Abstract
A new stationary phase which contains both negatively charged phosphate groups and positively charged amino groups was successfully synthesized by modification of amino-functionalized silica particles with trichlorophosphine oxide (POCl3) for hydrophilic interaction chromatography (HILIC). The composition of the surface grafts was determined by Fourier transform infrared spectroscopy and elemental analysis. Various parameters, such as column temperature, water content, pH values and ionic strength of the mobile phase were investigated to study the retention mechanism. The results demonstrated that the stationary phase involved a complex retention process including surface adsorption, partitioning and electrostatic interactions. Under optimized conditions, the separation of nucleobases and nucleosides, water-soluble vitamins, organic acids on the novel stationary phase could be achieved in the HILIC mode.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrophilic interaction liquid chromatography (HILIC) has emerged as a complementary technique to RPLC for separating polar compounds [1]. The acronym HILIC was first suggested by Alpert in 1990 when he studied silica with hydrophilic functional groups [2], although it has been used since 1975 for analysis of sugar and oligosaccharides on cyclodextrin and amino bonded phases [3, 4]. The HILIC stationary phases are polar materials, which are the same as NPLC, while the mobile phases for HILIC are similar to those used in RP-LC, and high organic solvent containing mobile phases are normally used to promote hydrophilic interaction. It overcomes the drawbacks of the poor retention in RPLC and the poor solubility encountered in NPLC of polar compounds. Furthermore, it can be conveniently coupled to electrospray ionization (ESI) mass spectrometry (MS) [5]. The high organic content mobile phase necessary to maintain retention in HILIC significantly increases ESI–MS sensitivity because of improved ionization efficiency [6].
Separation materials for HILIC consist of classical bare silica or silica gels modified with various polar functional groups, such as diol [7], amino [8], amide [9] and other polar ligands [10–13]. The structural variations of HILIC-type stationary phases are wider than those found in RPLC system; therefore, it may provide more choices for chromatographers to find an appropriate stationary phase for the desired separation. Although the types of commercially available HILIC columns are continuously growing in recent years, there is still no versatile stationary phase like C18 in RPLC. To meet the increasing demands, the development of novel HILIC stationary phases with improved hydrophilicity and separation efficiency is favorable for chromatographic science.
Zwitterionic HILIC (ZIC-HILIC) stationary phases, which consist of both positively and negatively charged groups, have been widely used for the separation of various polar analytes. This kind of phases has unique hydrophilic properties and exhibits higher selectivity over typical HILIC materials for the separation of polar analytes [9, 13–17]. And they have been proved to promote weak electrostatic interactions from the charged groups rather than strong ion exchange contributions, in addition to hydrophilic interactions [9, 17]. A typical example is the sulfobetaine phase, which contains both strongly acidic sulfonic groups and strongly basic quaternary ammonium groups separated by a short alkyl spacer, has found a wide range of applications in HILIC. With the sulfonic group at the distal end of the zwitterionic moiety, they exhibited slightly negative surface charge [18]. Another zwitterionic phosphorylcholine phase which was prepared by graft polymerization of 2-methacryloyloxyethyl phosphorylcholine onto the surface of silica gel has also been reported [19]. The major difference between those two phases is the spatial arrangement of positive and negative charged groups, resulting in different surface charge and chromatographic behaviors. Shen et al. [20] developed a new type of cysteine-bonded zwitterionic stationary phase with a uniform distribution of both positive and negative charges that are parallel to the surface of the silica gel. The new phase exhibited excellent performance in the separation of oligosaccharides, peptides and basic compounds. Consequently, other zwitterionic stationary phases with various functional groups and different spatial charge orientations are interesting to be studied.
Materials with terminated phosphate groups coordinating with metal ions have been widely used in enrichment and separation of phosphopeptides recently [21, 22]. And other phosphate-functionalized materials, such as polymer beads, magnetic nanoparticles, porous silicon, nanoplatelets, monolithic capillary and self-assembled monolayer, have also been applied in separation fields [23]. In this study, we successfully introduced phosphate groups on amino-functionalized silica particles to form a new zwitterionic stationary phase with both a terminated negatively charged phosphate group and a positively charged amino group. The retention characteristics of this new stationary phase were investigated in the HILIC mode using a set of charged as well as neutral polar compounds as test probes. The separation of nucleosides and nucleobases, water-soluble vitamins and organic acids was achieved on the new zwitterionic stationary phase, demonstrating the excellent application potential in the analysis of polar compounds.
Experimental
Materials
Spherical silica (5 μm particle size, 100 Å pore diameter and 300 m2g−1 specific surface area) was purchased from Welch materials, Inc (Maryland, USA). N-β-aminoethyl-γ-aminopropyl-trimethoxysilane was obtained from Wuhan University Silicone New Material (Wuhan, China). Vitamins (B1, B2, B3, B3-amide, B6, B12), deoxynucleosides, nucleosides, nucleobases and 2,4,6-collidine were purchased from Aladdin (Shanghai, China). Benzoic acid and its analogues, methanol (MeOH), triethylamine, acetonitrile (ACN), trichlorophosphine oxide (POCl3), acetic acid, ammonium acetate and ammonium formate were purchased from Sinopharm Chemical Reagent Factory (Shanghai, China). All other reagents were of analytical reagent grade unless otherwise indicated and were all obtained from various commercial sources. Deionized water was purified with a Milli-Q system (Waters, Milford, MA, USA) and used for all aqueous solutions.
Instrument and HPLC Conditions
All experiments were performed on the Agilent HPLC series 1200 system (Agilent Technologies, Palo Alto, CA), which consisted of G1311A Quaternary pump, a G1315B DAD detector, a G1329A autosampler, a G1330B thermostated column compartment, and a G1322A degasser. Elemental analysis was measured on a VarioEL III and 31P solid-state NMR MAS spectra was recorded on a VARIAN Infinityplus 300 solid-state NMR.
For chromatographic evaluations, the flow rate was 1.0 mL min−1 and the column temperature was 30 °C unless otherwise specified. Mobile phases were prepared by mixing ACN and buffer solution of ammonium acetate. The pH of mobile phase referred to the pH of buffer solution before mixing with acetonitrile. All probes in this study were prepared in water/acetonitrile (1/4, v/v). Toluene was used as marker of the dead time. Each measurement was replicated at least twice.
Preparation of Stationary Phase and Column Packing
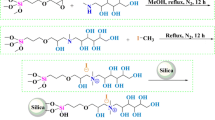
The synthesis procedure is shown in Fig. 1. Silica gel was firstly activated by rehydroxylation in hydrochloric acid–H2O (1:1) under reflux for 12 h, followed by washing with water to be neutral and drying at 160 °C for 8 h. Then the pretreated silica gel (10 g) was suspended in 30 mL of anhydrous toluene, 50 μL triethylamine and N-β-aminoethyl-γ-aminopropyl-trimethoxysilane (6.39 g) was added. Under nitrogen atmosphere, the mixture was kept at 110 °C with stirring for 13 h to form di-amine-modified silica gel (NAPTS). Then the NAPTS is filtered and washed with toluene and acetone. The obtained di-amino-modified porous silicon surface was further modified with a solution of 100 mM POCl3 and 100 mM 2,4,6-collidine in anhydrous acetonitrile for 12 h to form the phosphonate-terminated structure [24] following by rinsing with ACN, water, methanol and acetone in sequence. Finally, the obtained amino-phosphate silica (APS) sorbent was dried under vacuum at 60 °C overnight.
Both NAPTS and APS sorbents were dispersed in acetone, and then packed into 150 × 4.6 mm (i.d.) stainless-steel columns under 6,000 p.s.i. pressure for 30 min.
Results and Discussion
Characterization of Amino-Phosphate Silica Particle
The amino-phosphate silica particle was firstly characterized by FT-IR. As shown in Fig. S1 (Supporting Information), the signal at ν ≈ 3,300–3,600 corresponds to N–H stretching vibrations in amines and O–H vibrations in hydroxyl groups, absorptions at ν ≈ 3,000–2,800 cm−1 being from the C–H stretching bands. Siloxane bending vibrations are visible at ν ≈ 800 cm−1. In addition, the siloxane asymmetric stretching vibrations are observed at ν ≈ 1,100 cm−1. The characteristic peak at ν ≈ 1,640 cm−1 is attributed to bending vibrations of amino groups. The intensity of this signal decreases for APS when compared to NAPTS.
To quantitate the elemental composition and evaluate the reproducibility of the preparation strategy, we examined the elemental contents of nitrogen, carbon, and hydrogen of the APS silica particles with different batches by elemental analysis. The content of phosphate was determined by the molybdenum blue method. The stationary phase exhibited slightly positive surface charge because of the –NH– group and the unreacted terminated –NH2 group. These results clearly demonstrate the successful introduction of the organic phosphonic groups to the silica surface. Similar elemental composition of three batches of the APS stationary phase indicated satisfactory reproducibility, as shown in Table 1.
Comparison of the NAPTS and the APS Columns
Benzoic acid and VB1 were firstly chosen for the evaluation on the APS and NAPTS columns under the same chromatographic conditions. As shown in Fig. 2, benzoic acid showed entirely different trends on the NAPTS and the APS columns as the pH of the mobile phase decreased from 7.9 to 4.5. For NAPTS, the retention of benzoic acid weakened with the decreasing buffer pH. On the contrary, as for the APS, when pH decreased from 7.9 to 4.5, the phosphate ligands on the stationary phase as well as benzoic acid were gradually protonated, thus leading to decreased electrostatic repulsion and then increased retention time. An obviously decreased retention for benzoic acid was observed as the buffer pH decreased from 4.5 to 3.5 on both columns, which is because of that benzoic acid (pKa 4.2) existed in neutral form at lower pH values. The ionic exchange interaction with benzoic acid on APS stationary phase was much poorer than NAPTS phase, indicating that some amine groups on NAPTS phases were converted to PO3H2 groups. As for VB1, it contained positive charge in the range of studied pH. Therefore, the electrostatic repulsion interaction between analyte and APS phase increased when the pH decreased from 7.9 to 3.5, which resulted in the decreased retention for VB1. On the NAPTS, VB1 has poor retention and remains almost unchanged in the investigated pH range, resulting from a strong electrostatic repulsion interaction. Therefore, the developed APS phase could retain both acidic and basic analytes and could achieve their simultaneous separation.
Effect of Water Content
The strength of mobile phase in the eluent is considered as the dominant factor that affects retention of polar compounds in HILIC mode. We selected six polar compounds to study the HILIC properties of the APS stationary phase, including VB1, VB3, nicotinamide, melamine, clenbuterol and cytidine. Structures and pKa values of these compounds are listed in Fig. S2 (Supporting Information). The volume percentage of water in mobile phase was changed from 5 to 30 % while keeping ammonium acetate concentration constant at 15 mM and pH at 3.5, respectively. Decreasing retention factors were observed with the increase of water content, demonstrating a typical HILIC retention characteristic.
As shown in Fig. 3, we made an attempt to plot log k versus the linear and logarithmic function of the volume fraction of water in eluent to investigate the retention mechanism of APS phase under HILIC mode. Up to now, two principle retention mechanisms have been proposed: adsorption and partitioning. Alpert [2] suggested that the retention mechanism for HILIC was a partitioning between the bulk eluent and a water-rich layer, partially immobilized on the stationary phase. The relationship that is established for partitioning can be described in Eq. (1).
where k w is the retention factor for the weaker eluent component only as mobile phase, φ is the volume fraction (concentration) of the stronger member of a binary mobile phase mixture, and S is the slope of log k versus φ when fitted to a linear regression model [25].
The adsorption mechanism is in fact a displacement mechanism. It is assumed that the solute molecules and the modifier molecules are co-adsorbed on the tips of the hydrocarbon chains displacing solvent molecules. The relationship between the retention and the mole fraction N B of the stronger solvent B in the eluent should adhere to the following expression [26]:
where k B is the solute retention factor with pure B as eluent, A S and n B are the cross-sectional areas occupied by the solute molecule on the surface and the B molecules, respectively, and N B is the mole fraction of the stronger member B in the eluent. Plots made of log k versus the linear and logarithmical function of the water contents in the eluents used in HILIC separations should thus give an indication on whether partitioning or adsorption is the dominant retention mechanism.
Multiple regression analysis was performed as the results shown in Table 2. All the regression coefficients of the six tested probes were constructed based on the above two models. Different retention patterns were observed among these probes. The results showed that the adsorption model seemed to fit better for VB3, melamine and cytidine, while VB1, clenbuterol and nicotinamide exhibited partitioning mechanism on the stationary phase. The nature of analytes might affect the types of retention mechanisms on the APS column in HILIC mode. We can draw a conclusion that retention mechanism of the APS stationary phase is a combined process with partitioning and adsorption.
Effect of Buffer pH
The pH of the mobile phase plays an important role in the selectivity and retention of analytes in HILIC, especially for ionic solutes [27]. In the current study, seven compounds were chosen to investigate the effects of the mobile phase pH of ammonium acetate with pH values ranging from 3.5 to 7.9. As shown in Fig. 4, the retention factors of uncharged analytes, such as nicotinamide and cytidine, remained almost unchanged. The retention of basic compounds VB1 (pKa 9.0) and clenbuterol (pKa 9.6) increased with increasing buffer pH. This may be attributed to that both analytes and the stationary phase were gradually deprotonated with the increase of buffer pH, which led to weaker electrostatic repulsion and thus longer retention. The retention of another weak base melamine (pKa 8.0) was almost not changed with the increasing pH because its ionization did not change significantly in the pH range. For acid compounds VB3 (pKa 3.0) and 2-chlorobenzoic acid (pKa 2.9), when pH was decreased from 7.9 to 3.5, they lose their negative charge as well as the phosphate ligands in the stationary phase, weaker electrostatic repulsion then occurred, despite the increasing positive charge of the amino group in the stationary phase.
Effect of Ionic Strength of Mobile phase
The ionic strength of mobile phase also influences the retention of polar analytes in HILIC [28]. Ammonium acetate with different concentration ranging from 5 to 25 mM (ACN 80 %, pH 3.5) was employed to evaluate the effects of the ionic strength of mobile phase on the retention of polar analytes. The APS stationary phase was positively charged at this pH value. As shown in Fig. 5, for acidic analytes of VB3 and 2-chlorobenzoic acid, higher ionic strength weakened electrostatic attraction interaction, thus leading to decreasing retention. The basic compounds VB1, melamine and clenbuterol, showed increasing retention with the increasing buffer concentration. This is possibly because higher ionic strength weakened electrostatic repulsion interaction between the basic compounds and the positively charged stationary phase. Stronger retention was also observed in higher buffer concentration condition for uncharged solute cytidine. The possible reason was that more salt ions would be driven into the water-enriched layer with the increasing of salt concentration in HILIC condition, resulting in increasing hydrophilicity of the surface water-enriched layer and increasing retention of analytes [2, 10, 16, 28]. These results indicated that the separation mechanism of the APS might be involved in partitioning, surface adsorption and electrostatic interactions.
Effect of Column Temperature on Retention
Column temperature is an important parameter that affects analyte retention in HILIC [29]. The effect of temperature on the retention of model compounds can be expressed by the van’t Hoff equation [30].
where \( \Updelta H \) is the standard partial molar enthalpy of transfer; \( \Updelta S \) is the standard partial molar entropy of transfer; R is the gas constant; T is the absolute temperature, and \( \Upphi \) is the phase ratio of the chromatographic column, respectively. In this study, the relationship between ln k and 1/T was explored while column temperature varies from 20 to 60 °C. The plots (Fig. S3 in Supporting Information) showed retention values for four test probes decreased with an increase in column temperature. The good linearity for the plots indicated that retention mechanism remained unchanged as the temperature varied. Benzoic acid showed different tendency compared to the aforementioned four compounds. The possible reason was that at higher temperature the ionization degree of benzoic acid increased, more negative charge of benzoic acid then lead to a stronger retention.
Separation of Water-soluble Vitamins
The separation of six water-soluble vitamins which were difficult to be separated in RPLC was achieved within only 7 min in the isocratic HILIC mode. This performance was compatible with the previous reports [13, 31–33]. The mobile phase consists of acetonitrile/40 mM ammonium acetate at pH 7.9, 70/30 (v/v). As shown for the separation chromatogram in Fig. 6a, the new stationary phase exhibited excellent separation selectivity for vitamins.
Separation of vitamins (a), deoxynucleosides, nucleosides and nucleobases (b) and benzoic acid and its analogues (c) on APS column. a Mobile phase: acetonitrile/40 mM HCOONH4, 70/30 (v/v), pH 7.9; solutes: 1 nicotinamide, 2 VB2, 3 VB6, 4 VB1, 5 VB12, 6 VB3. b Mobile phase: acetonitrile/50 mM HCOONH4, 85/15 (v/v), pH 4.5; solutes: 1 thymine, 2 thymidine, 3 deoxyadenosine, 4 uridine, 5 cytosine, 6 deoxycytidine, 7 inosine, 8 deoxyguanosine, 9 cytidine, 10 guanosine. c Mobile phase: acetonitrile/50 mM HCOONH4, 90/10 (v/v), pH 4.5; solutes: 1 4-nitrobenzoic acid, 2 3,5-dinitrobenzoic acid, 3 salicylic acid, 4 3-nitrobenzoic acid, 5 anthranilic acid, 6 benzoic acid, 7 2-chlorobenzoic acid, 8 4-hydroxybenzoic acid. Other conditions were the same as Fig. 2
Separation of Nucleobases and Nucleosides
As shown in Fig. 6b, a set of deoxynucleosides, nucleosides and nucleobases were effectively separated on the APS column with a mixture of 85 % acetonitrile—50 mM ammonium acetate buffer solution (pH 4.5), showing a comparable performance with commercial ZIC-HILIC phase from Merck SeQuant [13]. This demonstrated a favorable hydrophilic property for the stationary phase.
Separation of Benzoic Acids
Eight benzoic acid and its analogues were chosen to test the selectivity of the APS column. Comparing previous reports [16, 34, 35], on the separation of benzoic acid and its analogues, the APS column exhibited excellent selectivity under similar mobile phase conditions. The separation chromatogram was shown in Fig. 6c. The mobile phase consists of 90 % ACN and 50 mM buffer solution (pH 4.5) of CH3COONH4.
Conclusions
A novel zwitterionic stationary phase (APS) was synthesized by further reaction on di-amino-modified porous silicon surface. This material possesses both a negatively charged phosphate group and a positively charged amino group. The successful grafting was further confirmed by FT-IR, elemental analysis and a chromatographic retention comparison between the NAPTS and APS. The chromatographic retention behaviors in the HILIC mode were investigated by varying factors such as the mobile phase composition, the buffer concentration, the buffer pH and the column temperature, revealing that the retention is a complex process composed of surface adsorption, partitioning and electrostatic interactions. At last, effective separation of several small polar compounds including water-soluble vitamins, nucleosides and nucleobases, and organic acids was achieved in the HILIC mode, demonstrating the excellent application potential of the APS stationary phase in the analysis of polar compounds.
References
McCalley DV (2007) J Chromatogr A 1171:46–55
Alpert AJ (1990) J Chromatogr 499:177–196
Wang C, Jiang C, Armstrong DW (2008) J Sep Sci 31:1980–1990
Liu Y, Urgaonkar S, Verkade JG, Armstrong DW (2005) J Chromatogr A 1079:146–152
Buszewski B, Noga S (2012) Anal Bioanal Chem 402:231–247
Nguyen HP, Schug KA (2008) J Sep Sci 31:1465–1480
Regnier FE, Noel R (1976) J Chromatogr Sci 14:316–320
Alpert AJ (2008) Anal Chem 80:62–76
Risley DS, Strege MA (2000) Anal Chem 72:1736–1739
Olsen BA (2001) J Chromatogr A 913:113–122
Tanaka H, Zhou X, Masayoshi O (2003) J Chromatogr A 987:119–125
Qiu H, Loukotková L, Sun P, Tesařová E, Bosáková Z, Armstrong DW (2011) J Chromatogr A 1218:270–279
Qiu H, Wanigasekara E, Zhang Y, Tran T, Armstrong DW (2011) J Chromatogr A 1218:8075–8082
Kawachi Y, Ikegami T, Takubo H, Ikegami Y, Miyamoto M, Tanaka N (2011) J Chromatogr A 1218:5903–5919
Chirita RI, West C, Zubrzycki S, Finaru AL, Elfakir C (2011) J Chromatogr A 1218:5939–5963
Guo Y, Gaiki S (2005) J Chromatogr A 1074:71–80
Kitano H, Mori T, Takeuchi Y, Tada S, Gemmei-Ide M, Yokoyama Y, Tanaka M (2005) Macromol Biosci 5:314–321
Jiang W, Irgum K (1999) Anal Chem 71:333
Jiang W, Fischer G, Girmay Y, Irgum K (2006) J Chromatogr A 1127:82–91
Shen A, Guo Z, Yu L, Cao L, Liang X (2011) Chem Commun 47:4550–4552
Johnson LN, Lewis RJ (2001) Chem Rev 101:2209–2242
Bollen M, Beullens M (2002) Trends Cell Biol 12:138–145
Li XS, Wu JH, Zhao Y, Zhang WP, Gao Q, Guo L, Yuan BF, Feng YQ (2011) J Chromatogr A 1218:3845–3853
Zhou H, Xu S, Ye M, Feng S, Pan C, Jiang X, Li X, Han G, Fu Y, Zou H (2006) J Proteome Res 5:2431–2437
Nikitas P, Pappa-Louisi A, Agrafiotou P (2002) J Chromatogr A 946:9–32
Scott RPW, Kucera P (1979) J Chromatogr 171:37–48
Hemstrom P, Irgum K (2006) J Sep Sci 29:1784–1821
Peng X-T, Yuan B-F, Feng Y-Q (2011) J Sep Sci 34:3123–3130
Shen A, Guo Z, Cai X, Xue X, Liang X (2012) J Chromatogr A 1228:175–182
Qiu H, Armstrong DW, Berthod A (2013) J Chromatogr A 1272:81–89
Wu J, Bicker W, Lindner W (2008) J Sep Sci 31:1492–1503
Karatapanis AE, Fiamegos YC, Stalikas CD (2009) J Sep Sci 32:909–917
Li Y, Feng Y, Chen T, Zhang H (2011) J Chromatogr A 1218:5987–5994
Guo Y, Srinivasan S, Gaiki S (2007) Chromatographia 66:223–229
Jiang Z, Smith NW, Ferguson PD, Taylor MR (2009) J Sep Sci 32:2544–2555
Acknowledgments
The authors thank the financial support from the National Key Basic Research Foundation of China (973 Program) (2013CB910702,2012CB720601), the National Natural Science Foundation of China (91017013, 31070327, 21005057), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, XD., Peng, XT., Yu, QW. et al. Preparation of a Novel Amino-Phosphate Zwitterionic Stationary Phase for Hydrophilic Interaction Chromatography. Chromatographia 76, 1569–1576 (2013). https://doi.org/10.1007/s10337-013-2534-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2534-3