invasion of the Huron River watershed in southeast Michigan, MS thesis. Ohio State University, Columbus
Sobek-Swant S, Kluza DA, Cuddington K, Lyons DB (2012) Potential distribution of emerald ash borer: what can we learn from ecological niche models using Maxent and GARP? For Ecol Manag 281:23–31. doi:10.1016/j.foreco.2012.06.017
Thompson JN (1988) Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol Exp Appl 47(1):3–14
Timms LL, Smith SM, de Groot P (2006) Patterns in the within-tree distribution of the emerald ash borer Agrilus planipennis (Fairmaire) in young, green ash plantations of south-western Ontario, Canada. Agr For Entomol 8(4):313–321. doi:10.1111/j.1461-9563.2006.00311.x
Tluczek AR, McCullough DG, Poland TM (2011) Influence of host stress on emerald ash borer (Coleoptera: Buprestidae) adult density, development, and distribution in Fraxinus pennsylvanica trees. Environ Entomol 40(2):357–366. doi:10.1603/en10219
Ulyshen MD, Klooster WS, Barrington WT, Herms DA (2011) Impacts of emerald ash borer-induced tree mortality on leaf litter arthropods and exotic earthworms. Pedobiologia 54(5–6):261–265. doi:10.1016/j.pedobi.2011.05.001
USDA-APHIS (2013). Emerald ash borer. http://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b
Wang X-Y, Yang Z-Q, Gould JR, Zhang Y-N, Liu G-J, Liu E-S (2010) The biology and ecology of the emerald ash borer, Agrilus planipennis, in China. J Insect Sci 10(128):1–23
Wei X, Wu Y, Reardon R, Sun TH, Lu M, Sun JH (2007) Biology and damage traits of emerald ash borer (Agrilus planipennis Fairmaire) in China. Insect Sci 14(5):367–373. doi:10.1111/j.1744-7917.2007.00163.x
Zhang YZ, Huang DW, Zhao TH, Liu HP, Bauer LS (2005) Two new species of egg parasitoids (Hymenoptera: Encyrtidae) of wood-boring beetle pests from China. Phytoparasitica 33(3):253–260. doi:10.1007/bf02979863
Acknowledgments
We thank Tim Watt, Tony Capizzo, and Jane Slater (all USDA-ARS student interns) for assistance in the egg survey. We also are grateful to Doug Luster and Roger Fuester (USDA-ARS), and four anonymous reviewers for helpful comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Jennings, D.E., Taylor, P.B. & Duan, J.J. The mating and oviposition behavior of the invasive emerald ash borer (Agrilus planipennis), with reference to the influence of host tree condition. J Pest Sci 87, 71–78 (2014). https://doi.org/10.1007/s10340-013-0539-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-013-0539-1