Abstract
A simple and effective method using ion chromatography was developed for the simultaneous determination of five monosaccharides (arabinose, glucose, fructose, xylose, and ribose) and two disaccharides (sucrose and lactose) in raw sugar samples. The separation was performed on a CarboPac PA 10 column using the gradient elution of sodium hydroxide and water as the mobile phase. Monosaccharides and disaccharides were detected by an integrated pulsed amperometric detection (IPAD) using gold working electrode. Acid hydrolysis was used for sample preparation before the analysis of glucose and fructose. All the studied sugars showed good linear ranges within 0.5–100 µg mL−1 with the correlation coefficients higher than 0.997. The limits of detection were all less than 0.5 µg mL−1. The RSDs of the method were less than 10 %. The recoveries of the sugars that spiked in raw sugar samples ranged from 96.1 to 102.4 %. The method was successfully applied for the analysis of sugars in raw sugar samples. Sucrose is the major constituent found in the samples at 97 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugar is a carbohydrate that occurs naturally in many kinds of food such as milk, fruits, vegetables, cereals, and grains. Sugar is an important nutrient that supplies energy to the body. In Thailand, sugar is made from sugar cane. Raw sugar is an intermediate product in cane sugar production. It is brown and coarse granulated product obtained from the evaporation of clarified sugar cane juice. The raw sugar producers ship this product to a refinery for final processing. However, people cannot eat raw sugar even though it is high in sucrose (about 98 %) because it may contain bacteria, molds, insect parts, and impurities [1]. Raw sugar consists of sugar and non-sugar materials. Several sugars are found in raw sugar including sucrose, glucose, fructose, arabinose, xylose, lactose, and ribose. Sucrose is the main constituent of the sugar material. Non-sugar materials are inorganic acid, carboxylic acid, amino acid, protein, starch, and wax, etc [2].
Manufacturers partially refine raw sugar to remove the impurities and sell the product as white sugar. The determination of sugars in sugar industry is of importance since it is concerned to all stages of production including the receipt of raw materials, throughout the processing (quality control) and for the final products (storage). In sugar industry, the degradation of sucrose during sugar processing is still a problem [3]. In addition, the content of sugars in different types of sugar products (such as white sugar, brown sugar, and raw sugars) is very useful for both nutrition application and commercial purpose. The analysis of sugars in raw sugar is a challenging task, because of the large different quantity of sucrose and the other sugars found in these samples.
Among various analytical techniques that have been used for the analysis of sugars, both gas chromatography(GC) and liquid chromatography (LC) are the most widely used techniques [4–8]. Besides high sensitivity and selectivity, chromatographic techniques also provide the ability for simultaneously determine multiple carbohydrates (sugars). However, GC requires derivatization of sugars before analysis. HPLC with refractive index detection is a common method used in sugar analysis; however, it is limited by poor sensitivity and selectivity. Although, HPLC equipped with UV–vis detection provides more selectivity, it is complicated and time consuming for the derivatization of sugars to facilitate the detection. Up to now, high-performance anion-exchange chromatography equipped with integrated pulsed amperometric detection (HPAEC-IPAD) is a direct method for simultaneous analysis of sugars. It has been applied successfully for the determination of sugars in various samples such as environmental sample [9], wood [10], serum [11], artichokes [12], and ryegrass [13]. However, there is no report on the analysis of sugars in raw sugar samples. As previously mentioned, a large different quantity of the interest analytes are concerned, and therefore sample preparation is required. Various sample preparation methods have been used for the analysis of raw sugar samples such as dialysis method [14–19], matrix elimination [10], ion-exchange resin (cartridge and column) [21, 22], and hydrolysis [23–26]. All of these methods have both advantages and disadvantages. However, all of the mentioned methods except hydrolysis method have disadvantages from the complicated operating procedure and time consuming. Hydrolysis is a chemical reaction of a compound with water, usually resulting in the formation of one or more new compounds. The hydrolysis of sucrose gives glucose and fructose. There are two methods for hydrolysis of sucrose namely acid hydrolysis [24, 26] and enzyme hydrolysis [27, 28]. Enzymatic hydrolysis is difficult to control and sometimes results in the damage and decrease in the enzyme efficiency. Acid hydrolysis is easier for the operating procedure, less time consuming, and cheaper than enzyme hydrolysis.
The aim of this work was to develop a simple method for the determination of sugars in raw sugar. The method consisted of direct analysis of sucrose and some minor constituent sugars and acid hydrolysis of sucrose before its subsequent analysis for glucose and fructose. HPAEC-IPAD was employed for the analysis of sugars. The experimental parameters affecting the separation of sugars and the acid hydrolysis of sucrose were investigated.
Materials and Methods
Chemicals and Reagents
All of chemicals and reagents used were at least analytical reagent grade. Sodium hydroxide, potassium hydrogen phthalate, potassium hydroxide, hydrochloric acid, and sodium azide were purchased from Carlo Erba (Italy). Phenolphthalein was purchased from Fluka (Switzerland). Monosaccharides: D-(−)-fructose, D-(+)-glucose anhydrous, and D-(+)-xylose were from Fluka (Switzerland), while D-(−)-arabinose and D-(−)-ribose were purchased from Sigma (Germany). Disaccharides: D-(+)-sucrose and D-(+)-lactose monohydrate were purchased from Fluka (Switzerland). All of aqueous solutions were prepared using deionized water from RiOs™ type I simplicity 185 (Millipore Waters, USA) with a resistivity of 18.2 MΩ cm.
Instruments
Chromatographic separations were performed on a Dionex Instrument DX-500 IC system (Sunnyvale, CA, USA). The system consisted of a GP40 gradient pump and ED40 electrochemical detector equipped with a thin-layer-type amperometric cell. The cell consisted of a 1.0-mm-diameter gold working electrode and platinum counter electrode in IPAD mode. The separations were carried out on a CarboPac PA 10 column set consisting of a guard column (50 mm × 2 mm ID) and an analytical column (250 mm × 2 mm ID). The sample injection volume was 25 μL. The flow rate was 1.0 mL min−1. The columns were placed inside a temperature controller. The chromatographic system control, the data acquisition, and analysis were performed using PeakNet 6.0 software (Dionex).
The Analysis of Raw Sugar Sample
Raw sugar samples were kindly provided by Mitrphol Factory, Khon Kaen, Thailand. An accurate amount of raw sugar sample (0.25 g) was dissolved in 25.00 mL of water. Sample solutions were filtered through a 0.22-μm membrane filter. The sample was diluted 100-fold with water and was directly injected to the chromatographic system for the analysis of sugars. The diluted sample solution was then subjected to hydrolysis (as described in the “Acid Hydrolysis” section) before being analyzed for glucose and fructose by the HPAEC-IPAD. The flow diagram for the analysis of sugars is summarized as follow:

Acid Hydrolysis
An aliquot (1.00 mL) of sucrose (100 μg mL−1) or 100-fold diluted raw sample was taken into a test tube, and 20 μL of 12 mol L−1 hydrochloric acid was added. The mixture was heated at 80 °C in a water bath for 5 min. After cooling, the pH of solution was adjusted to neutral with 5 mol L−1 sodium hydroxide. The hydrolysate was then analyzed by HPAEC-IPAD.
Results and Discussion
Sugars are weak acids which are partially ionized at high pH to anionic form [29]. Under alkaline solution, sugars are ionized and present in their anionic forms. In addition, sugars are electroactive species. Thus, high-performance anion exchange with integrated pulsed amperometric detection (HPAEC-IPAD) can be used for the analysis of sugars.
Ion-Exchange Chromatography of Sugars
In the present study, anion exchanger, CarboPac PA 10 column, was used as the stationary phase and sodium hydroxide was used as the mobile phase. The pH of the mobile phase has influences directly on the retention behavior of the analytes and also on the electrochemical reactions that occur at the working electrode [30]. IPAD is well known for its application to the direct and sensitive determination of carbohydrates [31]. The potential waveform used in this study was modified from Farin et al. [28]. The processes consisted of duration step (E1), current integration step (E2), and cleaning step (E3). Sugars are adsorbed on a gold working electrode and later detected by an oxidative desorption process. Following the detection, a second potential is used for oxidative cleaning of the electrode surface and a third potential pulse is then applied to reduce the surface oxide on the electrode. In this study, the potential applied for the integration was 0.05 V, and the integration time was set at 0.2–0.4 s, as shown in Fig. 1.
Optimization of Gradient Elution
The gradient conditions for the separation of the studied sugars were investigated by trial and error based on the isocratic elution, and deionized water was used as eluent A and 250 mmol L−1 sodium hydroxide as eluent B. The optimum gradient was step gradients with gradual increase in sodium hydroxide to obtain the best resolution between pairs. The gradient cycle was 40 min, as the optimum step gradient shown in Fig. 2.
Initial concentration of sodium hydroxide was kept constant at 2 mmol L−1 for 5 min and was increased to 5 mmol L−1 which was kept constant for 2 min. During this profile, two compounds were eluted including arabinose (10.08 min) and sucrose (12.12 min). Glucose and xylose were eluted at 13.70 and 15.32 min, respectively. During the time of 2 min from the 16th min to the 18th min, sodium hydroxide was kept constant at 31.25 mmol L−1 which fructose and ribose were separated at 17.04 and 18.10 min, respectively. The strong retained compound was lactose which was eluted at 22.10 min with sodium hydroxide higher than 40 mmol L−1.
Influence of Column Temperature on the Separation of Sugars
The column temperature affects the retention. Besides stationary phase type, mobile phase, and flow rate, column temperature also affects the retention behavior of the analytes in HPAEC [6, 31]. The column temperature was investigated in the range of 25–40 °C with 5 °C intervals. Figure 3 shows the effect of column temperature on the retention behavior of the studied sugars. It was found that individual sugar showed different relative changes in retention time with increased temperature. The retention of all the studied sugars (except glucose) exhibited exothermic behavior (i.e., retention to be decreased with increasing temperature) when the column temperatures were higher than 30 °C. However, the effect of temperature on retention is complicated [30, 31]. However, the influence of column temperature on the separation efficiency is considered to be a minor factor compared to the mobile phase composition [11]. In this study, the column temperature of 35 °C was chosen because it provided complete separation of peaks in a short analysis time with good baseline.
Analytical Performance and Validation of the Method
Figure 4 depicts the typical chromatogram under the optimum conditions. A calibration curve for each compound was constructed by plotting the peak area against the corresponding concentrations of the sugars from five concentration levels in triplicate. The calibration curve for each sugar was linear with the regression coefficients ranging from 0.997 to 0.999. The linear ranges of the studied sugars were in the range of 0.5–100 µg mL−1. Limit of detection (LOD) is the concentration of the sugar which gives the signal-to-noise ratio (S/N) of 3. Limit of quantitation (LOQ) is the concentration of the sugar which gives the signal-to-noise ratio (S/N) of 10.
The precision was expressed as relative standard deviations (RSD) of repetitive injections for both peak area and retention time. Intra-day precision was tested both to evaluate the constancy of instrumental response to a given analyte for both the quantitative (peak area) and qualitative (retention time). Standard mixture of seven sugars (10 µg mL−1 each) was injected ten replicates as the obtained chromatograms shown in Fig. 5. The RSD of retention time was less than 4 %, and RSD of peak area was less than 7 % for intra-day precision (data not shown). Inter-day precision was tested using the same mixture solution as the intra-day precision. The samples were analyzed 10 replicates in 3 days (3 × 10). The RSD of retention time was less than 5 %, and the RSD of peak area was less than 11 %.
Overlaid chromatograms for intra-day precision (10 replicates). Chromatographic conditions as described in Fig. 4
In order to evaluate the accuracy of the method, the recovery of the studied sugars was studied by spiking standard mixture of sugars (10 µg mL−1 each) into raw sugar samples. The average recoveries of sugars were varied from 96.14 to 102.48 %. Table 1 summarizes the analytical performance from ion chromatographic analysis.
Acid Hydrolysis of Sucrose
Since the amount of sucrose in raw sugar is much larger than the other sugars, it is difficult to determine sucrose and the other sugars in the same chromatographic run. Thus, sample preparation is required. In this study, acid hydrolysis was used for the sample preparation of raw sugar. Acid hydrolysis was chosen because it is simple, rapid, and low cost than enzyme hydrolysis. The hydrolysis of sucrose gives glucose and fructose [27, 28]. Parameters affecting the acid hydrolysis of sucrose were investigated and optimized. The results are shown in Fig. 6. The optimum conditions were as follow: sucrose of 1 mol was completely hydrolyzed using 20 µL of concentrated hydrochloric acid at 80 °C for 5 min.
Analysis of Sugars in Raw Sugar Samples
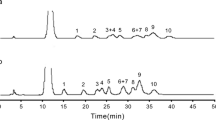
The proposed method was applied for the analysis of sugars in raw sugar samples. There are three steps to analyze sugars in raw sugar samples. Firstly, diluted raw sugar sample was analyzed for sucrose, while undiluted sample was directly analyzed for the other sugars. Secondly, known quantity of sucrose was prepared from raw sugar sample and was hydrolyzed under the optimum conditions. This experiment was performed in parallel to the standard sucrose at the same quantity. Finally, the hydrolysates (from known amount of sucrose of raw sugar sample and standard sucrose) were analyzed by ion chromatography. The quantities of glucose and fructose in samples were obtained by subtraction of their quantities from standard sucrose and samples. Figure 7 shows the chromatogram of sample before and after hydrolysis. After hydrolysis, the enormous peak of sucrose was eliminated, and thus the minor constituent sugars could be determined. The quantities of sugars in raw sugar samples along with their color are summarized in Table 2. Sucrose was detected ranging from 978.22 to 984.03 mg g−1 or 97.82 to 98.40 %. The other sugars including arabinose, glucose, fructose, xylose, ribose, and lactose were ranged from 5.07 to 5.72, 10.45 to 19.48, 10.23 to 19.04, 12.39 to 14.92, 11.25 to 15.98, and 5.44 to 6.26 mg g−1, respectively.
Chromatograms for the analysis of raw sugar. Peak assignments as described in Fig. 3
Conclusion
The present work demonstrates HPAEC-IPAD as a direct method for the analysis of monosaccharides and disaccharides in raw sugar samples. Seven sugars including arabinose, sucrose, glucose, xylose, fructose, ribose, and lactose were separated within 23 min by anion-exchange chromatography under step gradient elution of sodium hydroxide concentration ranged from 2 to 47 mmol L−1. The order of elution was arabinose (10.08 min), sucrose, glucose, xylose, fructose, ribose, and lactose (22.10 min). HPAEC-IPAD is a powerful technique for simultaneous analysis of sugars under the optimized condition. The use of acid hydrolysis as a sample preparation method facilitates the simultaneous analysis of sugars in a complicate sample such as raw sugar samples. The proposed method provided good analytical performance and has a potential to be used as a simple and efficient method for simultaneous analysis of sugars.
References
Wiggens LP, Honig P (1997) Principle of sugar technology. Elsevier, Philadelphia
Chen JCP, Chou CC (1997) Cane sugar handbook. John Wiley and Sons, Ames
Farine S, Villard C, Moulin A, Marchis Mouren G, Puigserver A (1997) Int J Biol Macromol 21:109–114
Ouchemoukh S, Schweitzer P, Bachir Bey M, Djoudad-Kadji H, Louaileche H (2010) Food Chem 121:561–568
Caseiro A, Marr IL, Claeys M, Kasper-Giebl A, Puxbauma H, Pio CA (2007) J Chromatogr A 1171:37–45
Yu H, Moub SF (2006) J Chromaogr A 1118:118–124
Chávez-Servín JL, Castellote AI, López-Sabater MC (2004) J Chromatogr A 1043:211–215
Medeiros PM, Simoneit BRT (2007) J Chromatogr A 1141:271–278
Caseiro A, Marr IL, Caeys M, Kasper-Giebl A, Puxbauma H, Pio CA (2007) J Chromatogr A 1171:37–45
Raessler M, Wissuwa B, Breul A, Unger W, Grimm T (2010) Anal Methods 2:532–538
Cai Y, Liu J, Shi Y, Liang L, Mou S (2005) J Chromatogr A 1085:98–103
Schütz K, Muks E, Carle R, Schieber A (2006) Biomed Chromatogr 20:1295–1303
Raessler M, Wissuwa B, Breul A, Unger W, Grimm T (2008) J Agric Food Chem 56:7649–7654
Buttler T, Nilsson C, Gorton L, Marko-Varga G, Laurell T (1996) J Chromatogr A 725:41–56
Pörtner R, Märkl H (1998) Appl Microbiol Biotechnol 50:403–414
Grudpan K, Jakmunee J, Sooksamiti P (1999) Talanta 49:215–223
Buldini PL, Mevoli A, Quirini A (2000) J Chromatogr A 882:321–328
Borba MBD, Brewer JM, Camarda J (2001) J Chromatogr A 919:56–65
Chiap P, Hubert PH, Crommen J (2002) J Chromatogr A 948:151–161
Bogenachütz G, Kolb T, Viehweger KH (2003) GIT Lab J:14–16
de Villiers A, Lynen F, Crouch A, Sandra P (2004) Chromatographia 59:403–409
Weitzhandler M, Pohl C, Rohrer J, Narayanan L, Slingsby R, Avdalovic N (1996) Anal Biochem 241:128–134
Moreau C, Durand R, Aliès F, Cotillon M, Frutz T, Théoleure MA (2000) Ind Crop Prod 11:237–242
Echeverria E, Burns JK (1989) Plant Physiol 90:530–533
Echeverria E (1990) J Plant Physiol 92:168–171
Pinheiro Torres A, Oliveira FAR (1999) J Food Eng 40:181–188
Gorin N, Zonneveld H (1974) J Agric Food Chem 22:709–712
Farine S, Versluis C, Bonnici PJ, Heck A, Peschet JL, Puigserver A, Biagini A (2001) J Chromatogr A 920:299–308
Jandik P, Cheng J, Avdalovic N (2004) J Biochem Biophys Meth 60:191–203
Fritz JS, Gjerde DT (2000) Ion Chromatography. Wiley-VCH, Ames
La Course WR (1997) Plused electrochemical detection in high-performance liquid chromatography. John Wiley and Sons, Ontario
Acknowledgments
Financial support from the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education, Thailand, is gratefully acknowledged. Raw sugar samples from Mitr Phol Factory, Khon Kaen, Thailand, are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection 20th International Symposium on Separation Sciences in Prague with guest editors Aleš Horna and Pavel Jandera.
Rights and permissions
About this article
Cite this article
Suksom, W., Wannachai, W., Boonchiangma, S. et al. Ion Chromatographic Analysis of Monosaccharides and Disaccharides in Raw Sugar. Chromatographia 78, 873–879 (2015). https://doi.org/10.1007/s10337-015-2865-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-015-2865-3