Abstract
Studies of the movements and home-ranges of houbara bustards (Chlamydotis undulata undulata) showed sexual and seasonal differences in the use of space, with a polygynous mating system similar to an ‘exploded-lek’ or a ‘resource-defence-polygyny’, that remains undefined. We used the arthropod biomass as an index of the trophic quality of six defined habitats and we radio-tracked 7 females and 13 males to test whether sexual and seasonal variations in habitat use were related to resource availability, and to verify if critical resources for breeding females were monopolised by males. We analysed habitat selection in both sexes separately. We used the habitat type composition of buffer zones around radio-locations to study annual and seasonal habitat selection and to identify preferred habitats, using the chi-square goodness-of-fit test. Habitat use between sexes and between seasons were compared using MANOVA based on log-ratios of habitat proportions. During the year, and in each season, both sexes appeared to be significantly selective for habitats in comparison to their availability. But males avoided esparto grass, while females used all habitats. Habitat use differed between sexes in the breeding season, but not in the non-breeding season. In spring, when food resources were abundant and uniformly distributed in space, males preferred ‘temporarily flooded areas’ and females preferred ‘reg with tall perennials’ that offered both food and cover for brooding. Critical resources were not monopolised by males and the mating system fulfilled the definition of the ‘exploded-lek’. Leks are key sites for reproduction and should be considered as priority areas in further conservation plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identifying habitat use is a fundamental ecological requirement in the understanding of a species’ biology. It is also essential for much population conservation and management, particularly in the case of endangered species where knowing what determines species distribution is necessary to mitigate its decline (Rushton et al. 2004).
The houbara bustard (Chlamydotis undulata) has suffered a major decline due to over-hunting, poaching and habitat degradation in all parts of its range, from the Canary Islands, through North Africa to Mongolia (Collar 1980; Lavee 1985; Combreau et al. 2001; Tourenq et al. 2005). To support conservation action plans on the houbara bustard (Combreau et al. 1995; Seddon et al. 1995), several studies have been undertaken in the last decade on houbara biology and ecology. However, all studies of habitat use and selection refer to the migratory Asian species, Macqueen's bustard (Chlamydotis macqueenii) (Seddon and Van Heezik 1996; Combreau and Smith 1997; Launay et al. 1997; Osborne et al. 1997; van Heezik and Seddon 1999; Combreau et al. 2000; van Heezik and Seddon 2002; Yang et al. 2002; Yang et al. 2003) and to the Canarian houbara bustard (Chlamydotis undulata fuerteventurae), which has adapted to cultivated land (Collins 1984; Martín et al. 1996; Medina 1999), but none concerned the African houbara bustard (Chlamydotis undulata undulata) which occupies semi-desert habitats in North Africa.
Previous studies on houbara bustard breeding biology reported the existence of a ‘polygynous’ or ‘promiscuous’ mating system (Ponomareva 1983; Collins 1984; Launay and Loughland 1995), with the absence of any male parental care (Gaucher 1995). In polygynous birds, habitat requirements might be different between sexes, at least during the breeding season (Emlen and Oring 1977). Despite this, all studies on houbara bustard habitat selection were performed at the population level without segregating sexes or ages.
Knowledge of the polygynous strategy of the houbara bustard was recently improved in eastern Morocco, where data collected on the distribution of breeding birds showed strong differences in the use of space, with an aggregation of males on traditional display sites that females visited for the purpose of mating before nesting elsewhere without any male parental care (Hingrat et al. 2004). These behaviours apparently fulfilled the definition of the lek mating system (Höglund and Alatalo 1995). However, males aggregated in a dispersed fashion, called ‘exploded-leks’, compared to true leks where display males are separated by only a few metres (Bradbury 1981; Oring 1982). Consequently, the space between males may offer suitable habitats where females can forage or even nest. In this case, when males defend habitats and the resources they shelter, the mating system is called a ‘resource-defence polygyny’, i.e. where males indirectly control access to females by monopolising critical resources (Emlen and Oring 1977). In the houbara bustard, owing to a lack of knowledge on habitats, food availability, and bird habitat requirements during the breeding season, no valid conclusion can as yet be drawn on the species’ mating system.
In this paper, we analysed habitat use and selection in male and female houbara bustards at different spatial and temporal scales in order to answer the following questions: (1) were there sexual differences and seasonal variations in habitat use in the houbara bustard in eastern Morocco and, (2), if so, are these variations related to food availability? This work helped us understand habitat selection in our population and identify suitable habitats for conservation planning. Furthermore, habitat use and bird behaviour during the breeding season, related to resource availability, improved our knowledge of the species’ mating system.
Therefore, throughout one complete year, we studied habitat use and selection of a wild population of adult male and female houbara bustards in an area were food availability was simultaneously measured. We used the arthropod activity biomass as an index of the overall food resource for two main reasons: (1) in semi-desert habitats, vegetation is patchily distributed and closely related to water availability (Le Houérou 1986; Ali et al. 2000; Kutiel et al. 2000), with strong positive relationships between vegetation and arthropods in terms of diversity, phenology and biomass (Crawford 1981; Seymour and Dean 1999); and (2), despite the omnivorous diet of the houbara bustard, arthropods probably represent the main daily energy intake in adults (Tigar and Osborne 2000), and females are supposed to feed their chicks entirely with arthropods in the first few weeks following hatching (Collar and Goriup 1983). We investigated annual habitat selection using the habitat type composition of buffer zones around radio-locations, and we studied whether selected habitat types were used disproportionately to their availability (study area composition) (Neu et al. 1974). We also tested whether males and females were selective in their habitat use in the four seasons (autumn, winter, spring and summer). Differences between sexes and seasonal variations in habitat use were then investigated using multiple comparisons based on log-ratios of habitat proportions (Aitchison 1986). Finally, in each season, habitat preferences or avoidances were compared to food availability. The conservation implications of our results are discussed, with special reference to houbara bustard habitat and population management and hunting pressure in eastern Morocco.
Methods
Study area
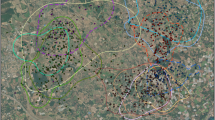
The study was undertaken in a piedmont named Al Baten, which encompasses 663 km² of desert steppe habitat situated on the east side of the Middle Atlas along the Moulouya river. It is centred at 33.23°N, 03.94°W, 130 km south-east of Fez (Fig. 1). The climate is semi-desertic with a hot summer and a cold winter. Rainfall is irregular with less than 200 mm per year, with a peak in autumn and a second in spring (April-May). The topography is gentle, with altitudes ranging from 800 to 1,700 m asl. Erosion processes have created undulating gravel plains, called reg, crossed by a dense drainage network of wadis temporarily flooded by irregular rainfalls (Raynal 1961). In this arid ecosystem, the vegetation is mostly concentrated in wadis and characterised by high bushes such as Zizyphus lotus (Ramnaceae) and Retama sphaerocarpa (Fabaceae). In contrast, the reg is covered by a shorter sparse shrubby vegetation dominated by associations of Lycium intricatum (Solanaceae) with various Salsolaceae. Steep slopes above 1,000 m asl are densely covered by esparto grass (Stipa tenacissima, Poaceae), while, in gently sloping areas, merging wadis create large temporarily flooded areas. In these irregularly flooded areas, sedimentation of silt and clay provide deeper soils densely covered by Salsola vermiculata associated with Atriplex halimus. These productive areas are often ploughed by locals to cultivate wheat in small ‘rainfed fields’. Otherwise, most of the human livelihood relies on livestock grazing. We chose Al Baten because it harbours a breeding population of houbara bustards, which has been surveyed and protected from hunting since 1996 (Lacroix 2003).
Al Baten study area (663 km²): location in eastern Morocco and habitat composition. The buffer zone represents the enlargement of a sampled area (1 km radius buffer zone) which highlight details on the delimitation of point and linear habitats (fields and wadis). The 100% Minimum Convex Polygon is based on all radio-locations of wild houbara bustards (Chlamydotis undulata undulata) collected since 1996 (n = 5,543)
Habitat types definition
To investigate relationships between habitat use and food availability, we used the results from a habitat classification of Al Baten made in 2002 (Hingrat et al. 2006). During this previous work, ordination methods (Anderson and Robinson 2003; Anderson and Willis 2003), based on vegetation structure and arthropod species assemblages, provided an a posteriori classification of six habitat types: (1) esparto grass steppes on hills, (2) cultivated fields, (3) temporarily flooded areas (TFA), (4) wadis, (5) reg with short perennials (RSP), and (6) reg with tall perennials (RTP). The average vegetation height and cover of each habitat types calculated in Hingrat et al. (2006) are summarised in the Table 1.
Habitat types were delineated during field surveys using a Global Positioning System (GARMIN 2+ GPS locator) and an habitat map was drawn using a Geographic Information System (GIS, ArcView 3.2; Environmental Systems Research Institute, Redland, Calif., USA). Linear habitat features such as wadis were extracted on the GIS from a topographic map at a 1:100,000 scale (Agence Nationale de la Conservation Foncière, du Cadastre et de la Cartographie) (Fig. 1).
Food availability
In each habitat type, arthropod availability was measured using pitfall traps of 10-cm diameter buried so that the upper opening just reached the ground surface. Note that because the six defined habitat types resulted from a previous habitat classification (see Habitat types definition), the number of traps per habitat type was not equal (Table 1). Traps were installed 25 m apart with one on bare soil and the other one at the foot of a bush. Traps were filled with 30 ml of ethylene glycol and emptied twice a month from November 2001 to October 2002. Except for spiders that were not identified, and Collembola and small Acarians that were not counted, all specimens were identified to species when possible or otherwise to morphospecies and labelled as “recognisable taxonomic units” (RTUs) (Oliver and Beattie 1993). This method avoids the use of fine-scaled formal taxonomy down to species level, and is considered adequate for the detection of differences among assemblages (Oliver and Beattie 1993; Oliver and Beattie 1996).
The overall sample of arthropods was dominated by ants (82%) and beetles (8%) (Hingrat et al. 2006). The dominance of these two groups is a characteristic of arthropod assemblages in semi-desertic and desertic areas (Tigar and Osborne 1997; Seymour and Dean 1999; Tigar and Osborne 1999; Dangerfield et al. 2003). The houbara bustard is an opportunistic bird and its diet reflects this dominance of ants and beetles (Collins 1984; Saint Jalme and Van Heezik 1996; Tigar and Osborne 2000). Thus, we used ants and beetles as indicators of food availability in the six habitat types.
Using pitfall traps to assess food availability, invertebrates caught are both diurnal and nocturnal (Tigar and Osborne 1999). Houbara bustards forage mostly after the sunset and before sunrise, but can also forage at night (Combreau and Launay 1996). In our study, direct searches of dwelling invertebrates conducted during the pitfall-trap sampling period showed that whether the two methods resulted in the collection of different arthropod assemblages, the dominant ones (ants and beetles) were shared by both (Hingrat et al. 2006). Thus, we assumed that ants and beetles caught using pitfall traps were representative of the food availability where houbara bustards forage during the day.
As stated above, the sampling effort was not equal between habitat types (Table 1). Thus, to test for seasonal and spatial variations in food availability we computed an index of ant and beetle activity-biomasses, from the biomass of individuals trapped per effective trap per trap day. The biomass was estimated by weighting the abundance of each RTU by its body length (in mm).
Bird trapping, tracking and breeding behaviour
We trapped adult houbara bustards in the Al Baten study area in three consecutive breeding seasons, from 2000 to 2002. We used nylon snares placed around the nest or surrounding a wild caught chick to catch females (Launay et al. 1999; Seddon et al. 1999; Hingrat et al. 2004), while snares were placed around a dummy female on displaying sites to catch males. Two different types of battery-powered transmitters were used: solar backpacks (45 g, Advance Telemetry System) and necklaces (18 g, 36 months, HSL RI-2CSP; Holohil System, ON, Canada). During 1 year, 7 females and 13 males were radio-tracked on Al Baten with a mean of 46 (±9.5) radio-locations [range (30–66)]. Houbara bustards were monitored from the ground and by aerial telemetry. Ground radio-tracking was undertaken by direct observation with four-wheel-drive vehicles using a portable scanner-receiver and a 3-element yagi antenna (AF Antronics, Urbana, Ill., USA). Aerial locations were taken from a Maule-7 B-235 aircraft using one 2-element yagi antenna fixed to each wing strut. A 4-element yagi antenna (AF Antronics) fixed on the right wing strut and pointed forward allowed signals to be received from as far as 45 km at an altitude of 1,800 m. Aerial and ground radio-tracking were both generally carried out weekly.
During the breeding season, males were regularly located early in the morning in order to assess their display site fidelity. In females, we tried to obtain at least two locations each week in order to locate their first nest and eventual replacement clutches.
In addition, we used nest and display site census data collected between 2001 and 2002 (Hingrat et al. 2004) to explore the breeding cycle of adult houbara bustards in relation to food availability (arthropod-activity biomass).
Annual habitat use and selection
In order to estimate habitat use, we calculated the proportions of habitat types within buffer zones of 100-m radius around birds’ radio-locations. We preferred using such zones instead of the exact locations themselves because of the following biases in location recordings: (1) observer accuracy, (2) avoidance movements of birds during ground tracking, (3) the speed and altitude of the plane during aerial tracking, and (4) the intrinsic accuracy of the global positioning system (White and Garrot 1990; Gantz and Stoddart 1997; Hulbert and French 2001).
Habitat selection was examined for males and females separately using the ‘Neu method’. This test is based on the chi-square goodness-of-fit analysis (Fleiss 1981), coupled with the placement of Bonferroni confidence intervals around proportional use, to estimate which habitats were selected disproportionately (preferred or avoided) to their availability (Neu et al. 1974). To circumvent problems linked to the definition of habitat availability due to the delineation of an arbitrary study area (Aebischer et al. 1993; Litvaitis et al. 1996), we followed the recommendation of McClean et al. (1998) by delimiting a minimum-sized study area using the outermost boundary of a 100% Minimum Convex Polygon (MCP) based on all houbara bustard locations recorded since 1996 (n = 5,543) on Al Baten (Fig. 1).
The difference in habitat use between sexes was compared using one-way MANOVA based on log-ratios of habitat proportions within buffer zones (Aitchison 1986). The log-ratio transformation overcomes the problem of lack of independence between the proportions of habitat types used that sum to 1 (unit-sum constraint) by converting the (n−1) log ratios, using one habitat proportion (here Field) as the denominator (Aitchison 1986; Aebischer et al. 1993).
Seasonal habitat use and selection
For comparisons between habitat use and seasonal food availability, all birds included in the analysis were resident males and females of Al Baten area, radio-tracked from November 2001 to October 2002. Each bird sample of radio-locations was divided into four seasons: autumn (October–December), winter (January–March), spring (April–June) and summer (July–September). In each season, we recorded a mean of 12 (±4.6) radio-locations per individual. For each sex and in each season, we used the ‘Neu method’ to investigate habitat selection (Neu et al. 1974). As in the annual habitat selection analysis, the habitat availability was restricted to the 100% MCP based on all houbara bustard locations (see above).
Differences in habitat use between sexes within seasons and between seasons for each sex were compared using one-way MANOVA based on log-ratios of habitat proportions within buffer zones (Aitchison 1986).
The areas of each habitat within the study area and the buffer zones were calculated using a Geographic Information System (GIS, ArcView 3.2; Environmental Systems Research Institute). Chi-square goodness-of-fit analyses were performed using RSW (Resource Selection Analysis Software for Windows) (Leban 1999). Mean comparisons were conducted using SYSTAT 7.0 (http://www.spss.com).
Results
Breeding behaviour
Display counts indicated that males started displaying in January and continued to late May, with a peak in April (Fig. 2). However, radio-tracking surveys showed that one male reached its display site earlier, in December. In addition, from November, we observed several tagged males performing courtship displays outside their respective display sites, when they were in flocks of both sexes. These displays were often followed by agonistic behaviours between males. In January, the 13 males behaved more solitarily and were faithful to the site where they were trapped in 2000 or in 2001.
Seasonal variation of ant (filled square) and beetle (open triangle) activity-biomasses between November 2001 and October 2002, related to the breeding cycle of adults male and female houbara bustards. Stars indicate a significant (P < 0.05) increase or decrease in the arthropod activity-biomass between months. White bars are the mean daily sightings of displaying males. Grey bars are the number of nests found each month
Radio-tagged females nested from 20 February to early June. During nest surveys, we did not find any houbara bustard nests before this date. The latest nest was found on 20 June, and the laying peak occurred in May (Fig. 2).
The reproductive success of females was very low, since only two females reared two chicks each to fledging. Chicks spent between 6 and 10 weeks with their mother. In cases of clutch or brood predation, most females laid replacement clutches. In fact, six of the seven females laid a second clutch at least 15 days after the loss of the first one, and four of them even laid a third clutch. The nest of the seventh female was found in June and we probably missed its first breeding attempts.
Annual habitat use and selection in males and females
During the year, males and females were observed mainly in the reg with short perennials (RSP), in temporarily flooded areas (TFA) and in the reg with tall perennials (RTP) (Fig. 3). Wadis and fields were less used and esparto grass totally unused by males.
Annual habitats used by 13 male and 7 female houbara bustards compared to habitat availability. Habitat availability was delimited by a 100% Minimum Convex Polygon based on all radio locations of wild houbara bustards collected since 1996 (n = 5,543). Bars represent mean proportions (±SD) of each habitat type within buffers (100 m radius) around annual birds’ radio-locations. Subscript letters indicate a significant difference between the use and the availability (P < 0.05), with P for habitats preferred and A for habitats avoided
The chi-square goodness-of-fit test showed that males were selective for habitats because habitat proportions within buffer zones differed significantly from habitat availability (χ 2 = 434.06, df = 5, P < 0.001). According to Bonferroni confidence intervals TFA was significantly preferred, whereas RSP and esparto grass were significantly avoided (Fig. 3).
Females were also selective for habitats in comparison to their availability (χ 2 = 18.16, df = 5, P < 0.05). But none of the habitat types appeared to be significantly avoided or preferred.
The comparison of log-ratios of habitat proportions within buffer zones, showed that males and females tended to differ in their habitat use (one-way MANOVA: F = 15.73, df = 5, P < 0.001). However, as esparto grass was totally unused by males, it may have skewed the analyses. Thus, we repeated the comparison with five habitat types only (by excluding the esparto grass) and still found a significant difference in habitat use between sexes (F = 13.94, df = 4, P < 0.001).
Seasonal variation in habitat use and selection by males and females
In the four seasons, habitat selection by each sex appeared to be significantly different from habitat availability (Fig. 4). Throughout the seasons, we observed a significant variation in habitat use in males (MANOVA: F = 1.85, df = 3, P < 0.05) and in females (F = 1.85, df = 3, P < 0.01). However, in males, pair-wise comparisons (Bonferroni adjustment) showed no significant differences in the use of each habitat type between the four seasons, indicating that males used them in similar relative proportions throughout the year. Compared to their availability, TFA was significantly preferred, while esparto grass was significantly avoided in all four seasons by males. Males also significantly avoided RSP in autumn, winter and spring. In winter, the preference for RTP appeared significant and wadis were significantly avoided (Fig. 4).
Habitat use by 13 male (white bars) and seven female (grey bars) houbara bustards compared to habitat availability (black bars) in the four seasons (seasons were defined as in Table 2). Habitat availability was delimited by a 100% Minimum Convex Polygon based on all radio locations of wild houbara bustards collected since 1996 (n = 5,543). Bars represent mean proportions (±SD) of each habitat type within buffer zones (100 m radius) around seasonal radio-locations. The results of the chi-square goodness-of-fit test (Neu et al. 1974) are shown on each graph and for each sex. This test indicates whether birds were selective for habitats in comparison of their availability. Subscript letters indicate a significant difference between the use and the availability (P < 0.05), with P for habitats preferred and A for habitats avoided. In each season, we indicated the mean ant (filled square) and beetle (open triangle) activity-biomass within the six defined habitats: esparto grass on hills (Esparto), fields, temporarily flooded areas (TFA), reg with short perennials (RSP), reg with tall perennials (RTP) and wadis
In females, the observed significant seasonal variation in habitat use was related to a significant increase in the use of RTP from winter to spring and a significant decrease in summer (Bonferroni adjustment). Indeed, in spring, RTP was significantly preferred by females. Otherwise, the use of other habitats did not differ significantly between seasons.
Comparisons between sexes in each season showed that habitat use differed significantly between males and females in winter (F = 5.32, df = 5, P < 0.001), spring (F = 8.48, df = 5, P < 0.001), and summer (F = 2.86, df = 5, P < 0.05), but did not differ in autumn (F = 1.63, df = 5, P = 0.15).
By excluding esparto grass, habitat use also differed significantly between males and females in winter (F = 6.46, df = 4, P < 0.001), spring (F = 6.75, df = 4, P < 0.001) and summer (F = 2.83, df = 4, P < 0.05), but did not differ in autumn (F = 0.42, df = 4, P = 0.79).
Food availability
During the annual cycle, the activity-biomass varied significantly for ants (ANOVA: F = 104, df = 11, P < 0.001) and beetles (F = 21.1, df = 11, P < 0.001). For both groups, we observed an increase in arthropod biomass from winter to summer with a subsequent decrease from summer to autumn (Fig. 2). However, these seasonal fluctuations differed between ants and beetles. Indeed, although the activity-biomass of the two groups increased significantly between April and June (Bonferroni adjustment, P < 0.001), it decreased suddenly from June to July for beetles (P < 0.001), whereas in ants the activity-biomass stayed at a high level during summer and only decreased in autumn (Fig. 2).
Seasonal variations of ant and beetle activity-biomasses were also significant in all habitat types (Tables 2, 3). A means comparison also showed significant variations in ant and beetle activity-biomass between habitats within seasons (Tables 2, 3).
However, for ants, among comparisons between habitats within seasons, only two means were significantly higher than all other habitats: the mean fields ant biomass in winter and the mean RSP ant biomass in summer. In this latter case, the mean had a high standard deviation (96.8 ± 193.1) (Table 2).
For beetles in autumn, the activity-biomass was similar in fields and TFA, and significantly greater than in all other habitats, which did not differ between themselves (Table 3). In winter, the beetle activity-biomass was also significantly greater in fields, but appeared similar across the five other habitats. Then, in spring, the biomass was homogeneous across most habitats, except in RSP which had a significant lower beetle activity-biomass than fields, TFA, RTP and wadis. In summer, the beetle activity-biomass was again significantly higher in fields, followed by TFA, which together were significantly different from all other habitats.
Thus, in autumn, winter, and summer, greater amounts of ants were found in fields and wadis and RSP (Table 2), whereas beetles were most abundant in fields, wadis and TFA (Table 3).
Note that, in most cases, esparto grass had the lowest ant and beetle activity-biomasses. In spring, these differences disappeared and, despite the overall significant variation in means of beetle and ant activity-biomasses, we did not find significant differences in pair-wise comparisons of habitat types. In fact, resource availability increased in all habitat types, but in an unequal manner. For example, in esparto grass and RTP, the ant activity-biomass was respectively 14-fold and 48-fold greater in spring than in winter, whereas in fields the increase was less than 4-fold (Table 2). Consequently food resources appeared to be uniformly distributed throughout the study area in spring.
Relationship between habitat use and food resources
Two main results emerged from comparisons of habitat use and food availability (Fig. 4). Firstly, despite their high ant and beetle activity-biomasses, fields were unused by both sexes most of the year, except in summer by females. Secondly, habitat use by adult houbara bustards seemed to better reflect variations in beetle activity-biomass than in ant activity-biomass, except in winter when arthropod resources were very low and when both taxonomic groups fluctuated similarly between habitats.
When fields were excluded, in autumn, spring and summer, habitat types with the highest beetle activity-biomass were preferred by houbara bustards and those with the smallest beetle biomasses were avoided. For example, in spring, RSP had the lowest beetles biomass and was significantly avoided by both sexes (Table 3; Fig. 4). The preference for esparto grass by females in autumn was an artefact due to one female which exclusively used this habitat type for several weeks.
For ants, we observed a weak relationship between the habitat types used by adult houbara bustards and their amount of food resources (Table 2; Fig. 4). In autumn, the richest habitat types (field and RSP) were respectively unused and even avoided by both sexes. However, in winter, TFA, RSP and wadis had a similar ant biomass, but both sexes preferred TFA, while males avoided RSP and wadis. In summer, the highest ant abundance occurred in RSP, but both sexes preferred TFA. In spring, despite the uniform availability of ants, the greatest increase was observed in RTP, which was preferred by females. At this time, most females were nesting, with a peak in May that preceded the peak in arthropod biomass in June (Fig. 2).
Discussion
The spatial and seasonal patterns of habitat use and selection by male and female houbara bustards in eastern Morocco supported our hypothesis of a sexual difference in habitat requirements linked to reproductive needs and social organisation.
The houbara bustard annual cycle
Clearly, male and female houbara bustards behaved differently most of the year. In adult males, the year could be divided in two distinct periods: (1) a non-breeding period, in summer and autumn, when males foraged in flocks of both sexes and juveniles, and (2) the display period, in winter and spring, when males were territorial and defended their display site, but could forage in small groups of neighbouring males (Hingrat et al. 2004). In adult females, the pattern was more complicated and variable throughout the year, and between individuals. In the most simple case, a female that bred successfully at its first attempt had four main periods in its annual cycle: (1) the non-breeding period, when the female foraged with males and juveniles, (2) the mating or pre-laying period, when the female visited males solitarily and made its mate choice, (3) the nesting period, when the female incubated its eggs for about 23 days (Gaucher 1995), and (4) the brooding period, when the female reared its chicks. However, this division of the year into four successive periods was not so simple because (1) females appeared asynchronous in their breeding attempts (from February to June), and (2) the breeding success was low with several replacement clutches. Thus, in a case of successive broods, periods (2) and (3) were repeated in the season, which increased the variability in habitat requirements between months and between females. Although replacement clutches have been reported for the Asian houbara bustard (Combreau and Launay 1999; Combreau et al. 2002), this remains poorly documented, especially in the case of the African houbara bustard. Here, we showed that replacement clutches were common in our population. Moreover, recent surveys of radio-tagged breeding females in spring 2004 (South-East Morocco) showed that, under highly favourable environmental conditions (heavy rain in spring), females can rear two successive broods to fledging (unpublished data).
Habitat use and selection
Owing to the observed differences in behaviour between sexes, we expected differences in habitat use between males and females. Our results confirmed our hypothesis, at least from winter to summer, i.e. a period that covers the entire breeding season for both sexes. In contrast, in autumn, habitat use in males and females did not differ. From autumn, we observed a significant decrease in food availability in all habitats, and houbara bustards formed foraging flocks composed of both sexes and juveniles. In birds, flocking occurs when resources are low and/or patchily distributed, and help individual survival by increasing foraging efficiency and predator detection (Pulliam 1973; Gardner 2004). As a consequence, males and females foraged in the same habitat types.
The next step of our study was to answer the following questions: which habitats were preferred by each sex, when and why, and can these preferences be related to food availability?
In terms of their annual habitat use, females were selective compared to habitat availability. However, they did not show any preference in their annual habitat requirements. This can be explained by the great diversity of behaviours in their annual cycle (see above). Habitat requirements might have varied between activities such as foraging, mating, nesting or feeding chicks. Consequently, the females’ annual range composition may have reflected this variability. In addition, as previously shown, females had larger home-ranges than males, owing to large movements when visiting males aggregate for the purpose of mating and when travelling from separate wintering and nesting areas (Hingrat et al. 2004). Consequently, during these movements they were able to cross the entire study area in a day and their range might have included all available habitat types.
When each season was considered separately, females appeared selective with regard to habitat availability and, despite their asynchrony in laying, we found a significant variation in habitat use throughout the seasons. This variation was mainly due to a significant preference for the reg with tall perennials (RTP) in spring. In fact, females increased their use of RTP when arthropod activity-biomass increased, indicating that this habitat type was probably used by females only when food resources were abundant. However, in spring, arthropods were abundant everywhere and RTP was probably chosen for additional key factors. Note that RTP was mainly used by the two females that reared broods. Because chicks are unable to fly until 1 month old, during the first few weeks following hatching they are easy prey for foxes or raptors (Saint Jalme and Van Heezik 1996; Combreau et al. 2002). At this stage, the female relies entirely on the chicks’ camouflage and ability to freeze motionless to avoid detection (Combreau et al. 2002). In addition, in the first weeks, the chicks’ diet is supposed to be essentially composed of invertebrates (Collar and Goriup 1983). In our study, the laying peak in May that preceded the peak in arthropod activity-biomass in June supported this hypothesis. Assuming an incubation period of 23 days, most hatchings on Al Baten probably coincided with the peak in arthropod availability. Thus, RTP (mainly composed of associations of Lycium intricatum with esparto grass, Launea arborescens, or Noaea mucronata) probably offered both a high abundance in food resources and a suitable vegetation cover and height (Tables 1, 2, 3).
Males appeared to be selective in the composition of their annual range and in each season. This was mainly due to their avoidance of esparto grass and preference for temporarily flooded areas. Surprisingly, whereas Hingrat et al. (2004) showed that the display behaviour in adult males resulted in a significant decrease in their home-range size, here we found that, throughout the seasons, males used the same habitats in similar relative proportions. Thus, despite changes in use of space and behaviour, the habitat type selected seemed to remain constant in males. In fact, we noted that even if males did not display from June to December, they were sedentary and faithful to their display site surroundings. These results, added to the clumping behaviour of display males, underlined the importance of display site selection in determining male distribution, home-range size, shape and composition. In China, Yang et al. (2002) studied display site selection in males and showed that, even though males conducted their courtship in open areas with a low vegetation cover, they were also close to well-vegetated patches for foraging or escaping from predators. In our study, during the breeding season, males avoided open habitats (reg with short perennials) and preferred temporarily flooded areas. However, in our sampling design, we deliberately located birds at different hours of the day in order to consider all their daily activities. Consequently, the preferences observed in males reflected a usage of habitats, not solely for displaying, but also for feeding, roosting, hiding, etc. As a result, the probable low proportion of radio-locations recorded while the males were displaying, added to the small sample of birds (n = 13), possibly underestimated the importance of open habitats such as reg with short perennials for displaying males in the breeding season. Obviously, more studies at the scale of the display site are needed to fully understand habitat selection in breeding males.
Mating system of the houbara bustard
All these results on behaviour and habitat use helped us to conclude that an exploded-lek mating system existed in our houbara bustard population. In lekking species, female choice of particular males for mating is based on characters that are not related to immediate gains such as access to resources (Emlen and Oring 1977). But these criteria appear to be unclear in exploded-lekking species, as male territories may contain critical resources for females (or their brood) (Bradbury 1981; Oring 1982). Emlen and Oring (1977) argued that, when resources are abundant and uniformly distributed in space, there is little opportunity for resource monopolisation by males. Such conditions were met in our study, with a similar amount of food resources among habitat types during the breeding season. However, breeding success might not rely solely on food availability, but also on cover and height of the vegetation in particular habitat types (here, RTP). In the breeding season, habitat use differed significantly between sexes and, while females selected preferentially RTP, males preferred temporarily flooded areas (TFA). This indicated that the critical resources used by females were not defended by males, and the mating system of the houbara bustard in eastern Morocco fulfilled the definition of a true exploded-lek.
Human disturbance
A surprising result in our study was the avoidance of fields by both sexes, although this habitat type had the highest ant and beetle activity-biomasses (Tables 2, 3). In the Canary Islands, Medina (1999) reported that houbara bustards foraged in cultivated fields which produce an environment with a high food availability (Dominguez-Casanova 1989). However, Medina (1999) failed to find any relationship between houbara bustard habitat use and arthropod availability and argued that birds used cultivated fields in relation to alfalfa (Medicago sativa) cover. In our study area, the main crop was wheat, which is probably less attractive for houbara bustards than alfalfa. In addition, these artificial habitats were probably less secure than other habitat types, since they were distributed close to roads, villages and isolated farms. Furthermore, a recent study in the same area highlighted the negative impact of the human presence on houbara bustard distribution (Le Cuziat et al. 2005).
Conservation implications
In eastern Morocco, concern over the decline of the houbara bustard led in 1996 to the establishment of the Emirates Center for Wildlife Propagation (ECWP) near Missour. The aim of the ECWP is to secure self-sustaining wild populations and it has identified as research priorities the ecology and behaviour of wild populations, and the study of their habitats (Lacroix 2003). Studying a species’ mating system and assessing mechanisms of male and female habitat selection, as well as the processes determining choice and the role of resources, is obviously of value for conservation (Höglund 1996; Morales et al. 2001).
Our study showed that male and female habitat requirements were not similar. Thus, conservation measures directed to increase female breeding success might be different from those associated with male occurrence.
In terms of habitat management, we showed that birds preferred temporarily flooded areas and avoided fields because of human disturbance. To date, fields account for approximately only 0.5% of the study area, and temporarily flooded areas 13% (Table 1). As farmers cultivate within temporarily flooded areas for their deep soil properties and higher moisture level (Fig. 1), we must pay attention to a possible expansion of cultivated areas which may lead to a loss of suitable habitats and an increase in human disturbance (Le Cuziat et al. 2005).
In terms of population management, the houbara bustard remains the favoured quarry of Arab falconers in eastern Morocco and excessive hunting is thought to be the major cause of population decline (Collar 1980; Goriup 1997; Bailey et al. 1998). Effective control of hunters is difficult and we do not have accurate estimates of past and present hunting pressures. An unpublished report by the local forestry authority (Ministère Délégué Chargé des Eaux et Forêts) related that falconers took on average 121 houbara bustards each year around Missour between 1983 and 1997 (range 35–443). On Al Baten, point-counts conducted in winter 2001 showed a density of 0.14 houbara bustards per km² (ECWP, unpublished data). Furthermore, Hingrat et al. (2004) reported that about 40 adult males displayed on traditional display sites in spring 2001. In view of the low population density, the breeding site fidelity of birds and their low reproductive rates, repeated hunting in the same area, without any game management, could have drastic effects on residual populations and rapidly lead to their local extinction. Fortunately, on Al Baten, hunting stopped following the creation of the ECWP and the area has been protected since 1996 (Lacroix 2003). In 1996, 10 displaying males were counted on the area compared to more than 50 today (ECWP, unpublished data), indicating a probable local increase of the population density.
However, a local increase in density is a poor indicator of population health, especially in lekking species. As pointed out by Alonso et al. (2004) for the lekking great bustard (Otis tarda), the fidelity to traditional sites and the strong conspecific attraction of birds might increase extinction risks. When a lek disappears, individuals do not tend to colonise vacant suitable areas but might concentrate in already occupied ones, i.e. joining remnant leks (Alonso et al. 2004). The result is an increased concentration of populations and, consequently, a greater vulnerability of the species to local catastrophes (Alonso et al. 2000, 2004).
Because of the importance of breeding sites in this lekking bird, leks should be recognised as priority areas for conservation planning, and we urgently need intensive surveys throughout eastern Morocco to locate traditional display sites.
Zusammenfassung
Habitatnutzung und Paarungssystem der Kragentrappe (Chlamydotis undulata undulata) in einer Halbwüste Nordafrikas: Konsequenzen für ihren Schutz
Untersuchungen zu Bewegungsmustern und Größe der Streifgebiete der Kragentrappe zeigten geschlechts- und jahreszeitenabhängige Unterschiede in der Flächennutzung mit einem polygynen Paarungssystem, das ähnlich einem Typ “erweiterte Balzarena” oder einer Ressourcenverteidigungs-Polygynie ist, aber bisher noch nicht geklärt war. Wir verwendeten die Arthropoden-Biomasse als Index für die trophische Qualität von sechs definierten Habitaten, und wir telemetrierten sieben Weibchen und 13 Männchen, um zu untersuchen, ob jahreszeitliche Variation in der Habitatnutzung mit der Verfügbarkeit von Ressourcen zusammenhing, und um festzustellen, ob für brütende Weibchen kritische Ressourcen von Männchen monopolisiert wurden. Wir untersuchten die Habitatwahl in beiden Geschlechtern getrennt. Die Zusammensetzung des Habitattyps in Pufferzonen um die telemetrierten Aufenthaltsorte herum nutzten wir, um jährliche und saisonale Habitatwahl und bevorzugte Habitate mit einem χ2-Anpassungstest zu untersuchen. Habitatnutzung zwischen Geschlechtern und zwischen Jahreszeiten verglichen wir mit einer MANOVA auf der Grundlage von log-Verhältnissen von Habitatanteilen. In allen Jahreszeiten schienen beide Geschlechter signifikant selektiv zu sein für bestimmte Habitate, gemessen an ihrer Verfügbarkeit. Jedoch mieden Männchen Espartogras, während Weibchen alle Habitate nutzten. Die Habitatnutzung unterschied sich zwischen den Geschlechtern während der Brutsaison, aber nicht außerhalb. Im Frühjahr, wenn Nahrung reichlich und gleichmäßig verteilt war, bevorzugten Männchen zweitweilig überflutete Gebiete, und Weibchen bevorzugten Gebiete mit hohen mehrjährigen Pflanzen, die sowohl Nahrung als auch Deckung fürs Brüten boten. Kritische Ressourcen wurden von den Männchen nicht monopolisiert und das Paarungssystem erfüllte die Definition des Typs “erweiterte Balzarena”. Balzarenen spielen eine Schlüsselrolle bei der Fortpflanzung und sollten bei zukünftigen Schutzmaßnahmen berücksichtigt werden.
References
Aebischer N, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Aitchison J (1986) The statistical analysis of compositional data, 1st edn. Chapman and Hall, New York
Ali MM, Dickinson G, Murphy KJ (2000) Predictors of plant diversity in a hyperarid desert wadi ecosystem. J Arid Environ 45:215–230
Alonso JC, Morales MB, Alonso JA (2000) Partial migration, and lek and nesting area fidelity in female Great Bustards. Condor 102:127–136
Alonso JC, Martin CA, Alonso JA, Palacin C, Magaña M, Lane SJ (2004) Distribution dynamics of the great bustard metapopulation throughout a decade: influence of conspecific attraction and recruitment. Biodiv Conserv 13:1659–1674
Anderson MJ, Robinson J (2003) Generalized discriminant analysis based on distances. Aust J Stat 45:301–318
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: an ecologically meaningful approach for constrained ordination. Ecology 84:511–525
Bailey TA, Samour JH, Bailey TC (1998) Hunted by falcons, protected by falconry: can the Houbara Bustard (Chlamydotis undulata macqueenii) fly in the 21st century? J Avian Med Surg 12:190–201
Bradbury JW (1981) The evolution of leks. In: Alexander RD, Tinkle DW (eds) Natural selection and social behavior. Chiron Press, New York pp 138–169
Collar NJ (1980) The World status of the Houbara: a preliminary review. In: Coles CL, Collar NJ (eds) Symposium Papers on the Houbara Bustard. Sydenhams, Athens
Collar NJ, Goriup PD (1983) The ICBP Fuerventura Houbara expedition. Bustard Stud 1:1–92
Collins D (1984) A study of the Canarian Houbara Bustard (Chlamydotis undulata fuertaventurae) with special reference to its behaviour and ecology. PhD Thesis, University of London, UK, p 119
Combreau O, Launay F (1996) Activity rhythms of Houbara Bustards (Chlamydotis undulata macquenii) in relation to some abiotic factors. J Arid Environ 33:463–472
Combreau O, Launay F (1999) An estimation of the nesting success in a Houbara Bustard Chlamydotis undulata macqueeni population in Kazakhstan. Sandgrouse 21:171–175
Combreau O, Smith TR (1997) Summer habitat selection by Houbara Bustards introduced in central Saudi Arabia. J Arid Environ 36:149–160
Combreau O, Saint Jalme M, Seddon PJ, Rambaud F, van Heezik Y, Paillat P, Gaucher P, Smith T (1995) A program for Houbara Bustard restoration in Saudi Arabia. In: Bissonet JA, Krausman PR (eds) Integrating people and wildlife for a sustainable future. Proceeding of the first International Wildlife Management Congress. The Wildlife Society, Bethesda, Maryland, p 697
Combreau O, Gelinaud G, Smith TR (2000) Home range and movements of Houbara Bustards introduced in the Najd Pediplain in Saudi Arabia. J Arid Environ 44:229–240
Combreau O, Launay F, Lawrence M (2001) An assessment of annual mortality rates in adult-sized migrant Houbara Bustards (Chlamydotis [undulata] macqueenii). Anim Conserv 4:133–141
Combreau O, Qiao J, Lawrence M, Gao Y, Yang W, Launay F (2002) Breeding success in a Houbara Bustard Chlamydotis [undulata] macqueenii population on the eastern fringe of the Jungar Basin, People’s Republic of China. Ibis 144:E45-E46
Crawford CS (1981) Biology of desert invertebrates. Springer, Berlin Heidelberg New York
Dangerfield JM, Pik AJ, Britton D, Holmes A, Gillings M, Oliver I, Briscoe D, Beattie AJ (2003) Patterns of invertebrate biodiversity across a natural edge. Austral Ecol 28:227–236
Dominguez-Casanova F (1989) The Houbara Bustard in the Canary Island (Spain): toward a recovery plan. Bustard Stud 4:42–51
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Fleiss JL (1981) Statistical methods for rates and proportions, Second edn. Wiley, New York
Gantz GF, Stoddart CL (1997) Accuracy of aerial telemetry locations in mountainous terrain. In: Forum on Wildlife Telemetry: Innovations, evaluations, and Research Needs, vol. 2001. USGS, Northern Prairie Wildlife Research Center. http://www.npwrc.usgs.gov/resource/tools/telemetry/mountain.htm, Snowmass Village, Colorado
Gardner JL (2004) Winter flocking behaviour of speckled warbler and the Allee effect. Biol Conserv 118:195–204
Gaucher P (1995) Breeding biology of the Houbara Bustard Chlamydotis undulata undulata in Algeria. Alauda 63:291–298
Goriup PD (1997) The world status of the Houbara Bustard Chlamydotis undulata. Bird Conserv Int 7:373–397
van Heezik Y, Seddon PJ (1999) Seasonal changes in habitat use by Houbara Bustards Chlamydotis undulata macqueenii in Northern Saudi Arabia. Ibis 141:208–215
van Heezik Y, Seddon PJ (2002) Patch use and exploratory movements of a resident Houbara Bustard in northern Saudi Arabia. J Arid Environ 50:683–686
Hingrat Y, Saint Jalme M, Ysnel F, Lacroix F, Seabury J, Rautureau P (2004) Relationships between home-range size, sex and season with reference on the mating system of the Houbara Bustard Chlamydotis undulata undulata. Ibis 146:314–322
Hingrat Y, Ysnel F, Saint Jalme M, Le Cuziat J, Béranger P-M, Lacroix F (2006) Assessing habitat and resources availability for an endangered desert bird species: the Houbara Bustard. Biodiv Conserv (in press)
Höglund J (1996) Can mating systems affect local extinction risks? Two examples of lek-breeding waders. Oikos 77:184–188
Höglund J, Alatalo RV (1995) Leks. Princeton University Press, Princeton
Hulbert IAR, French J (2001) The accuracy of GPS for wildlife telemetry and habitat mapping. J Appl Ecol 38:869–878
Kutiel P, Kutiel H, Lavee H (2000) Vegetation response to possible scenarios of rainfall variations along a Mediterranean-extreme arid climatic transect. J Arid Environ 44:277–290
Lacroix F (2003) The Emirates Center for Wildlife Propagation: developing a comprehensive strategy to secure a self-sustaining population of Houbara Bustards in eastern Morocco. Houbara News 5:2
Launay F, Loughland R (1995) Breeding system of Houbara Bustard Chlamydotis undulata macqueeni: preliminary observations. Sandgrouse 35:14–17
Launay F, Roshier D, Loughland R, Aspinall SJ (1997) Habitat use by Houbara Bustard (Chlamydotis undulata macqueenii) in arid shrubland in the United Arab Emirates. J Arid Environ 35:111–121
Launay F, Combreau O, Aspinall SJ, Loughland RA, Gubin B, Karpov F (1999) Trapping of breeding Houbara Bustard (Chlamydotis undulata). Wildl Soc Bull 27:603–608
Lavee D (1985) The influence of grazing and intensive cultivation on the population size of the Houbara Bustard in the Northern Negev in Israel. Bustard Stud 3:103–107
Le Houérou HN (1986) The desert and arid zones of northern Africa. In: Goodall DW, Evenari M, Noy-Meir I (eds) Hot deserts and Arid Shrublands, vol 12 B, 1st edn. Elsevier, Amsterdam, pp 101–147
Le Cuziat J, Lacroix F, Roche P, Vidal E, Médail F, Orhant N, Béranger P-M (2005) Landscape and human influences on the distribution of the endangered North African Houbara Bustard (Chlamydotis undulata undulata) in Eastern Morocco. Anim Conserv 8:143–152
Leban F (1999) RSW. Resource Selection Analysis Software. In, Beta 8.1 edn. Department of Fish and Wildlife Resources. http://www.cnrhome.uidaho.edu/fishwild/Garton/tools(fleban@artemisia.wildlife.uidaho.edu)
Litvaitis JA, Titus K, Anderson EM (1996) Measuring vertebrate use of terrestrial habitats and foods. In: Bookhout TA (eds) Research and management techniques for wildlife and habitats. The Wildlife Society, Bethesda, pp 254–274
Martín AM, Nogales MA, Hernández MA, Lorenzo JA, Medina FM, Rando JC (1996) Status, conservation and habitat selection of the Houbara Bustard Chlamydotis undulata fuertaventurae on Lanzarote (Canary Islands). Bird Conserv Int 6:229–239
McClean SA, Rumble MA, King RM, Baker WL (1998) Evaluation of resource selection methods with different definitions of availability. J Wildl Manag 62:793–801
Medina FM (1999) Foraging use of cultivated field by the Houbara Bustard Chlamydotis undulata fuerteventurae Rothschild and Hartert, 1894 on Fuerteventura (Canary Islands). Bird Conserv Int 9:373–386
Morales MB, Jiguet F, Arroyo B (2001) Exploded leks: what Bustards can teach us. Ardeola 48:85–88
Neu CW, Byers CR, Peek JM (1974) A technique for analysis of utilization-availability data. J Wildl Manag 38:541–545
Oliver I, Beattie AJ (1993) A possible method for the rapid assessment of biodiversity. Conserv Biol 7:572–578
Oliver I, Beattie AJ (1996) Designing a cost-effective invertebrate survey: a test of methods for rapid assessment of biodiversity. Ecol App 6:594–607
Oring LW (1982) Avian mating systems. In: Farner DJ, King JR, Parkes KC (eds) Avian biology, vol 6. Academic, New York, pp 1–92
Osborne PE, Launay F, Gliddon D (1997) Wintering habitat use by Houbara Bustards Chlamydotis undulata in Abu Dhabi and implications for management. Biol Conserv 81:51–56
Ponomavera TS (1983) Reproductive behaviour and distribution of Houbara Bustards on their breeding grounds. Zool Zh 4:592–602
Pulliam HR (1973) On the advantages of flocking. J Theor Biol 38:419–422
Raynal R (1961) Plaines et Piedmonts du bassin de la Moulouya (Maroc Oriental). Etude Géomorphologique, 1st edn. IMFRAMAR, Rabat
Rushton SP, Ormerod SJ, Kerby G (2004) New paradigms for modelling species distributions? J Appl Ecol 41:193–200
Saint Jalme M, Van Heezik Y (1996) Propagation of the Houbara Bustard, 1st edn. Keegan Paul, London
Seddon PJ, Van Heezik Y (1996) Seasonal changes in Houbara Bustard Chlamydotis undulata macqueenii numbers in Harrat Al Arrah, Saudi Arabia: implication for managing a remnant population. Biol Conserv 75:139–146
Seddon PJ, Saint Jalme M, van Heezik Y, Paillat P, Gaucher P, Combreau O (1995) Restoration of Houbara Bustard populations in Saudi Arabia: developments and future directions. Oryx 29:136–143
Seddon PJ, Launay F, Van Heezik Y, Al Bowardi M (1999) Methods for live trapping Houbara Bustards. J Field Ornithol 70:169–181
Seymour CL, Dean WRJ (1999) Effects of heavy grazing on invertebrate assemblage in the Succulent Karoo, South Africa. J Arid Environ 43:267–286
Tigar BJ, Osborne PE (1997) Patterns of arthropod abundance and diversity in an Arabian desert. Ecography 20:550–558
Tigar BJ, Osborne PE (1999) Patterns of biomass and diversity of aerial insects in Abu Dhabi’s sandy deserts. J Arid Environ 43:159–170
Tigar BJ, Osborne PE (2000) Invertebrate diet of the Houbara Bustard Chlamydotis [undulata] macqueenii in Abu Dhabi from calibrated faecal analysis. Ibis 142:466–475
Tourenq C et al (2005) Alarming Houbara Bustard population trends in Asia. Biol Conserv 121:1–8
White GC, Garrot RA (1990) Analysis of wildlife radio-tracking data. Academic, San Diego
Yang W, Qiao J, Combreau O, Gao X, Zhong W (2002) Display-sites selection by Houbara Bustard (Chlamydotis [undulata] macqueenii) in Mori, Xinjiang, People’s Republic of China. J Arid Environ 51:625–631
Yang W, Qiao J, Combreau O, Gao X, Zhong W (2003) Breeding habitat selection by the Houbara Bustard Chlamydotis [undulata] macqueenii in Mori, Xinjiang, China. Zool Stud 42:470–475
Acknowledgments
The authors would like to thank His Highness Sheikh Mohamed Bin Zayed Al Nahyan, founder and sponsor, and His Excellency Mohammed Al Bowardi, General Manager of the Office of Mohammed Bin Zayed Al Nahyan, for his supervision and guidance. We thank Jacques Renaud, Manager of the Emirates Center for Wildlife Propagation, for his support. Special thanks are given to all involved in data collection during field surveys: Joseph Le Cuziat, Pierre-Marie Béranger, Pierrick Rautureau, Nicolas Orhant, Sébastien Caron, Olivier Fontaine, Ahmed El Houki and Houcine El Hlilou. We also thank Chalah Toni, and Alain Canard for their support and comments. Many thanks to Dr H. Britton for improving the English text and anonymous reviewers for suggestions to improve the manuscript. The capture of birds and invertebrate sampling were under permit from the “Haut Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification”, kingdom of Morocco.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Hingrat, Y., Saint Jalme, M., Ysnel, F. et al. Habitat use and mating system of the houbara bustard (Chlamydotis undulata undulata) in a semi-desertic area of North Africa: implications for conservation. J Ornithol 148, 39–52 (2007). https://doi.org/10.1007/s10336-006-0098-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-006-0098-9