Abstract
In this study, a triazine-based polymeric network-modified magnetic nanoparticles (TPN/MNPs) was synthesized and applied as adsorbent for extraction of 1-naphthylacetic acid (NAA) from the fruit and vegetable samples followed by HPLC determination. The synthesized TPN/MNPs were characterized by the vibrating sample magnetometry (VSM), Fourier-transform infrared spectroscopy (FT-IR), field emission-scanning electron microscopy (FE-SEM) and thermogravimetric analysis (TGA). The factors influencing the extraction efficiency including pH, sorbent amount, salt concentration, extraction time, sample volume and desorption conditions were studied and optimized. Under the optimal conditions, good linearity was obtained in the range of 1.0–500 µg L−1. The limit of detection (LOD) and limit of quantification (LOQ) were 0.3 µg L−1 and 1.0 µg L−1, respectively. The relative standard deviations (RSDs) of intra-day and inter-day were less than 9.5% at 10 and 100 µg L−1 of NAA. The method was successfully applied for extraction and determination of NAA in the fruit and vegetable samples including cucumber, tomato and apple and good recoveries were obtained (94.5–96.5%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Naphthylacetic acid (NAA) is a naphthalene-derived plant growth regulator which is widely used in fruits and vegetables. Therefore, its residue in the plant is harmful to human and animal health due to its potential toxicity [1]. Several methods such as high-performance liquid chromatography with ultraviolet detector [2] and/or fluorescence detector [3], the gas chromatography (GC) with flame ionization detector [4], spectrofluorimetry [5], room temperature phosphorescence (RTP) [6, 7] have been applied for the determination of NAA.
However, sample treatment is still necessary for most applications. Several sample pretreatment methods such as solid-phase extraction (SPE) [8], miniaturized dispersive solid-phase extraction (MDSPE) [9], solid-phase microextraction (SPME) [10], dispersive liquid–liquid microextraction (DLLME) [11] and molecularly imprinted solid-phase extraction (MISPE) have been developed for extraction of NAA [12].
Recently, MSPE, a new type of solid-phase extraction based on magnetic sorbents with high extraction efficiency and convenient operation, has attracted significant attention [13]. In MSPE, the sorbents can be uniformly dispersed into the sample solution by vortexing or shaking that provides sufficiently large contact area between the sorbents and the analytes to ensure a fast mass transfer. Therefore, high extraction efficiency in a short time, which is desirable in high throughput sample preparations, can be obtained [14]. Magnetic nanoparticles (MNPs), generally Fe3O4 or γ-Fe2O3, are used as sorbents in MSPE. Compared to traditional SPE sorbents, MNPs possess high surface area and have unique magnetic properties. When MNPs are introduced to the solution, the equilibrium between sorbents and sample solutions can be reached quickly [16]. After extraction, MNP sorbents enriched with analytes are readily separated from suspension by applying an external magnetic field. Therefore, in contrast to the traditional cartridge SPE, MSPE eliminates the column packing and possible blockage during use. However, bare MNPs can easily aggregate that can alter their stability and extraction capacity [15].
Up to date, functionalized MNPs such as carbon nanotube (CNT) [1], graphene oxide (GO) [17], polypyrrole polymer [18], and β-cyclodextrin (β-CD) [9] have been used for extraction of the analyte. Hence, finding new and suitable functional groups for preparation of efficient MNP-based sorbents for extraction of analytes such as NAA is underway. In recent years, microporous organic polymers (MOPs) that formed by the polymerization of organic building blocks have attracted much research interest due to the combined superiority of porous materials and functional polymers. MOPs have well-defined porosity, high surface area and tunable surface chemistry. Furthermore, they are lightweight and can be easily functionalized [19]. In recent years, MOPs have been utilized to extract the hydroxylated polycyclic aromatic hydrocarbons [20], 5-nitro-imidazoles [21], imidacloprid and 2,4-dichlorophenoxyacetic acid [22], perfluorinated compounds [23] and for removal of methyl orange [24]. Poly(melamine–terephthalaldehyde) network (TPN) is a triazine-based MOP with high amount of amine functional groups that can absorb several cations. Therefore, if the surface areas and porosity of TPNs to be high, interesting applications for such TPNs are expected due to the presence of abundant amine groups on the pore of the walls. Functionalization of MNPs with MOPs could enhance chemical stability of MNPs and introduce porosity on the MNPs surface with proper functional groups that these properties can improve these adsorbents efficiency [24].
In this study, triazine-based polymeric network-modified magnetic nanoparticles (TPN/MNPs), which contain abundant amine groups, were synthesized and applied for the first time for extraction of NAA, as carboxylic acid containing analyte. The factors affecting the extraction efficiency such as pH, sorbent amount, salt concentration, extraction time, sample volume and desorption conditions were optimized. Finally, the proposed method was applied to determine NAA in cucumber, tomato and apple samples.
Experimental
Reagents and Chemicals
1-Naphthylacetic acid (NAA) was purchased from the Sigma–Aldrich (St. Louis, MO, USA). Ferric chloride (FeCl3.6H2O), ferrous chloride (FeCl2.4H2O), ammonia solution (28%), melamine, terephthaldehyde, dimethyl sulfoxide (DMSO), tetrahydrofuran (THF), dichloromethane, acetonitrile, methanol, acetone and acetic acid were purchased from Merck (Darmstadt, Germany). Stock standard solution of NAA at 1000 mg L−1 was prepared in acetonitrile and kept at 4 °C in a refrigerator. The working solution were prepared by proper dilutions of the stock solution with distilled water.
Instruments
Chromatographic analysis was performed on a Knauer HPLC system (Berlin; Germany) consisting degasser, a K-1100 HPLC quaternary pump, a manual injector with 20 µL sample loop and a K2600 UV detector. Data were processed by EZChrom software. The separation was performed on an analytical C8 column (5 µm, 4.6 mm i.d × 25 cm) (Knauer, Germany). Mixture of acetonitrile and water (60:40, v/v) was used under isocratic elution condition as mobile phase at flow rate 1.0 mL min−1. Finally, detection wavelength was set at 220 nm.
Field emission-scanning electron microscopy (FESEM) images were obtained by MIRA3 FESEM (Tescan, Czech Republic). The Fourier-transform infrared (FT-IR) spectra were recorded using a Thermo Nicolet Nexus 870 FT-IR spectrometer (Madison, USA). In addition, Thermogravimetric analysis (TGA) was performed on a NETZSCH TG 209 F1 (Selb, Germany). The magnetic properties were measured by vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir, Kashan, Iran).
Synthesis of Triazine-Based Polymeric Network-Modified Magnetic Nanoparticles
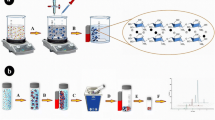
Iron oxide nanoparticles were prepared according to previously reported method [25]. Briefly, 2.4 g FeCl2.4H2O and 5.4 g FeCl3.6H2O were dissolved in 20 mL deionized water under nitrogen atmosphere with vigorous stirring using a mechanical stirrer. 25 mL NH3.H2O (28 wt%) was added dropwise to the solution. Then, the solution was heated at 70 °C for 5 h. The temperature was increased to 85 °C for vaporizing residual ammonia. The resulting black dispersion was isolated via a magnet, and washed with deionized water. The magnetic nanoparticles were modified by triazine-based polymeric network (TPN) through in-situ method as previously reported [24]. Briefly, 0.939 g melamine, 1.5 g terephthaldehyde and 45 mL DMSO were stirred and degassed by argon bubbling. The mixture was heated to 180 °C under the inert atmosphere. After 48 h, 1.5 g Fe3O4 nanoparticles were added and the reaction was continued for another 24 h. After it was cooled to room temperature, the resultant precipitation was collected by magnet, washed one time with 20 mL DMSO, acetone, THF and dichloromethane. Finally, the obtained TPN/MNPs were dried under vacuum at 60 °C. The preparation procedure of TPN/MNPs is shown in Fig. 1.
MDSPE Procedure
The MDSPE procedure is shown in Fig. 1. Initially, 50 mL of the sample solution containing NAA (100 µg L−1) was transferred to a beaker, and pH was adjusted to 6.5. Then, 10 mg of TPN/MNPs was added. Then, solution was shaken on a shaker at 200 rpm for 10 min to reach the equilibrium. Subsequently, a strong magnet was placed at the bottom of the beaker and TPN/MNPs were isolated from the solution. After about 1 min, the solution became limpid and the supernatant was decanted. The adsorbed analyte was desorbed with 500 µL of acetonitrile by shaking for 5 min. Finally, the supernatant was collected and 20 µL of the solution was injected into HPLC system for analysis.
Sample Preparation
The samples including cucumber, tomato and apple were purchased from local market (Tehran, Iran). The samples were ground and homogenized. 5.0 g of the homogenized samples was accurately weighed and 10 mL acetonitrile:water (20:80, v/v) was added. The samples were sonicated for 10 min and filtered with a 0.45 μm filter. Then, the filtered samples were diluted to 50 mL with distilled water.
For the spiked samples, proper volumes of standard solutions of NAA were added to the blank samples and then prepared as the mentioned procedure.
Results and Discussion
Characterization
Figure 2a shows FTIR spectra of the MNP and TPN/MNPs. In FTIR spectrum of MNPs, the band at 3390 cm−1 is attributed to O–H stretching vibration and a band at 572 cm−1 corresponds to Fe–O bond. The FTIR spectrum of TPN/ MNPs showed a broad coupling band around 3400 cm−1 related to N–H groups of TPN and O–H groups in surface of MNPs. The bands at 1554 cm−1 and 1485 cm−1 correspond to C=N group of TPN, and a band at 575 cm−1 corresponds to Fe–O [23].
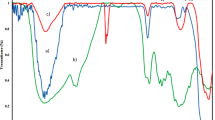
Figure 2b shows FE-SEM images of MNPs and TPN/MNPs. FE-SEM image of MNPs shows the nanoparticles have uniform size distribution. Some particles are agglomerated. The size of nanoparticles is 22–50 nm. It can be seen that TPN/MNPs sizes are 26–70 nm. The size of TPN/MNPs increased that may be attributed to modification with TPN. TGA curves of MNPs and TPN/MNPs are shown in Fig. 2c. In the TGA curve of MNPs, the total weight loss over the full temperature range is 1.6%, due to the loss of absorbed water and dehydration of the surface-OH groups [26]. In the TGA curve of TPN/MNPs, the weight loss 100 °C is attributed to the absorbed water loss. Finally, 53% weight loss in the range of 100–800 °C is probably due to decomposition of TPN.
Magnetization curves of MNPs and TPN/MNPs are shown in Fig. 2d. The saturation magnetization of the MNPs and TPN/MNPs are 70 and 31 emu/g, respectively. The saturation magnetization of TPN/MNPs decreased which is might be due to the present of TPN. However, it is enough for magnetic separation.
Optimization of MSPE Procedure
The parameters influencing the extraction efficiency including pH, extraction time, salt concentration, amount of adsorbent, and desorption conditions were investigated and optimized. The experiments were performed in triplicate. Distilled water spiked with 100 μg L−1 of NAA was used for optimization of parameters. All quantifications were done based on the peak area.
Effect of pH
The effect of pH on the extraction efficiency was studied in the range of 2.5–8.5. Based on Fig. 3a, when the pH increased from 2.5 to 6.5, the extraction efficiency increased and then decrease gradually at pH higher than 6.5. This result could be interpreted based on the pKa of the NAA which is around 4.5 [9]. The ionization of analyte occurred when pH exceeds pKa of the analyte. In the same conditions, amine groups of TPN/MNPs are protonated that causes electrostatic attractions between the NAA and TPN/MNPs surface. When pH increased above 6.5, the positive charge density on the surface of TPN/MNPs could be decreased which as result of that the extraction efficiency decreased. Moreover, extraction can be driven by hydrogen bonding [24].
Effect of Salt Concentration
The effect of salt concentration was investigated in the range from 0 to 10% (w/v) (Fig. 3b). The results indicated that the extraction efficiency decreased with increasing salt concentrations due to increasing the viscosity of the solution that causes difficult mass transfer [26]. Moreover, electrostatic interaction of chloride ions with positive sites of adsorbent can reduce the interaction of analyte with adsorbent. Therefore, the extraction efficiency decreased [27]. Therefore, no salt was added into the solution in subsequent experiments.
Effect of the Adsorbent Amount
The effect of the adsorbent amount on the extraction efficiency was studied in the range of 2.5–15 mg. As shown in Fig. 3c, extraction efficiency increased with increasing absorbent amount up to 10 mg due to increase of accessible sites of the adsorbent. The extraction efficiency decreased with further increasing of the adsorbent amount which is due to incomplete elution of the analyte by the fixed volume of eluent [28]. Therefore, 10 mg was selected for subsequent experiments.
Effect of Extraction Time
The extraction time was studied in the range of 2–15 min. Because of short diffusion route and magnetically assisted separation of the MNPs from the sample solutions, extraction of analyte can be achieved in shorter time even for larger volumes of samples. As shown in Fig. 3d, the extraction time increased by increasing time up to 10. After that, it remained almost constant. Thus, the extraction time of 10 min was selected [29].
Desorption Conditions
Various solvents such as methanol, acetone, acetonitrile and methanol-acetic acid were used for desorption of analyte from the adsorbent surface (Fig. 3e). Among these solvents, acetonitrile had highest desorption efficiency that might be due to polarity of solvent and the solvent is aprotic [1]. The adsorbent can interact with analyte through electrostatic attraction and hydrogen bonding. Also, acetonitrile can disrupt the interaction between the analyte and the adsorbent.
The volume of acetonitrile was investigated from 200 to 1000 µL. The desorption efficiency increased with increasing of the volume of acetonitrile up to 500 µL and then decreased as a result of dilution effect [28]. Therefore, 500 µL of acetonitrile was selected as the optimum volume. In addition, desorption time was investigated from 1 to 10 min and the results are shown in Fig. S1. The highest desorption efficiency was obtained when time was 5 min.
Effect of Sample Volume
The effect of sample volume on extraction efficiency was studied by different sample volumes (25–150 mL) containing 5.0 µg of analyte. As shown in Fig. 3f, the extraction efficiency decreased with enhancing the sample volume from 50 to 150 mL that can be as a result of more difficult transfer of the analyte from the sample solution to the surface of the sorbent because of decrease in the amount of sorbent in volume unit of the sample [27]. Therefore, sample volume of 50 mL was selected as optimum volume.
Method Validation
The analytical characteristics of the proposed method such as linearity, limit of detection (LOD), limit of quantification (LOQ) and preconcentration factor (PF) are summarized in Table 1. The linearity was in the range of 1.0–500 µg L−1 and determination coefficient (R2) was 0.9973. LOD and LOQ, calculated as 3 and 10 times the signal to noise ratio, were 0.3 µg L−1 and 1.0 µg L−1, respectively. The PF is defined as the ratio of the aqueous sample volume (50 mL) to the eluent volume (0.5 mL) which is 100. The EF was 90. The relative standard deviations (RSDs) of intra-day (repeatability, n = 6) and inter-day (reproducibility, n = 3) were less than 9.5% at 10 and 100 µg L−1 of NAA.
Analysis of Real Samples
The proposed method was applied for the determination of NAA in cucumber, tomato, and apple. The results are summarized in Table 2. 6.1 µg L−1 of NAA was detected in the apple sample. The trueness of the method was evaluated by analysis of the samples spiked with the analyte known amounts of the analyte. Samples analysis was performed before and after spike three times. The recoveries were in the range of 94.5–96.5% with RSDs in the range of 3.6–9.8. Figure 4 shows the chromatograms of NAA in the apple sample prior to and after the spiking with 20 and 40 μg L−1 of NAA.
Conclusion
In the present study, triazine-based polymeric network-modified magnetic nanoparticles (TPN/MNPs) were successfully synthesized and applied as efficient adsorbent for extraction of NAA. TPN/MNPs contains amine groups and can form hydrogen bond and electrostatic interaction with NAA. The adsorbent possess porosity, high surface area and good chemical stability. The magnetic separation greatly improved the separation rate and reduced the analysis time. The results indicated that TPN/MNPs can be used as efficient adsorbent for extraction of NAA in fruit and vegetable samples.
The proposed method was compared with other published methods. As shown, based on the results in Table 3, this the method has wide linear range. In addition, this method has lower LOD in comparison with other methods [8,9,10, 12]. The advantage of magnetic separation is easy separation and adsorbent can be isolated from the sample solution rapidly by applying an external magnetic field. The method is simple and requires low amount of organic solvent. The proposed method has lower extraction time in comparison with the other reported methods [9, 10]. Also, the adsorbent has high adsorption capacity.
References
Li WK, Chen J, Zhang HX, Shi YP (2017) Selective determination of aromatic acids by new magnetic hydroxylated MWCNTs and MOFs based composite. Talanta 168:136–145
Maiti B, Desai SR, Krishnamoorthy TS (1988) Determination of naphthaleneacetic acid residue in apples by high-performance liquid chromatography. Analyst 113(4):667–668
Moye HA, Wheaton TA (1979) Determination of naphthaleneacetic acid (NAA) in orange, tangerines, and processed products: high performance liquid chromatography with fluorometric detection. J Agric Food Chem 27(2):291–294
Zweig G, Gutnick DL, Gulli R, Archer TE, Hartmann HT (1964) Residue determination of naphthaleneacetic acid in olives. J Agric Food Chem 12:59–61
Vilchez JL, Blanc R, Navalón A (1997) Simultaneous spectrofluorimetric determination of 1-naphthylacetic acid and 1-naphthalenacetamide in commercial formulations and soil samples. Talanta 45(1):105–111
Murillo Pulgarín JA, Fernández López P, García Bermejo LF, Martín Alfonso F (2003) Fast kinetic determination of 1-naphthylacetic acid in commercial formulations, soils and fruit samples using stopped-flow phosphorimetry. J Agric Food Chem 51(22):6380–6385
Segura Carretero A, Cruces Blanco C, Ales Barrero F, Fernández Gutiérrez A (1998) Method for the quantitative determination of 1- naphthaleneacetic acid in spiked canned pineapple samples by micelle-stabilized room temperature phosphorescence. J Agric Food Chem 46(2):561–565
Wang ZH, Xia JF, Han Q, Shi HN, Guo XM, Wang H, Ding MY (2013) Multi-walled carbon nanotube as a solid phase extraction adsorbent for analysis of indole-3-butyric acid and 1-naphthylacetic acid in plant samples. Chin Chem Lett 24(7):588–592
Li N, Chen J, Shi YP (2016) Magnetic reduced graphene oxide functionalized with β-cyclodextrin as magnetic solid-phase extraction adsorbents for the determination of phytohormones in tomatoes coupled with high performance liquid chromatography. J Chromatogr A 1441:24–33
Han XF, Chen J, Shi YP (2018) N-doped carbon nanotubes-reinforced hollow fiber solid-phase microextraction coupled with high performance liquid chromatography for the determination of phytohormones in tomatoes. Talanta 185:132–140
Lu Q, Chen L, Lu M, Chen G, Zhang L (2010) Extraction and analysis of auxins in plants using dispersive liquid–liquid microextraction followed by high-performance liquid chromatography with fluorescence detection. J Agric Food Chem 58(5):2763–2770
Yan H, Wang F, Han D, Yang G (2012) Simultaneous determination of four plant hormones in bananas by molecularly imprinted solid-phase extraction coupled with high performance liquid chromatography. Analyst 137(12):2884–2890
Nekoeinia M, Kabiri Dehkordi M, Kolahdoozan M, Yousefinejad S (2016) Preparation of epoxidized soybean oil-grafted Fe3O4–SiO2 as a water dispersible hydrophobic nanocomposite for solid-phase extraction of rhodamine B. Microchem J 129:236–242
de Souza KC, Andrade GF, Vasconcelos I, Viana IMO, Fernandes C, Sousa EMB (2014) Magnetic solid-phase extraction based on mesoporous silica-coated magnetic nanoparticles for analysis of oral antidiabetic drugs in human plasma. Mater Sci Eng C 40:275–280
Ye L, Wang Q, Xu J, Shi ZG, Xu L (2012) Restricted-access nanoparticles for magnetic solid-phase extraction of steroid hormones from environmental and biological samples. J Chromatogr A 1244:46–54
Tahmasebi E, Yamini Y, Seidi S, Rezazadeh M (2013) Extraction of three nitrophenols using polypyrrole-coated magnetic nanoparticles based on anion exchange process. J Chromatogr A 1314:15–23
Chen J, Cao S, Zhu M, Xi C, Zhang L, Li X, Wang G, Zhou Y, Chen Z (2018) Fabrication of a high selectivity magnetic solid phase extraction adsorbent based on β-cyclodextrin and application for recognition of plant growth regulators. J Chromatogr A 1547:1–13
Miah M, Iqbal Z, Lai EPC (2012) Rapid CE-UV evaluation of polypyrrole-coated magnetic nanoparticles for selective binding of endocrine disrupting compounds and pharmaceuticals by aromatic interactions. Anal Methods 4(9):2866–2878
Li Q, Razzaque S, Jin S, Tan B (2017) Morphology design of microporous organic polymers and their potential applications: an overview. Sci China Chem 60(8):1056–1066
Zhou L, Hu Y, Li G (2016) Conjugated microporous polymers with built-in magnetic nanoparticles for excellent enrichment of trace hydroxylated polycyclic aromatic hydrocarbons in human urine. Anal Chem 88(13):6930–6938
Yang W, Wu X, Liu T, Wang T, Hou X (2018) A triazine-based conjugated microporous polymer composite for magnetic solid phase extraction of 5-nitroimidazoles coupled with UPLC-MS/MS for quantification. Analyst 143(23):5744–5753
Moradi Shahrebabak S, Faraji M, Saber-Tehrani M, Aberoomand-Azar P (2019) Simultaneous magnetic solid phase extraction of acidic and basic pesticides using triazine-based polymeric network modified magnetic nanoparticles/graphene oxide nanocomposite in water and food samples. Microchem J 146:630–639
Ren JY, Wang XL, Li XL, Wang ML, Zhao RS, Lin JM (2018) Magnetic covalent triazine-based frameworks as magnetic solid-phase extraction adsorbents for sensitive determination of perfluorinated compounds in environmental water samples. Anal Bioanal Chem 410:1657–1665
Faraji M, Shabanian M, Aryanasab F (2018) Efficient removal of anionic dyes from aqueous media using newly in situ synthesized triazine-based nitrogen-rich network modified magnetic nanoparticles. J Iran Chem Soc 15(3):733–741
Shabanian M, Khoobi M, Hemati F, Khonakdar HA, Ebrahimi SES, Wagenknecht U, Shafiee A (2015) New PLA/PEI-functionalized Fe3O4 nanocomposite: preparation and characterization. J Ind Eng Chem 24:211–218
Poursaberi T, Hassanisadi M, Torkestani K, Zare M (2012) Development of zirconium (IV)-metalloporphyrin grafted Fe3O4 nanoparticles for efficient fluoride removal. Chem Eng J 189–190:117–125
Tahmasebi E, Yamini Y (2012) Facile synthesis of new nano sorbent for magnetic solid-phase extraction by self assembling of bis-(2,4,4-trimethyl pentyl)-dithiophosphinic acid on Fe3O4@Ag core@shell nanoparticles: Characterization and application. Anal Chim Acta 756:13–22
Tahmasebi E, Yamini Y, Mehdinia A, Rouhi F (2012) Polyaniline-coated Fe3O4 nanoparticles: an anion exchange magnetic sorbent for solid-phase extraction. J Sep Sci 35(17):2256–2265
Tahmasebi E, Yamini Y, Moradi M, Esrafili A (2013) Polythiophene-coated Fe3O4 superparamagnetic nanocomposite: synthesis and application as a new sorbent for solid-phase extraction. Anal Chim Acta 770:68–74
Acknowledgements
This study researchers would appreciate the Standard Research Institute and Islamic Azad University research for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sepideh Moradi Shahrebabak would declare that she would not have any conflicts of the interests. Mohammad Faraji would also declare that he would have not any conflicts of the interests. Moreover, Mohammad Saber-Tehrani would disclose that he would not have any conflicts of interests. In addition, Meisam Shabanian discloses that he would not have any conflicts of interests. Finally, Parviz Aberoomand-Azar would declare that he would not have any conflicts of interests.
Ethical approval
The present paper did not include any research on the humans or animals subject.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moradi Shahrebabak, S., Faraji, M., Saber-Tehrani, M. et al. Triazine-Based Polymeric Network-Modified Magnetic Nanoparticles (NPs) as an Efficient Sorbent to Extract 1-Naphthylacetic Acid in Fruit and Vegetable Samples. Chromatographia 83, 863–871 (2020). https://doi.org/10.1007/s10337-020-03905-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03905-z