Abstract
Objective
To investigate the variation in T2 at different zones of normal hip cartilage in children and the relationship between T2 value and age.
Materials and methods
Nineteen children with 30 normal hip joints were evaluated with a coronal T2 mapping sequence at a 3-Tesla MRI system. The femoral cartilage and acetabular cartilage were firstly segmented by mask-based interactive method and then equally divided into eight and six radial sections, respectively. Moreover, each radial section was further divided into two layers referring to the superficial and deep halves of the corresponding cartilage. Cartilage T2 of these sections and layers were measured and subsequently analyzed.
Results
There was a negative correlation between the T2 values in the hip cartilage and the age of children (rs < − 0.6, P1 < 0.05). Articular cartilage T2 increased at angles close to the magic angle (54.7°). Femoral cartilage and acetabular cartilage had a relatively shorter T2 in the radial sections near the vertex of the femoral head. The T2 values in superficial layers of both cartilages were significantly higher than those in deep layers (P < 0.05).
Conclusion
The T2 value decreases as the cartilage developing into a more mature state. Cartilage T2 values in the weight-bearing areas are relatively low due to an increase of collagen density and the loss of interstitial water. The restriction of the water molecules by solid components in the deeper layer of cartilage may decrease the T2 values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal articular cartilage is an elastic tissue with smooth and resilient surface, which is comprised of chondrocytes and the extracellular matrix (ECM) [1, 2]. The ECM molecules, primarily including water, proteoglycan and collagen, determine the cartilage functions such as loading-bearing, lubrication and antifriction. The biochemical assessment of articular cartilage by quantitative magnetic resonance imaging (qMRI) can provide a non-invasive and non-ionizing radiation way to reveal the early pathological changes [3, 4] for orthopedic conditions in children such as hip osteoarthritis (OA) [5], hip dysplasia [6], avascular necrosis (AVN) of the femoral head [7], etc. These disorders will have a serious impact on children, mainly related to limb development, joint function and quality of life. There are many reports on knee cartilage [8,9,10], but few are devoted to normal hip cartilage and related lesions, especially in children.
The most common qMRI techniques for articular cartilage include T1 mapping [11], \(T1\rho\) mapping [12], T2 mapping [13], \(T2*\) mapping [14] and so on. Among them, T2 mapping is frequently used to characterize the changes of water and collagen in articular cartilage [15]. Soellner et al. [13] found that T2 distribution is sensitive to early changes in articular cartilage injures. Watanabe et al. [16] performed T2 quantitative imaging based on multi-echo sequence on the hip joints of 12 healthy adults and found that there were differences in T2 values of different sub-regions of hip cartilage.
Clinically, it is not only time-consuming for experienced doctors to manually draw the region of interest (ROI) to calculate T2 value of a specific tissue, but also may lead to inaccurate T2 measurement and even misdiagnosis [17]. This study proposed a semi-automatic segmentation of hip cartilage in children and then analyzed the average T2 values of both femoral cartilage and acetabular cartilage at different zones to indirectly reflect the distribution of biochemical components in cartilage.
Materials and methods
Participants and their hip joints
From September 2019 to October 2020, 19 cases under 9-year-old were enrolled from all children who underwent 3-Tesla MRI (GE Discovery MR750) examination of hip joint in the Children’s Hospital of Soochow University (Suzhou, China). We used the following inclusion criteria: (1) bilateral hips scanned by MRI, (2) at least one normal healthy hip joint clinically diagnosed without any abnormalities and (3) no previous treatment. All parents of children signed informed consent to participate in this study with the approval of the hospital institutional review board.
Data acquisition by T2 mapping technology
Half an hour before MRI examination, all subjects were given 10\(\%\) chloral hydrate sedative orally at a dose of 0.5ml/kg to avoid the influence of some factors such as motion artifacts on image quality. During the scanning, the children were in the supine position and kept both legs in a symmetrical neutral position. Quantitative T2 mapping based on the fast spin echo (FSE) sequence was used to perform a coronal scan of bilateral hip joints. The parameters were set as follows: echo train length 8, slice thickness 3 mm; spacing between slices 4.5 mm; matrix size 256\(\times\)256; field of view (FOV) 300\(\times\)300 mm; excitation 1; bandwidth 244 kHz; flip angle 90\(^{\circ }\); repetition time 1500 ms; the first echo time (TE) 7.49 ms; echo spacing 7.49 ms.
Interactive semi-automatic segmentation
Due to the complex morphology of femoral cartilage and acetabular cartilage, an interactive semi-automatic algorithm based on mask was proposed to achieve a hip cartilage segmentation and the flow chart was shown in Fig. 1. Firstly, the first echo from the eight echo images was eliminated to improve the T2 accuracy [18] and the remaining echo images were used for T2 measurement to generate a pseudo-color T2 map based on the mono-exponential function method (SI=\(S_0\) \(\times\) \(exp(-TE/T2))\). Secondly, a binary image without hip cartilage was generated by the maximum inter-class variance segmentation algorithm (Otsu, 1978), in which the gray-scale T2 map converted from pseudo-color map was used. Then, other regions far from hip cartilage were manually selected into the above binary image, which form the inverted mask image. Finally, because of the features that T2 value decreases from the superficial to the deep layer in articular cartilage [19] and the femoral head is close to a 2/3 sphere, the femoral cartilage and acetabular cartilage could be separated from the whole hip cartilage. The T2 map of hip cartilage could be obtained by applying the mask on the original T2 map. The specific implementation of the segmentation method was further integrated into the graphical user interface (Fig. 2) developed by the MATLAB 2018b (The MathWorks, Natick, MA, USA) program.
Graphical user interface (GUI) for semi-automatic segmentation of hip cartilage. a–c were three parts, which were respectively used to display case information, the image processing manipulates and the original image and ROI. d mainly completed the segmentation and filling of holes based on the grayscale images. e was firstly obtained by multiplying the mask in d after inversion with original T2 map and fitted the central circle of the femoral head, and then relied on line 1 and line 2 to determine the acetabular cartilage region, which was similar to the femoral cartilage. f was mainly used to display the segmentation results, including mask, pseudo-color map and T2 value
ROI setting and T2 measurement
To evaluate the T2 distribution of hip cartilage, the femoral cartilage and acetabular cartilage were individually divided into eight radial sections (sections F1\(\sim\)F8) and six radial sections (sections A1\(\sim\)A6) with equal angles from the lateral to the medial, as shown in Fig. 3. Moreover, each radial section was further divided into two layers representing the superficial layer and deep layer. For T2 measurements, all the above ROIs were automatically separated from the whole cartilage. For the division of radial sections, the angle (denoted as \(\alpha\)) between the outermost and innermost margin line of femoral cartilage was firstly calculated, and then eight equal angular intervals were set to divide cartilage into eight radial sections. The similar approach was applied to the radial sections setting of acetabular cartilage. Furthermore, at each radial section, a thickness of half the cartilage thickness was calculated to determine both the superficial and deep layers for femoral cartilage and acetabular cartilage, respectively.
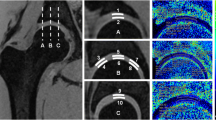
Division of femoral cartilage and acetabular cartilage. a Femoral cartilage (donated as F). b Acetabular cartilage (donated as A). Both femoral cartilage and acetabular cartilage were divided into radial sections and each radial section was further divided into superficial (transparent yellow area) and deep (transparent red area) layers. The central circle of the femoral head was found by fitting the points at the junction of the two types of cartilage. A center line that was parallel to the static magnetic field \(B_0\) and through the center of the femoral head was drawn. The magic angle 54.7\(^{\circ }\) relative to \(B_0\) was located in the section F6 of femoral cartilage or in the section A4 of acetabular cartilage
Statistical analysis
IBM@ SPSS@ Statistics (Version 26; IBM Corp., Illinois, USA) was used for all analyses. The measurement data in accordance with normal distribution were expressed as mean ± standard deviation. The unit of T2 value is ms. The relationship between the T2 value of each zone in normal hip cartilage and age was calculated by Spearman correlation coefficient (denoted as \(r_s\)). One-way ANOVA was used to compare the differences of T2 values in each zone at different age groups, and the least significant difference method (LSD) was used to make pairwise comparisons between groups. Independent sample t-test was used to compare the differences of T2 values between surface and deep layers. When \(P<0.05\), it can be considered that the difference is statistically significant.
Results
A total of 19 children were included in the collection of T2 mapping images, of which 30 hip joints were diagnosed as normal by clinical assessment. According to the age, the cases were divided into three groups as 0\(\le\)age<3 (\(n=9\)), 3\(\le\)age<6 (\(n=11\)) and 6\(\le\)age<9 (\(n=10\)).
According to one echo image of Fig. 4, we found that the head of femur was completely enclosed by articular cartilage to improve stability and the femoral cartilage in the shape of a semicircular ring clung to the femoral head. In early childhood, the acetabulum is a cartilage complex consisting of acetabular cartilage and Y-shaped cartilage due to the skeletal immaturity of the pelvis composed of the ischium, ilium and pubis bone [20]. The pseudo-color T2 map showed that cartilage T2 was about between 20\(\sim\)80 ms, and gradually decreased from the surface to the deep layer indicating that the water content of cartilage surface was higher than that of layer close to bone. For T2 measurement, both the femoral cartilage and the true acetabular cartilage were included in our study.
As shown in Table 1, results of rank correlation and one-way ANOVA analysis illustrated negative correlations between T2 values at various sections of femoral cartilage and the age of children (\(r_s<-0.60\), \(P1<0.05\)). With respect to the pairwise comparison of femoral cartilage T2 between various radial sections, some results obtained by the LSD post hoc test (P) were given in the following statements. In three age groups, femoral cartilage in the deep layers showed the shortest T2 values at section F3 except for the medial peripheral sections (\(P<0.05\)). There was no significant difference of cartilage T2 at superficial sections (F3\(\sim\)F5) near the vertex of femoral head (\(P<0.05\)). Femoral cartilage T2 in both superficial and deep layers had the tendency of an increase from section F3 to both sides. Furthermore, in the three age groups, femoral cartilage T2 in the superficial layer was higher than in the deep layer for each regional cartilage (\(P2<0.05\)).
As shown in Table 2, T2 values at all sections of acetabular cartilage were negatively correlated with age (\({r_s}\, < \, - 0.60\), \(P1<0.05\)). To investigate the difference of acetabular cartilage T2 between various radial sections, the data were analyzed statistically. In age < 3 group, there was no significant difference of acetabular cartilage T2 at sections A1\(\sim\)A4 in both superficial and deep layers (\(P>0.05\)). In 3\(\le\)age < 6 and 6\(\le\)age < 9 groups, acetabular cartilage T2 at sections A3 and A4 in both superficial and deep layers was higher than that at section A1 (\(P<0.05\)), and cartilage T2 at section A4 in both layers was also higher than that at sections A2, A5 and A6 (\(P<0.05\)). Furthermore, in the three age groups, acetabular cartilage T2 in the superficial layer was higher than in the deep layer for each regional cartilage (\(P2<0.05\)).
Discussion
Conventional morphological MRI techniques are not sensitive to the changes of biochemical components in the early stage of articular cartilage damage. The qMRI approaches could reveal certain biochemical parameters (\(T1, T2, T2*, T1\rho\), etc.) which were with respect to the inherent characteristics of cartilage with early degenerative changes. T2 mapping is a technique to evaluate the structural integrity and water content of cartilage. Previous studies have shown that the increase of T2 value is closely related to the loss of collagen fiber integrity and the increase of water content in cartilage matrix, so T2 value can be used as a biomarker for early observation of cartilage injury [10, 21].
Image segmentation of hip cartilage is an important prerequisite for T2 measurement. At present, the clinical measurement of cartilage T2 of hip joints mainly depends on doctors to draw the ROIs on the T2 map generated by post-processing workstation, which not only takes a lot of time and energy, but also has low reliability (repeatability). Scott et al. [17] proposed a semi-automatic segmentation approach for cartilage segmentation of hip MRI images, which mainly used a bespoke segmentation method applied recursively to automatically divide the data volume generated, and then the user selected the ROIs using the region editor. Ying Xia et al. [22] proposed a fully automatic scheme for segmenting individual femurs and acetabular cartilage plates in human hips from high-resolution 3D MR images. In our study, a semi-automatic segmentation algorithm based on mask was integrated into MATLAB GUI, and cartilage T2 was automatically measured at various ROIs. With the combination of human-computer interaction and automatic segmentation, femoral cartilage and acetabular cartilage could be segmented accurately, which would effectively reduce the complexity of the algorithm. Additionally, the interactive method improved the objectivity of T2 quantitative analysis to some certain degree and was friendly to low resolution images.
The T2 distribution of hip cartilage in children can provide an effective auxiliary diagnosis for early quantitative evaluation of related diseases from a long-term point of view. In this study, the distribution of T2 values was mainly conducted on the normal hip cartilage of children. It was found that cartilage T2 decreases gradually with the growing of age, which might be attributed to the increasing of collagen content during the maturation process of children’s bones. Watrin et al. [23] found that the T2 values progressively decreased with the maturation of cartilage, mainly because both the decrease of proteoglycan and the increase of collagen limited the space available for free water, which was consistent with our result.
In a research on knee joint, Meng et al. [24] pointed out that articular cartilage might show anatomical regional differences and spatial heterogeneity due to different load distribution in physiological activities. Ferro et al. [25] found that there was a significant difference between patients with femoroacetabular impingement (FAI) and asymptomatic volunteers by comparing the T2 values of lateral and medial acetabular cartilage under load and they believed that this was due to the increased load of medial acetabular cartilage. For children’s hip joints, because of the orientation-dependent dipolar interaction [26], cartilage T2 in both superficial and deep layers increased in radial sections oriented close to the magic angle relative to the static magnetic field \(B_0\) except for innermost sections. Furthermore, the hip belongs to a ball-and-socket joint, and the main stress points are concentrated on the vertex of the femoral head and the upper-inner edge of the acetabulum during joint activities. In these loading-bearing areas, we found femoral cartilage and acetabular cartilage showed the relatively low T2 values compared with cartilage T2 at the lateral peripheral sections and the section of magic angle 54.7\(^{\circ }\) relative to \(B_0\). Superficial cartilages located in the loading-bearing areas are more often worn with pressure during joint activities, which may lead to the increased density of collagen fibers and the exudation of interstitial water from the articular cartilage surface [27]. Changes from these biochemical components would result in a decrease of cartilage T2. However, when coupled with an increase of the angle relative to \(B_0\), the T2 values still showed an increasing trend. In a study of children with developmental dysplasia of the hip (DDH) under 2 years old, Sun et al. [28] also concluded that the femoral cartilage at the apex of the femoral head and acetabular cartilage in the surface of the ilium are the most characteristic changes.
Unlike mature articular cartilage [29], children’s cartilage can be depicted as three different layers under the high-resolution high-field-strength imaging [30]. The superficial zone is thin with collagen fibers parallel to the cartilage surface and the transitional zone is thicker with randomly oriented fibers. However, articular cartilage was actually divided into superficial and deep layers with a split of 50/50 in our study. The “superficial” layer in our study may contain the superficial zone and large part of transitional zone. As other reports [8, 31] have revealed from spatial variety, our study also showed that the T2 values of femoral cartilage and acetabular cartilage might decrease gradually from superficial layer to deep layer. In the superficial layer, the content of chondrocytes with flattened shape and randomly distribution is relatively high and the collagen fibers arranged parallel to the surface or in an oblique and randomized orientation to the surface. There are some variations in biochemical components of articular cartilage with depth far from the superficial layer. The content of proteoglycan is high and collagen fibers with larger diameter are arranged perpendicular to the joint surface. These changes may lead to a low T2 because of the solid components restricting the free water.
Our research also has some limitations. First, only normal hip joints with a small sample size were included in this study. In future studies, we need to expand the sample size to investigate differences in cartilage T2 of hip-related diseases, especially developmental dysplasia of the hip that was common seen in pediatric orthopedics. Second, there were still some subjective factors under the condition of semi-automatic segmentation, which might lead to the inaccuracy of T2 measurement. We are considering to develop a deep learning algorithm based-segmentation for automatic segmentation of articular cartilage. Third, large FOV was used for the acquisition of hip articular cartilage in children to obtain MRI images with bilateral hip joints. The optimization of T2 mapping parameters should be scheduled to improve the image resolution, and high-resolution images will be subsequently available to realize automatic segmentation and to calculate T2 map accurately.
Conclusion
The T2 value decreases as the cartilage developing into a more mature state. Cartilage T2 values in the weight-bearing areas are relatively low due to an increase of collagen density and the loss of interstitial water. The restriction of the water molecules by solid components in the deeper layer of cartilage may decrease the T2 value.
References
Lehner KB, Rechl HP, Gmeinwieser JK et al (1989) Structure, function, and degeneration of bovine hyaline cartilage: assessment with MR imaging in vitro. Radiology 170(2):495–499
Ulrich-Vinther M, Maloney MD, Schwarz EM et al (2003) Articular cartilage biology. J Am Acad Orthop Surg 11(6):421–430
Chiamil SM, Abarca CA (2016) Imaging of the hip: a systematic approach to the young adult hip. Muscles Ligaments Tendons J 6(3):265–280
Mascarenhas VV, Caetano A (2018) Imaging the young adult hip in the future. Ann Jt 3(5):47
Houghton KM (2009) Review for the generalist: evaluation of pediatric hip pain. Pediatr Rheumatol Online J 7(1):1–9
St George J, Kulkarni V, Bellemore M et al (2021) Importance of early diagnosis for developmental dysplasia of the hip: A 5-year radiological outcome study comparing the effect of early and late diagnosis. J Paediatr Child Health 57(1):41–45
Madadi F, Shamsian BS, Alavi S et al (2011) Avascular necrosis of the femoral head in children with acute lymphoblastic leukemia: a 4-to 9-year follow-up study. Orthopedics 34(10):e593–e597
Hongyue T, Yang Q, Yiwen H et al (2018) Quantitative T2-mapping and T2*-mapping evaluation of changes in cartilage matrix after acute anterior cruciate ligament rupture and the correlation between the results of both methods. Biomed Res Int 2018:7985672
Verschueren J, Meuffels DE, Bron EE et al (2018) Possibility of quantitative T2-mapping MRI of cartilage near metal in high tibial osteotomy: A human cadaver study. J Orthop Res 36(4):1206–1212
Waldenmeier L, Evers C, Uder M et al (2019) Using cartilage MRI T2-mapping to analyze early cartilage degeneration in the knee joint of young professional soccer players. Cartilage 10(3):288–298
Shiguetomi-Medina JM, Ramirez-GL JL, Stødkilde-Jørgensen H et al (2017) Systematized water content calculation in cartilage using T1-mapping MR estimations: design and validation of a mathematical model. J Orthop Traumatol 18(3):217–220
Heckelman LN, Smith WAR, Riofrio AD et al (2020) Quantifying the biochemical state of knee cartilage in response to running using T1 magnetic resonance imaging. Sci Rep 10(7):1870
Soellner ST, Goldmann A, Muelheims D et al (2017) Intraoperative validation of quantitative T2 mapping in patients with articular cartilage lesions of the knee. Osteoarthr Cartil 25(11):1841–1849
Yang LL, Liu P, Man YQ et al (2017) Correlation of MRI T2-star mapping and the expression of \(\gamma\)-glutamyl carboxylase in degenerative knee cartilage. Int J Clin Exp Med 10(1):739–745
Li XJ, Majumdar S (2013) Quantitative MRI of articular cartilage and its clinical applications. J Magn Reson Imaging 38(5):991–1008
Watanabe A, Boesch C, Siebenrock K et al (2007) T2 mapping of hip articular cartilage in healthy volunteers at 3T: a study of topographic variation. J Magn Reson Imaging 26(1):165–671
Fernquest S, Park D, Marcan M et al (2018) Segmentation of hip cartilage in compositional magnetic resonance imaging: A fast, accurate, reproducible, and clinically viable semi-automated methodology. J Orthop Res 36(8):2280–2287
Milford D, Rosbach N, Bendszus M et al (2015) Mono-exponential fitting in T2-relaxometry: relevance of offset and first echo. PLoS One 10(12):e0145255e0145255
Xu J, Xie G, Di Y et al (2011) Value of T2-mapping and DWI in the diagnosis of early knee cartilage injury. J Radiol Case Rep 5(2):13–18
Lu W, Li L, Zhang L et al (2021) Development of acetabular anteversion in children with normal hips and those with developmental dysplasia of the hip: a cross-sectional study using magnetic resonance imaging. Acta Orthop 92(3):341–346
Golditz T, Steib S, Pfeifer K et al (2014) Functional ankle instability as a risk factor for osteoarthritis: using T2-mapping to analyze early cartilage degeneration in the ankle joint of young athletes. Osteoarthr Cartil 22(10):1377–1385
Xia Y, Chandra SS, Engstrom C et al (2014) Automatic hip cartilage segmentation from 3D MR images using arc-weighted graph searching. Phys Med Biol 59(23):7245–7266
Watrin A, Ruaud JP, Olivier PT et al (2001) T2 mapping of rat patellar cartilage. Radiology 219(2):395–402
Meng C, Lin Q, Si S et al (2017) The influences of walking, running and stair activity on knee articular cartilage: Quantitative MRI using T1 rho and T2 mapping. PLoS One 12(11):e0187008
Ferro FP, Ho CP, Dornan GJ et al (2015) Comparison of T2 values in the lateral and medial portions of the weight-bearing cartilage of the hip for patients with symptomatic femoroacetabular impingement and asymptomatic volunteers. Arthroscopy 31(8):1497–1506
Wang N, Xia Y (2012) Depth and orientational dependencies of MRI T2 and T1\(\rho\) sensitivities towards trypsin degradation and Gd-DTPA2- presence in articular cartilage at microscopic resolution. Magn Reson Imaging 30(3):361–370
Nishii T, Shiomi T, Tanaka H et al (2010) Loaded cartilage T2 mapping in patients with hip dysplasia. Radiology 256(3):955–965
Niannian S, Qi L, Lei W et al (2020) T2 mapping of hip joint cartilage in infants under 2 year-old with developmental dysplasia of the hip. Int J Med Radiol 43(2):168
Mahbub SB, Guller A, Campbell JM et al (2019) Non-invasive monitoring of functional state of articular cartilage tissue with label-free unsupervised hyperspectral imaging. Sci Rep 9(1):1–10
White CL, Chauvin NA, Waryasz GR, March BT, Francavilla ML (2017) MRI of native knee cartilage delamination injuries. Am J Roentgenol 209(5):W317–W321
Kaneko Y, Nozaki T, Yu H et al (2015) Normal T2 map profile of the entire femoral cartilage using an angle/layer-dependent approach. J Magn Reson Imaging 42(6):1507–1516
Funding
This work was supported by Social Development Project in Jiangsu Province (Grant no.BE2017670).
Author information
Authors and Affiliations
Contributions
HZ: measurement, analysis and interpretation of the data, drafting of the manuscript; HJ: explanation of critical clinical problems, acquisition of the data; GL: design of the interactive semi-automatic segmentation; TZ: design of the interactive semi-automatic segmentation; JW: acquisition of the data; YC: critical revision; LW: design of the interactive semi-automatic segmentation; MS: study conception and design; and XY: study conception and design.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Children’s Hospital of Soochow University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Each participant had obtained consent from a parent or guardian. The patients gave written consent to the submission of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, H., Jia, H., Lei, G. et al. Quantitative assessment of normal hip cartilage in children under 9 years old by T2 mapping. Magn Reson Mater Phy 35, 459–466 (2022). https://doi.org/10.1007/s10334-021-00962-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-021-00962-9