Abstract
Shifting to fallback food (FBF) consumption and crop raiding are behavioral adjustments that support primates’ ability to endure in human-altered habitats. Nutritional models predict that the consumption of preferred foods leads to increased competition, while consumption of staple fallback foods results in decreased competition. We analyzed the competitive regime faced by individuals in a group of 133 blond capuchin monkeys (Sapajus flavius), an endangered species that inhabits a 270-ha fragment of Atlantic forest in northeast Brazil. During the study year, quantitative analyses show that fruits were a preferred food, while sugarcane was used as a staple FBF. As predicted by primate fallback foraging models, the consumption of sugarcane helped the group to survive in this fragment by providing these animals with half of the food they consumed throughout the year. Contrary to predictions, group dispersion increased with greater fruit abundance, while direct competition peaked during the consumption of sugarcane. We suggest that, although it is abundant and scattered in the area, the long handling time required to process sugarcane before consumption facilitates the direct competition. Overall, the pattern found indicates that consumption of a staple FBF does not directly translate into decreased competition and increased stability of social groups in forest fragments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At a time when humans are changing the environment more and more rapidly, the survival of many primate populations depends on the use of anthropogenic landscapes (Estrada et al. 2017). By 2013, at least 57 primate taxa make use of and/or are partially dependent on agroecosystems, such as agricultural crops and forestry plantations (Bracebridge et al. 2013). Almost half of the primate species that make use of anthropogenic landscapes are listed as threatened by the IUCN Red List. Consequently, there is increased interest in how primates adjust their behavior to make use of fragmented or disturbed habitats and how this flexibility can help in the population endurance of a certain area (McLennan et al. 2017).
Caloric intake is a primary selective pressure, and primates adjust their behavior according to food availability by increasing the diversity of the food items consumed (Tutin et al. 1997; Gautier-Hion 1980; Lopes and Ferrari 1994; Lambert and Rothman 2015), their travel time and route, and their home ranges (Poulsen et al. 2001; O’Brien and Kinnaird 1997; Overdorff 1996). These adjustments in dietary and foraging behaviors are accompanied by changes in demography and social dynamics. Strum (2010) describes how the opening of agricultural fields facilitated the fission of a baboon group. A daughter group composed of individuals exhibiting raiding behavior split off from the original group of non-raider animals. By having their nutritional constraints relaxed due to the use of human food, the animals from the raiding group traveled less and socialized more, the males and females grew faster, and the females reproduced faster and had lower interbirth intervals than those of the original non-raiding group. Due to the trade-off between high nutritional values and predation-conflict risks, Strum (2010) argues that crop raiding should be considered a foraging strategy for animals that are living in human-dominated landscapes, and it should be included in the optimal foraging and socioecological models for the evolution, distribution, and management of primate populations.
Primate nutritional studies are aimed at modeling how nutrient requirements and intake influence the niche separation, biogeography, species life history, morphological, physiological, and behavioral traits of primates (Rothman et al. 2012). Recent models point out the importance of food consumed by primates during periods of shortages of preferred food to the evolution of the clade (Laden and Wrangham 2005; Rosenberger 2013). According to Marshall and Wrangham (2007, p. 1221), food preference is defined as the relationship between two parameters: availability and usage. Preferred food items are those that are selected disproportionately more often relative to their abundance within the habitat. In contrast, fallback foods “are consumed by a particular taxon in inverse proportion to the availability of their preferred food” (Marshall et al. 2009 p. 604).
Primate fallback foraging models (Marshall and Wrangham 2007; Altmann 2009; Constantino and Wright 2009) distinguish two types of fallback food. Staple FBFs are defined as those that may seasonally compose 100% of the animal’s diet, exhibit low seasonality, and are uniformly distributed in the environment. Filler FBFs present moderate seasonal fluctuations, are more patchily distributed, never comprise 100% of the diet, and can be seasonally absent from an animal’s diet (Marshall and Wrangham 2007). Food items such as fruits are preferred because they provide a fast intake of calories (i.e., a better energy/handling time ratio), but they are costly for the plant to produce and tend to be rare. FBFs provide low energy gain, are hard to process, or both (Marshall and Wrangham 2007 p. 1223).
Consumption of preferred foods is predicted to be a selective force on harvesting adaptations, such as visual acuity and spatial navigation, or other cognitive and locomotor abilities, while consumption of abundant and nutritionally poorer FBFs select processing adaptations, such as thick dental enamel and digestive capabilities, as well as behavioral adaptations, such as fission–fusion social systems and tool use (Marshall and Wrangham 2007; Marshall et al. 2009; Lambert 2007). Primate fallback foraging models predict that a reliance on staple FBFs would allow increased social stability via decreased competition, while species relying on preferred or filler FBFs would form fewer stable groups that face more competition (Marshall and Wrangham 2007, p. 1230; Marshall et al. 2009 p. 605; Lambert 2007, p. 761; Harrison and Marshall, 2011, p. 541).
In a review of the traits associated with foods consumed by apes, Harrison and Marshall (2011) found support for the primate fallback foraging model’s predictions by showing that a heavy reliance on preferred or filler FBFs is associated with indices of competition, such as a longer day range, higher travel efficiency/speed, higher feeding of direct competition, lower group stability, and slower life history. The opposite traits were associated with species that have a heavy reliance on staple FBFs. The Marshall and Wrangham (2007) model has been corroborated in other studies, such as those on orangutans (Smith et al. 2012), bonobos (Serckx et al. 2015), and chimpanzees (Watts et al. 2012; Eckhardt et al. 2015; Dominy et al. 2016). However, some findings are controversial. For instance, contrary to the Marshall and Wrangham (2007) model, travel time was positively correlated with the consumption of FBFs (lianas), but not the consumption of fruits by two groups of howler monkeys inhabiting fragments of a tropical rain forest in Mexico (Dunn et al. 2012). The authors suggest that as for preferred food, consumption of FBFs imposes ranging costs and may also be involved in selection for harvesting traits. In their more recent review, Harrison and Marshall (2011) also found high direct competition between gorillas, despite the high dependency of this species on the utilization of staple FBFs.
Capuchin monkeys (Cebus and Sapajus) are Neotropical primates with enlarged neocortices, high dietary plasticity, and a capacity for customary tool use. They are commonly used as models for comparative cognition, socioecology, and nutritional ecology research (Moura and Lee 2004; Visalberghi et al. 2005; Vogel and Janson 2007, 2011; Sugiura et al. 2011; Emídio and Ferreira 2012). Although omnivorous, capuchins prefer fruits, while insects and other vegetable parts are used as fillers and staple FBFs, respectively (Garber et al. 2012). Several authors have shown that direct competition is higher during the periods of highest fruit availability (Janson 1985, 1988; Phillips 1995a, 1995b; Vogel and Janson 2007, 2011; Izar et al. 2012), with increased avoidance and less group cohesion when this availability decreased (Rose 1994; Phillips 1995a, 1995b; Izar 2004; Vogel et al. 2007; Gogarten et al. 2014). Izar (2004) showed that the population of capuchin monkeys in a well-preserved area of the Atlantic Forest showed an increase in the interindividual distances and followed the fission–fusion pattern during periods of low fruit supply, as was predicted by ecological models.
The blond capuchin monkey (Sapajus flavius) is a critically endangered species that occupies Atlantic forest fragments in northeast Brazil (Oliveira and Langguth 2006; IUCN 2015). The fragments are found in human-altered landscapes, and the matrix surrounding the forest patches is dominated by a sugarcane agroecosystem of industry plantations or by crops that are traditionally farmed (Lynch-Alfaro et al. 2014). The species has only approximately 2000 individuals left, with little data available regarding blond capuchin feeding habits, demography, or social dynamics. A review of the literature reveals fewer than ten papers published on the species, and most of them are records of occurrence or descriptions that are based on indirect methods, such as camera traps (Bezerra et al. 2014) or tool-use sites (Ferreira et al. 2009).
In this study, we investigated food competition in a group of blond capuchin monkeys. We first tested the hypothesis that in the fragment studied, fruits are preferred food items, while sugarcane is a staple FBF. Then, we tested the hypothesis that consumption of preferred foods leads to increased competition while consumption of staple FBFs leads to decreased competition.
Methods
Study site and group
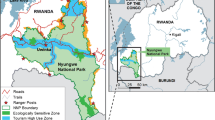
The study group lives in a 270-ha U-shaped fragment of the Atlantic Forest, in Caaporã, Paraiba, Brazil (S 07º52′85.2″ W 034º96′29.4″). The climate is tropical with a mean rainfall of 139.8 mm per month and 1678.0 mm per year (over the last 3 years, IPA 2014). The study area has been almost entirely surrounded by a sugarcane matrix for the last 25 years. Our team has followed the group since 2009; the group is habituated to the presence of observers, and it currently consists of over 133 individuals, including at least 16 adult males, 11 adult females, 13 subadults, and 43 juveniles.
Sampling methods
Between February and December 2014, we followed the PB group on foot for 10 days a month. We utilized the focal-scan method (Altmann 1974), in which one individual was observed continuously for 10 min, with its behavior and distance from neighbors recorded on a minute-by-minute basis. As not all animals were individually identified, we chose the next focal animal to ensure that sampling was equally distributed across individuals of each sex–age class (cf. Cords et al. 2010).
The behavioral and proximity data were recorded on a voice recorder and then later transcribed to Microsoft Excel sheets. An ethogram (Electronic Supplementary Material 1) was used to register behaviors, including locomotion, foraging (searching for food), food manipulation, food ingestion, and affiliative and agonistic interactions (Ferreira et al. 2016). When a focal animal was observed foraging or manipulating food, we recorded every minute what food item was being handled or ingested. While following the group, we also recorded all occurrences of direct agonistic interactions (Altmann 1974), including the location, the type of aggression observed (e.g., vocalizations, threat display, chases, bites, hits, overt aggression), the behavioral context in which it occurred, and the age–sex classes of the participants. As the number of partners available can bias social interaction, we used rates of agonism per individuals in sight as an index of direct competition.
While behaviors that are indicative of contest competition are easily observable (namely, aggressive conflicts, Sterck et al. 1997; Janson and van Schaik 1988), the indicators of indirect or scramble competition vary between studies: (a) increases in home-range and/or day-range size (e.g., larger groups have larger home ranges or increases in day ranges during periods of food scarcity (Isbell et al. 1999); (b) lower fertility in larger groups or in subordinate individuals (Oates 1987); (c) differential allotment of time to daylight activities (Janson and van Schaik 1988; Isbell et al. 1999); (d) differential use of space by subordinate and dominant individuals (Ron et al. 1996); and (e) decreased cohesiveness between individuals in groups that face increased competition (White and Chapman 1994; Hall and Fedigan 1997; Vogel and Janson 2007) or periods of food scarcity (Dunbar 1988). We used this last approach, the spacing between individuals, to derive two indices of indirect competition.
To calculate one index, we recorded the number and sex–age class of neighbors at distances of 1, 5, and 10 m from the focal animal (NN1, NN5, NN10) at every minute. Second, at intervals of 1.5 h, we searched for all of the individuals in view and recorded the spatial configuration of individuals within the group during a scan sampling period of 20 min. To do this, the observer (PGL) recorded her geographical location (with GPS receptor Garmin GPSmap 62sc) and the relative position of each individual to her location. The mean spacing between the individuals was calculated from the minimum convex polygon (MCP) area formed by the animals displayed in each scan. This method is similar to that employed by Janson (1990) and Robinson (1981) and is based on analyses of collective movement and differential use of spacing and leadership (Sueur et al. 2009; Smith et al. 2015). Here, we use the MPC as an indicator of indirect competition in the group. Because larger polygons are expected when more individuals could be observed, this MCP was standardized by dividing by the number of animals in each scan (MCPn). The spatial data visualization and MCP analyses were performed using the software QGIS (Nanni et al. 2013).
Food availability
We plotted 30 sampling areas with a 5-m diameter, which was equivalent to 1% of the study area (90 ha). The sampling areas were randomly distributed, with a minimum distance of 60 m and a maximum distance of 265 m between each other, including patches of vegetation in advanced and middle regeneration stages, as well as the interior and border of the fragment. At each point, we placed fruit traps 1 m high and 50 cm in diameter, and 10 cm diameter ground pitfall traps for arthropods. Fruits and arthropods were weighed monthly using a balance accurate to 1 g (ER2856st model). This method was used in other studies of wild capuchin monkeys and, therefore, allows comparability of the data (Izar et al. 2012, 2018).
In these 30 sampling areas, the abundance of fruit was inferred by registering the monthly phenology of a total of 104 trees with DBHs above 12.5 cm and representing 30 species. Using the Fournier method, the crown of the tree was divided into four quadrants, and for each quadrant, we determined on an ordinal scale whether it contained 0, 1 (up to 25%), 2 (up to 50%), 3 (up to 75%), or 4 (100%) ripe fruits or flowers (Bencke and Morellato 2002). The relative frequency of the fruit availability for each species was obtained by the Fournier index: \({\text{FI}} = \sum {nk} = 1({{\text{Fi}} \mathord{\left/ {\vphantom {{\text{Fi}} {4N)}}} \right. \kern-0pt} {4N)}}\) , where FI = Fournier index, Fi = phenophase intensity for each plant individual (0–4 scale), and N = total number of observed plants (Silva et al. 2014).
The monthly availability of fruits (Fm) were calculated by the following formula: \({\text{Fm}}{\kern 1pt} \; = \;\sum {nk} = 1\;{\text{Fk}} \times {\text{Dk}} \times {\text{Sk}}\) , where Fk denotes the mean availability of ripe fruit of individuals in species k (i.e., the Fournier index), Dk denotes the density of adult trees of species k (as the mean of the density of each species in the 30 plots), and Sk is the mean DBH size of the trees of species k (McLennan 2013; Huang et al. 2015). Therefore, we used the composite index as an estimate of the availability of tree quadrants with ripe fruits in an area of 5 m.
To capture aspects of phenology that were potentially most relevant to blond capuchins (Gogarten et al. 2014), we also inferred availability by using only data from nine of the ten most commonly consumed fruits from these species (a list of all fruits consumed can be found in the Electronic Supplementary Material 2).
We evaluated food consumption by the number of instantaneous records of food ingestion of each item within the general activity budget. This index was calculated per day and provides a measure of the overall representation of each item within the total diet of the animal (McLennan 2013).
Data analysis
To verify if fruits are a preferred food item and whether sugarcane is a staple FBF, we conducted two types of analyses. First, using regression analyses, we employed the Akaike information criteria to select the best models for predicting time spent foraging on fruit/sugarcane/insects based on the availability of these respective items. Second, we conducted a cluster analysis using rainfall and productivity as grouping factors to define periods of high, medium, and low availability of fruits. Then, we employed a multivariate generalized linear model (GLM) to test whether time spent foraging on fruits differed between these periods. If fruits are consumed according to or above their availability, and other food items are consumed in inverse proportion to the fruit availability, then we may assume that fruits are preferred items while others are fallback foods.
To test whether direct competition increases with decreasing fruit availability, we conducted regression models using rainfall and fruit productivity as predictors and indices of direct and indirect competition (rate of agonism/ind, NN1, NN5, NN10, and MCP/ind) as response variables. We also conducted a multivariate GLM to test if the indices of direct and indirect competition varied between seasons and behavioral contexts using rainfall and productivity as random factors. Data were analyzed using the R 3.1.1 (R Development Core Team 2011), and the significance level α = 0.05 was two tailed.
Results
A total of 721 h of field effort was conducted, which resulted in 407 contact hours with the blond capuchin group. We obtained a total of 6511 focal-scan samples and 200 group scans.
Food availability
The two methods to infer fruit availability (Fournier and fruit traps) were positively correlated (rs = 0.565, N = 10 months, p = 0.089), showing that the methods of obtaining data relative to fruit availability were consistent. The fruit availability of the 30 species monitored varied from 0.25 to 1.67 (Fournier index corrected by diameter and number of trees). Our estimate for the total production in the fragment was 78.36 kg/month/ha of fruits and 1302.5 kg/month/ha of arthropods during the year studied.
Fruit and arthropod productivities exhibited quadratic and cubic relations with rainfall, respectively. Cluster analyses indicated that one could roughly divide the year into three periods based on the rainfall intensity and the food productivity: Humid: February–April, rainfall = 101–157 mm, great availability of both fruits (135.3 kg/month/ha) and arthropods (1879.4 kg/month/ha); Rainy: May–September, the most severe rainfall (303 mm), but lower availability of ripe fruit (56.2 kg/month/ha) and arthropods (1312.4 kg/month/ha); Dry: October–December, precipitation less than 100 mm, with low availability of fruit (58.3 kg/month/ha) and arthropods (709.2 kg/month/ha).
Fruit occupied 24.1% of the capuchin diet. Other vegetable parts (OVP), including bulbs, leaves, flowers, petioles, bark and resin, and contributed to 12.7% of the diet, which was little more than animal prey (arthropods and vertebrates) with 12.6%. Sugarcane was the most consumed resource, contributing with a mean of 50.3% to the annual diet with a monthly range of 25.9–71.8%.
More time was spent ingesting food during the humid period (F = 5.441, p = 0.004, df = 2; Fig. 1) compared to more time spent foraging (F = 53.032, p = 0.001, df = 2) and less locomotion during the dry period (F = 6.479, p = 0.002, df = 2). Time spent in social behavior did not vary across periods (F = 0.096, p = 0.909, df = 2; Fig. 1). Females searched less and moved and socialized more, while juveniles ingested more than all other age categories (FFOR = 10.263, p = 0.001, df = 2; FING = 4.252, p = 0.005, df = 2; FLOC = 13.869, p = 0.001, df = 2; FSOC = 3.039, p = 0.028, df = 2). There was a significant interaction between periods and age–sex class, with females altering their searching time during dry periods (F = 3.578, p = 0.002, df = 2).
Consumption versus availability
The best model to predict fruit consumption included the availability of the nine most-consumed fruits and the availability of arthropods and rainfall (Table 1). All three variables loaded positively, in that with increased availability of fruit and insects and more rainfall, a greater number of fruits were consumed. The best model to predict arthropod consumption included the weight of arthropods, in very weak negative relation, and positive relation with availability of fruit. The best model to predict sugarcane consumption only included the availability of the top nine most consumed fruits in a negative relationship, in that the less availability of the top nine preferred fruits, the more sugarcane was consumed. This result corroborates predictions regarding fruits as a preferential food item and sugarcane as a staple fallback food (Fig. 2).
Indirect competition
The mean minimum convex polygon (MCP) area formed by the group of subjects was 450 ± 476 m2, with a mean spacing between individuals 25 ± 27 m2. The best model to predict MPCn included the total and top nine fruit availability and rainfall (Table 1), with greater distances among individuals occurring in periods of higher fruit availability. Similarly, the number of neighbors showed a negative relationship to the availability of fruits (Table 1), with individuals spreading out more during periods of higher fruit productivity.
Multivariate GLM corroborates regression analyses, indicating that the MPCn is greater in the humid period and lower in the dry period (F = 14.125, p = 0.001; Fig. 3). Although MPCn did not vary among behavioral contexts (F = 1.551, p = 0.174), there was a tendency for individuals to be closer during the consumption of sugarcane during the dry and rainy periods (Tukey post hoc, p = 0.073; Fig. 3). Overall, there was no significant interaction between periods and behavioral contexts (F = 0.414, p = 0.940).
Contest competition
We observed 996 agonistic interactions in a ratio of 2.45 events per hour of contact with the group, or 0.06 events of aggression per individual in sight per hour. Most aggression behaviors were committed by males (68.4%), and the most frequent victims were other adult males (54%) and subadult males or females (28.8%).
The total fruit and the weight of arthropods were included in the best model to predict rates of direct competition, but only the second one had significance (Table 1), with less aggression occurring in periods of greater weight of arthropods. There was a significant influence of the context and season on the exhibition of direct competition (context × season F = 2.896, p = 0.002), and post hoc analyses indicated that increased direct competition occurs during sugarcane consumption in all seasons (Fig. 4).
Discussion
Primates in fragmented forests may face severe nutritional shortfalls with frequent reports of animals shifting to crop raiding (Siemers 2000; Williams-Guilllén et al. 2006; Sabbatini et al. 2008; McKinney 2011; McLennan 2013). However, the resilience of species or populations to these changes in diet is by definition limited, and behavior can be an indicator of the feeding plasticity and competitive regime faced by the animals (McLennan et al. 2017).
The studied fragment hosts the densest population that has yet been found for the endangered blond capuchin monkey: at least 133 animals occupying 270 ha. This high density occurs despite the fact that the forest exhibited fruit productivity that was lower than that described in other Atlantic fragments containing capuchin monkeys (78 kg/month/ha vs. 123 kg/month/ha at UNA, S. xanthosternos; 235 kg/month/ha at parque estadual carlos botelho, S. nigritus) (Izar et al. 2018).
Rain had a quadratic relation to the fruit availability; that is, too little or too much rain diminished the fruit availability. In the humid period, the diet of the monkeys consisted of a wider variety of species of fruit (N = 27) and reduced dependence on consumption of insects, with capuchins spending 4.6% of their time foraging on the ground. During the rainy period, the monkeys increased their time eating sugarcane and arthropods, and thus increased their time on the ground (8.4%) and decreased their consumption of fruits. In the dry period, the diet of primates was dominated by a single species of food, sugarcane Saccharum sp. (44–72% of the feeding time and 18.4% of time on the ground). Use of the ground to consume fallback foods during periods of scarcity is described for other groups (Galetti and Pedroni 1994; Freitas et al. 2008). As for these other groups, animals ventured out of the fragment, collected the sugarcane, and rapidly returned to consume it on trees. Increased terrestriality is also described as a condition for tool use for cracking open nuts in arid areas, although these nuts are not fallback foods for the populations studied (Visalberghi et al. 2005; Ottoni and Izar 2008). We did not record any tool-use behaviors in this group, possibly because sugarcane can be peeled and processed by hands and teeth.
We confirmed that fruits are a preferred food item, with fruit consumption predicted by the availability of the top nine most consumed fruit species and increased during the humid period. However, fruits composed only one-quarter (25%) of the animals’ diet. Conversely, sugarcane composed a large portion of the monthly food consumed by blond capuchin monkeys, and its consumption was inversely correlated to the total and top nine fruit species availability, which indicates that sugarcane is a staple FBF for these animals.
Sugarcane composed one- to three-quarters of the animals’ diets throughout the year, which is much higher than the amount of crop-food consumed by Sapajus nigritus (14%—Galetti and Pedroni 1994), but is similar to the consumption rate of sugarcane and other crops in other Atlantic forest fragments (10%, but reaching 70% in dry season—Freitas et al. 2008; 20% of diet—Rimoli et al., Rímoli et al. 2008) and bromeliads in the Andes (72%—Brown and Zunino 1990). Sugarcane is a highly fibrous food (62% of dry matter), poor in proteins (< 4%, less than that found in bromeliads, 8.8%, or in nuts consumed in caatinga, 17%); however, it is composed of 32–57% sugar (Pate et al. 2001). Capuchin monkeys do not eat the sugarcane, but suck it and discard the chewed material, which indicates that, although the caloric intake of animals can be supplied by sugarcane, its composition may not satisfy all of the nutritional requirements of capuchin monkeys.
The animals’ mean interindividual distance was 25 ± 27 m2, but, contrary to predictions, individuals did not become more cohesive during periods of fruit consumption; rather, the area formed by the group (MPCn) increased, and the number of neighbors at 1, 5, and 10 m decreased with the increased availability of fruits. One possibility is that the animals are spread over several fruit trees during periods of higher fruit abundance/consumption and are grouped during periods of low fruit availability because the animals cluster to use the same few fruit-producing trees. Our data also show that a significant increase in group cohesion and in the proximity between individuals occurred during sugarcane consumption.
The rate of direct competition found here was higher than that described in other robust capuchin monkey studies [present study 0.064 eV/h/ind vs. 0.025 eV/h/ind for S. libidinosus at Caatinga; 0.038 eV/h/ind for S. nigritus at Parque Estadual Carlos Botelho Atlantic Forest (Izar et al. 2018); 0.048 eV/h/ind for S. libidinosus at mangrove (Cutrim 2013); 0.027 for S. apella at moist tropical forest in Peru (Janson 1985); 0.045 eV/h/ind for a reintroduced and food supplemented Sapajus spp. at Parque Ecológico do Tietê (Ferreira et al. 2008); and an estimated mean of 0.03 male–male aggression bouts/hour in S nigritus at Atlantic Forest fragment (Lynch et al. 2002)]. This indicates high direct competition within the group due to the increased local density of competitors for limited resources (Janson and van Schaik 1988; Wheeler et al. 2013). However, the direct competition rate did not correlate with the consumption of preferred food items. Rather, a peak in direct competition was detected during the consumption of sugarcane, which is a staple FBF. There are three possible explanations, which are not mutually exclusive, for this pattern.
First, as the distance between individuals becomes greater when they move faster (Sugiura et al. 2011), it is possible that the increased spacing between individuals increased due to the greater mobility between the productive trees in periods of high fruit availability. Studies by Phillips (Phillips 1995a, b) show that individuals of the genus Cebus disperse naturally while they are foraging, but not enough to form subgroups in response to decreased environmental quality.
A second alternative is that agonism increases due to ground use while foraging for sugarcane because animals do not experience the limitations of the arboreal environment that sometimes prevents individuals from engaging in high-intensity agonistic interaction. Also, a higher proportion of occurrences of agonistic interactions are more likely to be missed by observers of arboreal relative to terrestrial primates due to the decreased visibility in the former context (Wheeler et al. 2013). If this was the case, agonism should be higher during both sugarcane and arthropod consumption. Our data do not support this alternative (see Fig. 4).
One final possibility is that sugarcane, although abundant, is a food that requires extended processing from pulling it out of the ground to remove (with teeth and hands) the external parts to access the edible core. During processing, several fights, threats, and coalitions occurred between males competing for an edible, exposed piece of sugarcane. From this perspective, Isbell’s (1991) concepts of the feeding site depletion time and usurpability seem to capture the dynamics of competition in this blond capuchin group. As described for other crop raiding primates (Siemers 2000; Sabbatini et al. 2008; McKinney 2011), the risk associated with collection makes individuals become more cohesive when venturing out from the forest into the crop fields. The fact that sugarcane stalks are only 50 cm apart from each other does not diminish competition, since individuals are competing for an already collected and processed piece of sugarcane brought by one individual to the edge of the forest. This indicates that, although widely distributed and constant year round, sugarcane is a monopolizable, usurpable food item, and its consumption increases the probability of direct competition (Janson 1985; Hirsch 2007; Chancellor and Isbell 2009).
In their review of the conservation status of the genus, Lynch-Alfaro et al. (2014) list 53 sites where research on Cebus (17) and Sapajus (35) occurs. While human–monkey conflicts are registered in sites located near traditional crop areas (Siemers 2000; Sabbatini et al. 2008; McKinney 2011), major conflicts are not commonly described for fragments bordered by agroecosystems or stockbreeding activities (but see Pagno et al. 2015). Capuchin monkeys are not major targets in traditional/leisure hunting in the ways that have been observed for some other mammals. Crop raiding and the use of human food is widely described for capuchin monkeys. This is the case of the present fragment, where crop raiding is minimal relative to the agroecosystem size, and no monkey hunting has been observed in the last 10 years.
Knowing that the use of human-disturbed fragments is important to the survival of many primate populations, we tested the hypothesis that the consumption of fallback food dispersed in the environment relates to decreased competition and stability of social groups. We conclude that this high-density population (133 individuals living in 270 ha) in a low productive fragment is made possible by capuchins that exhibit a risky and costly behavior: terrestrial excursions out of the forest for sugarcane crop raiding and manipulation. The consumption of sugarcane as a staple FBF that comprises more than half of the food ingested by the animals throughout the year allows for the permanence of these animals in this fragment by diminishing the overall caloric deficit. However, contrary to predictions, sugarcane use was associated with increased direct competition, which may lead to instability in social relations and associated wound-death risks. Refinements of nutritional and socioecological models, including crop raiding as a foraging strategy (Strum 2010), will help the management of small and large fragments where primates are found, thereby avoiding the extirpation of a local population from the remaining habitats.
References
Alfaro JWL, Izar P, Ferreira G (2014) Capuchin monkey research priorities and urgent issues. Am J Primatol 76(8):705–720
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Altmann SA (2009) Fallback foods, eclectic omnivores, and the packaging problem. Am J Phys Anthropol 140:615–629
Bencke CSC, Morellato LPC (2002) Comparação de dois métodos de avaliação da fenologia de plantas, sua interpretação e representação. Revista Brasileira de Botânica 25:269–275
Bezerra BM, Bastos M, Souto A, Keasey MP, Eason P, Schiel N, Jones G (2014) Camera trap observations of nonhabituated critically endangered wild blonde capuchins, Sapajus flavius (formerly Cebus flavius). Int J Primatol 35:895–907
Bracebridge CE, Davenport TRB, Mbofu VF, Marsden SJ (2013) Is there a role for human-dominated landscapes in the long-term conservation management of the critically endangered kipunji (Rungwecebus kipunji)? Int J Primatol 34:1122–1136
Brown AD, Zunino GE (1990) Dietary variability in Cebus apella in extreme habitats: evidence for adaptability. Folia Primatol 54:187–195
Chancellor RL, Isbell LA (2009) Female grooming markets in a population of gray-cheeked mangabeys (Lophocebus albigena). Behav Ecol 20:79–86
Constantino PJ, Wright BW (2009) The importance of fallback foods in primate ecology and evolution. Am J Phys Anthropol 140:599–602
Cords M, Sheehan MJ, Ekernas LS (2010) Sex and age differences in juvenile social priorities in female philopatric, nondespotic blue monkeys. Am J Primatol 72:193–205
Cutrim FHR (2013) Padrão comportamental e uso de ferramentas em macacos-prego (Sapajus libidinosus) residentes em manguezal. Doctoral thesis. Universidade de São Paulo, São Paulo, Brazil. p 114
Dominy NJ, Yeakel JD, Bhat U, Ramsden L, Wrangham RW, Lucas PW (2016) How chimpanzees integrate sensory information to select figs. Interf Focus 6:1–9
Dunbar RIM (1988) Primate social systems. Croom Helm, London
Dunn JC, Asensio N, Arroyo-Rodriguez V, Schnitzer S, Cristobal-Azkarate J (2012) The ranging costs of a fallback food: liana consumption supplements diet but increases foraging effort in howler monkeys. Biotropica 44:705–714
Eckhardt N, Polansky L, Boesch C (2015) Spatial cohesion of adult male chimpanzees (Pan troglodytes verus) in Tai national park, Côte D’lvoire. Am J Primatol 77:125–134
Emídio RA, Ferreira RG (2012) Energetic payoff of tool use for capuchin monkeys in the caatinga: variation by season and habitat type. Am J Primatol 74:332–343
Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Fiore AD, Nekaris A, Nijman V, Heymann EW, Lambert JE, Rovero F, Barelli C, Setchell JM, Gillespie TR, Mittermeier RA, Arregoitia LV, Guinea M, Gouveia S, Dobrovolski R, Shanee S, Shanee N, Boyle SA, Fuentes A, MacKinnon KC, Amato KR, Meyer ALS, Wich S, Sussman RW, Pan R, Kone I, Baoguo L (2017) Impending extinction crisis of the world’s primates: why primates matter. Sci Adv 3:1–16
Ferreira RG, Lee PC, Izar P (2008) Food competition in a semi-free-ranging Cebus apella group. Folia Primatol 79:463–475
Ferreira RG, Jerusalinsky L, Silva TCF, Fialho MS, Roque AA, Fernandes A, Arruda F (2009) On the occurrence of Cebus flavius (Schreber 1774) in the Caatinga, and the use of semi-arid environments by Cebus species in the Brazilian state of Rio Grande do Norte. Primates 50:357–362
Ferreira RG, Mendl M, Wagner PGC, Araújo T, Nunes D, Mafra AL (2016) Coping strategies in captive capuchin monkeys (Sapajus spp.). Appl Anim Behav Sci 176:120–127
Freitas CH, Setz EZF, Gobbi N (2008) Agricultural crops in the diet of bearded capuchin monkeys, Cebus libidinosus spix (Primates: Cebidae), in forest fragments in southeast Brazil. Rev Bras Zool 25(1):32–39
Galetti M, Pedroni F (1994) Seasonal diet of capuchin monkeys (Cebus apella) in a semideciduous forest in south-east Brazil. J Trop Ecol 10(1):27–39
Garber PA, Gomes DF, Bicca-Marquez JC (2012) Experimental field study of problem-solving using tools in free-ranging capuchins (Sapajus nigritus). Am J Primatol 74:344–358
Gautier-Hion A (1980) Seasonal variations of diet related to species and sex in a community of cercopithecus monkeys. J Anim Ecol 49:237–269
Gogarten JF, Bonnel TR, Brown LM, Campenni M, Wasserman MD, Chapman CA (2014) Increasing group size alters behavior on a folivorous primate. Int J Primatol 35:590–608
Hall CL, Fedigan LM (1997) Spatial benefits afforded by high rank in white-faced capuchins. Anim Behav 53:1069–1082
Harrison ME, Marshall AJ (2011) Strategies for the use of fallback foods in apes. Int J Primatol 32:531–565
Hirsch BT (2007) Cost and benefits of within-group spatial position: a feeding competition model. Quart Rev Biol 82:9–27
Huang Z, Huang C, Tang C, Huang L, Tang H, Ma G, Zhou Q (2015) Dietary adaptations of Assamese macaques (Macaca assamensis) in limestone forest in southwest China. Am J Primatol 77:171–185
IPA (2014) Instituto Agronômico de Pernambuco. Resource document. http://www.ipa.br/indice_pluv.php. Accessed 22 Dec 2014
Isbell LA (1991) Contest and scramble competition: patterns of female aggression and ranging behavior in primates. Behav Ecol 2:143–155
Isbell LA, Pruetz JD, Lewis M, Young TP (1999) Rank differences in ecological behavior: a comparative study of patas monkeys (Erythrocebus patas) and vervets (Cercopithecus aethiops). Int J Primatol 20:257–272
IUCN (2015) Sapajus flavius. The IUCN Red List of threatened species 2015. Resource document. http://dx.doi.org/10.2305/IUCN.UK.2015.RLTS.T136253A70612549.en. Accessed 2 May 2016
Izar P (2004) Female social relationships of Cebus apella nigritus in a southeastern Atlantic forest: an analysis through ecological models of primate social evolution. Behaviour 141:71–99
Izar P, Verderane MP, Peternelli-dos-Santos L, Mendonça-Furtado O, Presotto A, Tokuda M, Visalberghi E, Fragaszy D (2012) Flexible and conservative features of social systems in tufted capuchin monkeys: comparing the socioecology of Sapajus libidinosus and Sapajus nigritus. Am J Primatol 74:315–331
Izar P, Resende BD, Ferreira RG (2018) Proximate causes of tool use in feeding in the genus Sapajus. In: Urbani B, Kowalewski M, Cunha RGT, Torres S, Cortés-Ortriz (eds) La primatologia en Latinoamérica 2, Tomo I Argentina–Colombia. Ediciones IVIC. Instituto Venezolano de Investigaciones Científicas (IVIC), Caracas, pp 239–249
Janson C (1985) Aggressive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behav Ecol Sociobiol 18:125–138
Janson C (1988) Food competition in brown capuchins monkeys (Cebus apella): quantitative effects of group size and tree productivity. Behaviour 105:53–76
Janson C (1990) Social correlates of individual spatial choice in foraging groups of brown capuchin monkeys, Cebus apella. Anim Behav 40:910–921
Janson CH, van Schaik CP (1988) Recognizing the many faces of primate food competition: methods. Behaviour 105:165–186
Laden G, Wrangham R (2005) The rise of the hominids as an adaptive shift in fallback foods: plant underground storage organs (USOs) and australopith origins. J Human Evol 49:482–498
Lambert JE (2007) Seasonality, fallback strategies, and natural selection: a chimpanzee and cercopithecoid model for interpreting the evolution of the hominin diet. In: Ungar PS (ed) Evolution of the human diet: the known, the unknown, and the unknowable. Oxford University Press, Oxford, pp 324–343
Lambert JE, Rothman M (2015) Fallback foods, optimal diets, and nutritional targets: primate responses to varying food availability and quality. Annu Rev Anthropol 44:493–512
Lopes MA, Ferrari SF (1994) Foraging behavior of a tamarin group (Saguinus fuscicollis weddelli) and interactions with marmosets (Callithrix emiliae). Int J Primatol 15(3):373–387
Lynch JW, Ziegler TE, Strier KB (2002) Individual and seasonal variation in fecal testosterone and cortisol levels of wild male tufted capuchin monkeys, Cebus apella nigritus. Horm Behav 41:275–287
Lynch-Alfaro JW, Izar P, Ferreira RG (2014) Capuchin monkey research priorities and urgent issues. Am J Primatol 76:1–16
Marshall AJ, Wrangham RW (2007) Evolutionary consequences of fallback foods. Int J Primatol 28:1219–1235
Marshall AJ, Boyko CM, Feilen KL, Boyko RH, Leighton M (2009) Defining fallback foods and assessing their importance in primate ecology and evolution. Am J Phys Anthropol 140:603–614
McKinney T (2011) The effects of provisioning and crop-raiding on the diet and foraging activities of human-commensal white-faced capuchins (Cebus capucinus). Am J Primatol 73:439–448
McLennan MR (2013) Diet and feeding ecology of chimpanzees (Pan troglodytes) in Bulindi, Uganda: foraging strategies at the forest–farm interface. Int J Primatol 34:585–614
McLennan MR, Spagnoletti N, Hockings KJ (2017) The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. Int J Primatol 38:105–121
Moura AC, Lee PC (2004) Capuchin stone tool use in caatinga dry forest. Science 306:1909
Nanni AS, Descovi Filho L, Virtuoso MA, Montenegro D, Willrich G, Machado PH, Sperb R, Dantas GS, Calazans Y (2013) Quantum GIS-Guia do Usuário, Versão 1.7.4’Wroclaw’. 291p. Resource document. http://qgisbrasil.org. Accessed 30 Sept 2013
O’Brien TG, Kinnaird MF (1997) Behavior, diet, and movements of the sulawesi crested black macaque (Macaca nigra). Int J Primatol 18:321–351
Oliveira MM, Langguth A (2006) Rediscovery of marcgraves capuchin monkey and designation of a neotype for Simia flavia chreber, 1774 (primates, Cebidae). Bol do Mus Nac 523:1–16
Ottoni EB, Izar P (2008) Capuchin monkey tool use: overview and implications. Evol Anthropol 17(4):171–178
Overdorff DJ (1996) Ecological correlates to activity and habitat use of two prosimian primates: Eulemur rubriventer and Eulemur fulvus rufus in Madagascar. Am J Primatol 40:327–342
Pagno L, Cândido JF Jr, Brocardo CR (2015) Seed predation of Araucaria angustifolia by Sapajus nigritus. Neotropical Primates 22(1):1–6
Pate FM, Alvarez J, Phillips JD, Eiland BR (2001) Sugarcane as a cattle feed: production and utilization. Resource document. http://corn.agronomy.wisc.edu/Crops/SugarCane.pdf. Accessed 10 June 2018
Phillips KA (1995a) Resource patch size and flexible foraging in white-faced capuchins (Cebus capucinus). Int J Primatol 16:509–519
Phillips KA (1995b) Foraging-related agonism in capuchin monkeys (Cebus capucinus). Folia Primatol 65:159–162
Poulsen JR, Clark CJ, Smith TB (2001) Seasonal variation in the feeding ecology of the grey-cheeked mangabey (Lophocebus albigena) in Cameroon. Am J Primatol 54:91–105
Rímoli J, Strier KB, Ferrari SF (2008) Seasonal and longitudinal variation in the behavior of free-ranging black tufted capuchins Cebus nigritus (Goldfuss, 1809) in a fragment of Atlantic forest in southeastern Brazil. In: Ferrari SF, Rímoli J (eds) A primatologia no Brasil-9. sociedade brasileira de primatologia, biologia geral e experimental—UFS, Aracaju, pp 130–146
Robinson G (1981) Spatial structure in foraging groups oh wedge-capped capuchin monkeys Cebus nigrittatus. Anim Behav 29:1036–1056
Ron T, Henzi P, Motro U (1996) Do female chacma baboons compete for a safe spatial position in a southern woodland habitat? Behaviour 133:475–490
Rose LM (1994) Sex differences in diet and foraging behavior in white-faced capuchins (Cebus capucinus). Int J Primatol 15:95–114
Rosenberger AL (2013) Fallback foods, preferred foods, adaptive zones, and primate origins. Am J Primatol 75:1–8
Rothman JM, Chapman CA, Van Soest PJ (2012) Methods in primate nutritional ecology: a user’s guide. Int J Primatol 33:542–566
Sabbatini G, Stammati M, Tavares MCH, Visalberghi E (2008) Behavioral flexibility of a group of bearded capuchin monkeys (Cebus libidinosus) in the National Park of Brasília (Brazil): consequences of cohabitation with visitors. Brazil J Biol 68:685–693
Serckx A, Kuhl H, Beudels-Jamar RC, Poncin P, Bastin J, Huynen M (2015) Feeding ecology of bonobos living in forest-savannah mosaics: diet seasonal variation and importance of fallback foods. Am J Primatol 77:948–962
Siemers BM (2000) Seasonal variation in food resource and forest strata use by brown capuchin monkeys (Cebus apella) in a disturbed forest fragment. Folia Primatol 71:181–184
Silva AG, Freitas L, Pires JPA (2014) A Fournier Index upgrade as a new approach for quantitative phonological studies in plant communities. Trop Ecol 55:137–142
Smith TM, Kupczik K, Machanda Z, Skinner MM, Zermeno JP (2012) Enamel thickness in Bornean and Sumatran orangutan dentitions. Am J Phys Anthropol 147:417–426
Smith JE, Estrada JR, Richards HR, Dawes SE, Mitsos K, Holekamp KE (2015) Collective movements, leadership and consensus costs at reunions in spotted hyaenas. Anim Behav 105:187–200
Sterck EHM, Watts DP, VanShaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41:291–310
Strum SC (2010) The development of primate raiding: implications for management and conservation. Int J Primat 31:133–156
Sueur C, Petit O, Deneubourg JL (2009) Selective mimetism at departure in collective movements of Macaca tonkeana: a theoretical and experimental approach. Anim Behav 79:1087–1095
Sugiura H, Shimooka Y, Tsuji Y (2011) Variation in spatial cohesiveness in a group of Japanese macaques (Macaca fuscata). Int J Primatol 32:1348–1366
R Development Core Team (2011) R: a language and environment for statistical computing. Resource document. R foundation for statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org/. Accessed 10 Mar 2015
Tutin CEG, Ham RM, White LJT, Harrison MJS (1997) The primate community of the Lopé Reserve, Gabon: diets, responses to fruit scarcity, and effects on biomass. Am J Primatol 42:1–24
Visalberghi E, Fragaszy DM, Izar P, Otonni E (2005) Terrestriality and tool use. Science 308:951
Vogel ER, Janson CH (2007) Predicting the frequency of food-related agonism in white-faced capuchin monkeys (Cebus capucinus) using a novel focal-tree method. Am J Primatol 69:533–550
Vogel ER, Janson CH (2011) Quantifying primate food distribution and abundance for socioecological studies: an objective consumer-centered method. Int J Primatol 32:737–754
Vogel ER, Munch SB, Janson CH (2007) Understanding escalated aggression over food resources in white-faced capuchin monkeys. Anim Behav 74:71–80
Watts DP, Pots KB, Lwanga JS, Mitani JC (2012) Diet of chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale national park, Uganda, 2. Temporal variation and fallback foods. Am J Primatol 74:130–144
Wheeler BC, Scarry CJ, Koenig A (2013) Rates of agonism among female primates: a cross-taxon perspective. Behav Ecol 24:1369–1380
White F, Chapman C (1994) Contrasting chimpanzees and bonobos: nearest neighbour distances choices. Folia Primatol 63:181–191
Williams-Guilllén K, McCann C, Sánchez JCM, Koontz F (2006) Resource availability and habitat use by mantled howling monkeys in a Nicaraguan coffee plantation: can agroforests serve as core habitat for a forest mammal? Anim Conserv 9:331–338
Acknowledgements
We would like to thank José Henrique Barbosa Filho for his valuable company in the forest, Clayton Jeronimo for his help during fieldwork, and Yuri for the time spent habituating the monkeys. We are also grateful to the Usina TABU, especially the manager, Paulo, for the structural help to conduct this research. This research was supported by grants of the Fundação Grupo Boticario (0973-2013-8) and of the CNPq. Permission to work was granted by IBAMA-SISBIO 38855).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lins, P.G.A.d.S., Ferreira, R.G. Competition during sugarcane crop raiding by blond capuchin monkeys (Sapajus flavius). Primates 60, 81–91 (2019). https://doi.org/10.1007/s10329-018-0698-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-018-0698-z