Abstract
Snakes present a hazard to primates, both as active predators and by defensive envenomation. This risk might have been a selective pressure on the evolution of primate visual and cognitive systems, leading to several behavioral traits present in human and non-human primates, such as the ability to quickly learn to fear snakes. Primates seldom prey on snakes, and humans are one of the few primate species that do. We report here another case, the wild capuchin monkey (Sapajus libidinosus), which preys on snakes. We hypothesized that capuchin monkeys, due to their behavioral plasticity, and cognitive and visual skills, would be capable of discriminating dangerous and non-dangerous snakes and behave accordingly. We recorded the behavioral patterns exhibited toward snakes in two populations of S. libidinosus living 320 km apart in Piauí, Brazil. As expected, capuchins have a fear reaction to dangerous snakes (usually venomous or constricting snakes), presenting mobbing behavior toward them. In contrast, they hunt and consume non-dangerous snakes without presenting the fear response. Our findings support the tested hypothesis that S. libidinosus are capable of differentiating snakes by level of danger: on the one hand they protect themselves from dangerous snakes, on the other hand they take opportunities to prey on non-dangerous snakes. Since capuchins and humans are both predators and prey of snakes, further studies of this complex relationship may shed light on the evolution of these traits in the human lineage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snakes are potential predators of primates, even of those that are primarily arboreal (Cisneros-Heredia et al. 2005; Quintino and Bicca-Marques 2013; Teixeira et al. 2015; Ribeiro-Júnior et al. 2016). Primates’ fear of snakes is acquired by a fast, strong and persistent conditioned learning process (Cook and Mineka 1989; Vitale et al. 1991; Emile and Barros 2009; Kawai and Koda 2016). One hypothesis explaining such quick learning is that recognition of snakes (and the elicitation of adequate responses) was so important for survival during primate evolution that learning to fear snakes is a facilitated response mechanism in primates. Snakes are a stimulus more strongly and persistently conditioned as aversive stimulus than are neutral ones, and snakes appear to have attentional priority in visual search tasks (Öhman 2009). Studies have shown that experimental lesions in early life of specific neural circuits, such as the superior colliculus, impair fast detection of threat responses by primates, as revealed by the lack of fear of a rubber snake in a threat-reward conflict task by young capuchin monkeys (Maior et al. 2011). In Japanese macaques (Macaca fuscata) an experiment showed an attentional bias toward snakes, with quick detection and rapid fear learning limited to snake visual stimulus (Kawai and Koda 2016). Wild vervet monkeys (Chlorocebus pygerythrus) can easily detect snake skin patterns, even when only a couple of centimeters are visible (Isbell and Etting 2017). In wild white-faced capuchins (Cebus capucinus) subjects of all ages show anti-predatory behavior towards snake models, but infants show less discrimination between predatory and non-predatory snake models (Meno et al. 2013). In humans (Homo sapiens) the visual system is capable of detecting and reacting to snake visual stimuli quicker than to other fear-related stimuli (Soares et al. 2017). Humans identify and react to a snake visual stimulus more readily than to a spider visual stimulus, even when distracting neutral stimuli are presented (Soares et al. 2014).
According to Isbell’s snake detection theory (Isbell 2006, 2009), snakes were an important evolutionary pressure for the evolution of primates’ visual and perceptual adaptations that increased their ability to detect and quickly react to snakes. Mobbing on snakes by primates usually involves approaching, gathering around, intently observing and harassing the snake, and sometimes vocalizing and attacking it (Crofoot 2012). Vervet monkeys react to snakes with distinct vocalizations and looking to the ground (Seyfarth et al. 1980), and white-faced capuchins usually present mobbing behavior (Perry et al. 2003; Meno et al. 2013), and calls directed at dangerous snakes (Digweed et al. 2005).
Isbell (2006, 2009) also argues that the pressure of venomous snakes was different during the evolution of platyrrhine and catarrhine primates. Since the various genera of platyrrhines had already differentiated by the time venomous snakes arrived in America, platyrrhines present more variability in the ability to detect and respond to snakes than catarrhines. In addition, since platyrrhines had less time to evolve under the pressure of venomous snakes, their visual system may not be as capable of detecting snakes; this might explain why platyrrhines are less terrestrial than catarrhines (Isbell 2006, 2009). However, studies concerning wild primates’ encounters with snakes are still limited (McGrew 2015).
Although several primate species frequently hunt and eat vertebrates (Butynski 1982), they almost never eat snakes, possibly because of the life-threatening risk involved. In fact, we found only three reports of primate snake predation: one by wild Tarsius bancanus preying on Calliophis intestinalis (Niemitz 1973), and two by lion tamarins (Leontopithecus sp.), one captive Leontopithecus chrysomelas and one reintroduced Leontopithecus rosalia, that preyed on coral snakes (Pissinatti et al., in press). Both tamarins died as a consequence of envenomation. To our knowledge, humans are the only primate that regularly reverse this predator–prey relationship, and include snakes in their diet (Headland and Greene 2011).
We report here a compendium of 6 years of fieldwork, with several observations of snake interactions, including predation and consumption, with different species of snakes, by four groups of bearded capuchin monkeys (Sapajus libidinosus) from two wild populations living in northeastern Brazil. Our hypothesis is that Sapajus monkeys, due to their cognitive capabilities, omnivorous diet, opportunistic foraging, high behavioral plasticity (Fragaszy et al. 2004) and having the most catarrhine-like vision (Isbell 2009), would be able to discriminate dangerous (capable of causing great harm or death) from non-dangerous snakes (capable of causing no or little harm). We predicted that capuchin monkeys would:
-
1.
Mob dangerous snakes more often than non-dangerous snakes.
-
2.
Kill and consume non-dangerous snakes more often than dangerous ones.
-
3.
Present a sex/age difference, with adult capuchins, especially males, being more involved than juveniles in snake consumption since it is a potentially dangerous activity, with youngsters being more involved in scrounging pieces of snake killed by older individuals.
Methods
Study sites
We collected data on four bearded capuchin monkey groups living in two distinct study sites. Both sites are located in the south of Piauí State, Brazil, and are 320 km apart. Serra da Capivara National Park (SCNP; 8°50′S, 42°33′W) is the location of the Pedra Furada (PF) and Bocão (BC) neighboring groups. The climate is semi-arid, with dry bush vegetation (Caatinga biome), and a long annual dry season from May to October/November (Falótico and Ottoni 2013).
Fazenda Boa Vista (FBV; 9°39′36″S, 45°25′10″W) is the location of the Chicão (CH) and Zangado (ZA) groups. FBV is a semiarid Cerrado-Caatinga ecotone, where rainfall is seasonally distributed, with a clear dry season from May to September and a wet season from October to April (Spagnoletti et al. 2012).
There are reports from both sites on the presence of snakes, some of them posing a threat to capuchin monkeys by predation or accidental envenomation (Rodrigues and Prudente 2011; Cavalcanti et al. 2014; Pessis et al. 2014; see Supplementary material 1). Details on studied groups, locality, and hours of observation are reported in Table 1.
Data collection
We recorded all occurrences of encounters between snakes and capuchin monkeys (Martin and Bateson 2007) during the data collection phase of several different behavioral studies (see Table 1) (Falótico and Ottoni 2016; Verderane et al. 2013; Mendonça-Furtado et al. 2014; Spagnoletti et al. 2011). For each event, we recorded time and day, subject(s) involved (and/or age and sex), type of snake (visually identifying species when possible), type of interaction (capuchins killing and eating snakes or behaving defensively), and the monkeys’ behavioral responses (prey sharing with group members, in the predation case, or mobbing behavior toward a snake, in the defense case). We used Fisher’s exact tests (two-tailed, α-value 0.05) to compare events by sex and age.
Results
Snakes as prey
Three out of the four studied groups were observed preying on snakes, and altogether there were 23 recorded events (Table 2; Fig. 1; Supplementary material 2). In 11 of them we identified the snake by sight, before the monkeys ate or took it away. All 11 were non-venomous colubrids.
Snake predation was performed more by adult males (16 of 23 events for all groups, 70%), but the difference was only significant for the ZA group (p = 0.007) after controlling the expected values by group sex ratio. Females and juveniles were also recorded killing and eating snakes (Table 2; Supplementary material 3).
In nine of 23 of the predation events (39%), we also observed scrounging from group members. Eleven out of the 15 scroungers (73%) were infants and juveniles (Supplementary material 3). However, this age difference (adults/subadults vs. juveniles/infants) in scrounging events was not statistically significant (p = 0.449).
Snakes as danger
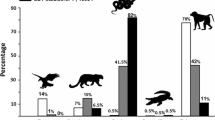
We recorded 35 snake encounters in which capuchin monkeys were alarmed and did not prey on the snake (Table 3; Supplementary material 4, 5).
We could identify 25 of 35 snakes (71%) that were attended to but not eaten, and 20 of those identified were potentially dangerous (Table 3; Fig. 2). The monkeys responded mainly to dangerous snakes (96%; 24 of 25 events) by vocalizing, shaking and dropping branches, threatening, and even poking them with sticks [poking with sticks occurred only in the PF group in two events out of 13 (Falótico and Ottoni 2014); see Supplementary material 4]. Threat displays were mostly collective: 91% (n = 35) of the observed events involved mobbing by an average of 7.9 individuals, of all ages (SCNP, 11.3, range 1–40; FBV, 4.6, range 1–16).
Mobbing events (Supplementary material 5) were variable in duration (median 17 min, range 1–365 min). Most defensive responses were of short duration. However, two events lasted at least 1 h and involved the entire group, and another, directed at an injured snake, lasted several hours.
Although we did not measure snake length, the snakes that were eaten appeared to be shorter than those that were mobbed. Exceptions were the coral snakes (Micrurus sp.) we observed, which were both small (10–20 cm in length) and dangerous, that the monkeys mobbed and did not eat (see Supplementary material 4). We never witnessed a predation event by any predator on the monkeys.
Discussion
Although events of encounters with dangerous and non-dangerous snakes were rare, bearded capuchin monkeys presented different responses when interacting with these types of snake. When dealing with predatory and potentially dangerous non-predatory snakes (i.e., venomous snakes, such as coral snakes), or with mimics of venomous snakes (i.e., Xenodon merremi and Thamnodynastes sp., genus Bothrops mimics), capuchins displayed mobbing behavior, including threat vocalizations and postures, looking for other monkeys nearby to join the threat (recruiting), and even using sticks to poke the snakes [15% events in the PF group (Falótico and Ottoni 2014)]. In contrast, non-dangerous snakes were readily killed and eaten, with no other behavioral display other than the predatory one.
Predation on snakes
The rate of predation on snakes by PF was double that of the CH and ZA groups. This is probably due by the PF group being the largest group studied. If the PF group had the same group size as the FBV groups, then its estimated snake predation rate would have been 0.26 events/100 h; this value is close to the average values observed for snake predation in FBV groups (0.28 events/100 h). Other factors, such as variation in snake population density, not sampled at this time, could also affect the snake encounter and predation rate. We did not observe snake predation by a BC group that was equally habituated but observed for less time.
Snakes that were killed were often not identifiable because the monkeys took the carcass with them or consumed it before we could identify it. However, in the cases where examination of the remains and/or visual assessment of the snake while it was being consumed was possible, they were clearly non-venomous snakes.
Adult males killed snakes more often (70%) than adult females and juveniles. In other primate species, e.g., chimpanzees (Pan troglodytes) and baboons (Papio spp.) a bias toward adult males in predatory behavior has also been reported (Butynski 1982; Watts and Mitani 2002; Pruetz et al. 2015). These sex-biased predation rates could be due to males’ risk-prone behavior to obtain high-risk, energetically valuable food, independently of abundance of food. This behavior is predicted by the energetic costs and fitness potential from males’ and females’ different sexual roles, as females have more costs—pregnancy, lactation, and carrying infants—and are risk averse (Fedigan 1990). We did not have enough data to relate rates of predation on snakes to food abundance, thus future research should examine this issue.
Defensive behavior toward snakes
Most defensive behaviors involved the participation of several individuals and were directed largely at venomous snakes. Nevertheless, capuchins also behaved defensively toward two non-venomous snakes, the vine snake (Chironius sp.) in one instance and the chicken snake (Spilotes pullatus) in two instances. Although these snakes are non-venomous, both of them predate small vertebrates (usually rodents); moreover, S. pullatus is large (up to 2.7 m), aggressive (Freitas 2003) and has black and yellow aposematic colors (Fig. 1d) to warn predators.
The use of tools as weapons in mobbing contexts has been rarely observed in capuchins, e.g., using a club to attack a Bothrops snake [wild C. capucinus (Boinski 1988)], or to explore the response of the snake [captive Sapajus sp. (Vitale et al. 1991)]. The SCNP population exhibited the snake poking behavior probably because they regularly use stick tools (Mannu and Ottoni 2009; Falótico and Ottoni 2014), and individuals were capable of generalizing the use of sticks from the usual foraging context to defensive behavior. The FBV population did not use poke sticks, possibly because they do not use stick tools (Cardoso and Ottoni 2016).
Learning
Our data indicate that adult capuchin monkeys respond differently to snakes that can be preyed upon and snakes that can kill them. Several cues may account for this (Isbell 2006). The size of the snake is a good predictor, especially of constrictors. However, small snakes, e.g., coral snakes, can also be dangerous (Pissinati et al., in press). For coral snakes, color (for trichromatic individuals), or contrast (for dichromatic individuals), could be a potential cue (Fig. 1b). Both types of information are available for the detection of S. pullatus, a large and aposematic snake. A third stimulus is the sound that some venomous snakes produce, e.g., the rattling sound of rattlesnakes (Crotalus spp.).
The learning of this discrimination must be fast, especially when dealing with dangerous snakes. Primates have a bias to learn a fear of snakes (Cook and Mineka 1989; Öhman 2009; Maior et al. 2011; Kawai and Koda 2016), and it has been argued that their visual system has evolved in response to the pressure to detect snakes (Isbell 2006). The social and conspicuous nature of capuchins’ defensive behavior toward snakes may provide a good learning context for a young monkey. Our data show that most (91%) of the threatening events were collective. Observing older individuals dealing with dangerous snakes creates the opportunity to associate some characteristics, such as size, skin pattern, coloration and sound of these snakes to the threatening behavior exhibited by expert group members.
Similarly, scrounging and allowing others to share killed snakes, which occurred in at least one-third of predation events, provides information about which snakes to eat, and food reward. Immatures experiencing these opportunities may learn faster and in a less hazardous way than those that do not. An ontogenetic study examining how capuchins learn to distinguish between dangerous versus non-dangerous snakes is crucial to fully appreciate this phenomenon.
Evolution
The capacity to detect snakes appears to be especially developed in primates that have a long evolutionary history of snake predation pressure (Isbell 2006). Isbell’s snake detection theory proposes that (1) different taxonomic groups of primates had different amounts of evolutionary time coexisting with venomous snakes; and that (2) differences in time would affect the behavioral response, making Catarrhini more uniformly “wired” to detect and avoid snakes than Platyrrhini. Our data support Isbell’s view, showing that a New World monkey, although fearful of snakes, learns to discriminate non-dangerous snakes as potential prey.
Isbell also argued that the habitual terrestriality reported in some catarrhines (e.g., baboons, vervets, and patas monkeys) may not occur in platyrrhines because their visual systems in general are not as sensitive as those of catarrhines to snakes. Snakes can be extremely difficult to see, especially on the ground where they may be camouflaged by leaf litter. Thus, platyrrhines may be more hesitant to go onto the ground because they may not be able to detect snakes as well as catarrhines. Interestingly, among the platyrrhines studied to date, capuchins have the most catarrhine-like vision (Isbell 2009), and the groups studied here are among the most terrestrial of known populations of capuchin monkeys (Spagnoletti et al. 2009; Verderane 2010; Falótico 2011). Therefore, the high level of terrestriality could also be a consequence of the ability to detect snakes, although ecological factors can also affect ground use by platyrrhines (Tabacow et al. 2009). Further studies should investigate the level of terrestriality in the genus Sapajus and the ability to prey on terrestrial vertebrates, as well as to discriminate dangerous from non-dangerous snakes.
Overall, our findings support the hypothesis that these populations of S. libidinosus can discriminate between dangerous and non-dangerous snakes and behave accordingly, protecting themselves when necessary, but taking the opportunity to prey on snakes when it is safe to do so. This study shows that capuchin monkeys are, like humans, both predators and prey of snakes. As such, capuchins can offer a non-human primate model for further investigation into the evolution of complex predator–prey relationships in the human lineage.
References
Boinski S (1988) Use of a club by a wild white-faced capuchin (Cebus capucinus) to attack a venomous snake (Bothrops asper). Am J Primatol 14:177–179. doi:10.1002/ajp.1350140208
Butynski TM (1982) Vertebrate predation by primates: a review of hunting patterns and prey. J Hum Evol 11:421–430. doi:10.1016/s0047-2484(82)80095-x
Cardoso RM, Ottoni EB (2016) The effects of tradition on problem solving by two wild populations of bearded capuchin monkeys in a probing task. Biol Lett 12:20160604. doi:10.1098/rsbl.2016.0604
Cisneros-Heredia DF, Cisneros-Heredia DF, León-Reyes A et al (2005) Boa constrictor predation on a titi monkey, Callicebus discolor. Neotrop Primates 13:11–12. doi:10.1896/1413-4705.13.3.11
Cook M, Mineka S (1989) Observational conditioning of fear to fear-relevant versus fear-irrelevant stimuli in rhesus monkeys. J Abnorm Psychol 98:448–459. doi:10.1037/0021-843X.98.4.448
Crofoot MC (2012) Why mob? Reassessing the costs and benefits of primate predator harassment. Folia Primatol 83:252–273. doi:10.1159/000343072
de Queiroga Cavalcanti LB, Costa TB, Colli GR et al (2014) Herpetofauna of protected areas in the Caatinga. II. Serra da Capivara National Park, Piauí, Brazil. Check List 10:18–27
Digweed S, Fedigan L, Rendall D (2005) Variable specificity in the anti-predator vocalizations and behaviour of the white-faced capuchin, Cebus capucinus. Behaviour 142:997–1021. doi:10.1163/156853905774405344
Emile N, Barros M (2009) Recognition of a 3D snake model and its 2D photographic image by captive black tufted-ear marmosets (Callithrix penicillata). Anim Cogn 12:725–732. doi:10.1007/s10071-009-0234-z
Falótico T (2011) Uso de ferramentas por macacos-prego (Sapajus libidinosus) do Parque Nacional Serra da Capivara—PI. Thesis, University of São Paulo. doi: 10.11606/T.47.2011.tde-04112011-171428
Falótico T, Ottoni EB (2013) Stone throwing as a sexual display in wild female bearded capuchin monkeys, Sapajus libidinosus. PLoS ONE 8:e79535. doi:10.1371/journal.pone.0079535
Falótico T, Ottoni EB (2014) Sexual bias in probe tool manufacture and use by wild bearded capuchin monkeys. Behav Process 108:117–122. doi:10.1016/j.beproc.2014.09.036
Falótico T, Ottoni EB (2016) The manifold use of pounding stone tools by wild capuchin monkeys of Serra da Capivara National Park, Brazil. Behaviour 153:421–442. doi:10.1163/1568539X-00003357
Fedigan LM (1990) Vertebrate predation in Cebus capucinus: meat eating in a Neotropical monkey. Folia Primatol 54:196–205. doi:10.1159/000156444
Fragaszy DM, Visalberghi E, Fedigan LM (2004) The complete capuchin: the biology of the genus Cebus. Cambridge University Press, Cambridge
Freitas MA (2003) Serpentes brasileiras. Malha-de-Sapo, Lauro de Freitas
Headland TN, Greene HW (2011) Hunter-gatherers and other primates as prey, predators, and competitors of snakes. Proc Natl Acad Sci USA 108:E1470–E1474. doi:10.1073/pnas.1115116108
Isbell LA (2006) Snakes as agents of evolutionary change in primate brains. J Hum Evol 51:1–35. doi:10.1016/j.jhevol.2005.12.012
Isbell LA (2009) The fruit, the tree, and the serpent. Harvard University Press, Cambridge
Isbell LA, Etting SF (2017) Scales drive detection, attention, and memory of snakes in wild vervet monkeys (Chlorocebus pygerythrus). Primates 58:121–129. doi:10.1007/s10329-016-0562-y
Kawai N, Koda H (2016) Japanese monkeys (Macaca fuscata) quickly detect snakes but not spiders: evolutionary origins of fear-relevant animals. J Comp Psychol 130:299–303. doi:10.1037/com0000032
Maior RS, Hori E, Barros M et al (2011) Superior colliculus lesions impair threat responsiveness in infant capuchin monkeys. Neurosci Lett 504:257–260. doi:10.1016/j.neulet.2011.09.042
Mannu M, Ottoni EB (2009) The enhanced tool-kit of two groups of wild bearded capuchin monkeys in the Caatinga: tool making, associative use, and secondary tools. Am J Primatol 71:242–251. doi:10.1002/ajp.20642
Martin P, Bateson P (2007) Measuring behaviour: an introductory guide, 3rd edn. Cambridge University Press, Cambridge
McGrew WC (2015) Snakes as hazards: modelling risk by chasing chimpanzees. Primates 56:107–111. doi:10.1007/s10329-015-0456-4
Mendonça-Furtado O, Edaes M, Palme R et al (2014) Does hierarchy stability influence testosterone and cortisol levels of bearded capuchin monkeys (Sapajus libidinosus) adult males? A comparison between two wild groups. Behav Process 109:79–88. doi:10.1016/j.beproc.2014.09.010
Meno W, Coss RG, Perry S (2013) Development of snake-directed antipredator behavior by wild white-faced capuchin monkeys. I. Snake-species discrimination. Am J Primatol 75:281–291. doi:10.1002/ajp.22106
Niemitz C (1973) Tarsius bancanus (Horsfields tarsier) preying on snakes. Lab Primate Newsl 12:18–19
Öhman A (2009) Of snakes and faces: an evolutionary perspective on the psychology of fear. Scand J Psychol 50:543–552. doi:10.1111/j.1467-9450.2009.00784.x
Perry S, Manson JH, Dower G, Wikberg E (2003) White-faced capuchins cooperate to rescue a groupmate from a Boa constrictor. Folia Primatol 74:109–111. doi:10.1159/000070008
Pessis A-M, Martin G, Guidon N (2014) Os biomas e as sociedades humanas na pré-história da região do Parque Nacional Serra da Capivara, Brasil, vol II-A. A&A Comunicação, São Paulo
Pissinatti A, Chagas WN, da Cruz JB, et al (in press) Snake incident as a limiting factor in the process of reintroduction of lion tamarins to their habitat. Leontopithecus Lesson, 1840 (Callitrichidae-Primates). In: Silva VL, Ferreira RG, Oliveira MAB (eds) A Primatologia no Brasil, vol. 14. UFPE, Recife, pp 212–225
Pruetz JD, Bertolani P, Ontl KB et al (2015) New evidence on the tool-assisted hunting exhibited by chimpanzees (Pan troglodytes verus) in a savannah habitat at Fongoli, Senegal. R Soc Open Sci 2:140507. doi:10.1098/rsos.140507
Quintino EP, Bicca-Marques JC (2013) Predation of Alouatta puruensis by Boa constrictor. Primates 54:325–330. doi:10.1007/s10329-013-0377-z
Ribeiro-Júnior MA, Ferrari SF, Lima JRF et al (2016) Predation of a squirrel monkey (Saimiri sciureus) by an Amazon tree boa (Corallus hortulanus): even small boids may be a potential threat to small-bodied platyrrhines. Primates 57:317–322. doi:10.1007/s10329-016-0545-z
Rodrigues FDS, Prudente ALDC (2011) The snake assemblage (Squamata: Serpentes) of a Cerrado-Caatinga transition area in Castelo do Piauí, state of Piauí, Brazil. Zoologia 28:440–448. doi:10.1590/S1984-46702011000400005
Seyfarth RM, Cheney DL, Marler P (1980) Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim Behav 28:1070–1094. doi:10.1016/S0003-3472(80)80097-2
Soares SC, Lindström B, Esteves F, Öhman A (2014) The hidden snake in the grass: superior detection of snakes in challenging attentional conditions. PLoS ONE 9:e114724–e114726. doi:10.1371/journal.pone.0114724
Soares SC, Maior RS, Isbell LA et al (2017) Fast detector/first responder: interactions between the superior colliculus-pulvinar pathway and stimuli relevant to primates. Front Neurosci 11:158–190. doi:10.3389/fnins.2017.00067
Spagnoletti N, Izar P, Visalberghi E (2009) Tool use and terrestriality in wild bearded capuchin monkey (Cebus libidinosus). Folia Primatol 80:142
Spagnoletti N, Visalberghi E, Ottoni EB et al (2011) Stone tool use by adult wild bearded capuchin monkeys (Cebus libidinosus). Frequency, efficiency and tool selectivity. J Hum Evol 61:97–107. doi:10.1016/j.jhevol.2011.02.010
Spagnoletti N, Visalberghi E, Verderane MP et al (2012) Stone tool use in wild bearded capuchin monkeys, Cebus libidinosus. Is it a strategy to overcome food scarcity? Anim Behav 83:1285–1294. doi:10.1016/j.anbehav.2012.03.002
Tabacow FP, Mendes SL, Strier KB (2009) Spread of a terrestrial tradition in an arboreal primate. Am Anthropol 111:238–249. doi:10.1111/j.1548-1433.2009.01116.x
Teixeira DS, dos Santos E, Leal SG et al (2015) Fatal attack on black-tufted-ear marmosets (Callithrix penicillata) by a Boa constrictor: a simultaneous assault on two juvenile monkeys. Primates 57:123–127. doi:10.1007/s10329-015-0495-x
Verderane MP (2010) Socioecologia de macacos-prego (Cebus libidinosus) em área de ecótono Cerrado/Caatinga. Thesis, University of São Paulo. doi: 10.11606/T.47.2010.tde-27072010-084124
Verderane MP, Visalberghi E, Izar P, Fragaszy DM (2013) Socioecology of wild bearded capuchin monkeys (Sapajus libidinosus): an analysis of social relationships among female primates that use tools in feeding. Behaviour 150:659–689. doi:10.1163/1568539X-00003076
Vitale AF, Visalberghi E, de Lillo C (1991) Responses to a snake model in captive crab-eating macaques (Macaca fascicularis) and captive tufted capuchins (Cebus apella). Int J Primatol 12:277–286. doi:10.1007/BF02547588
Watts DP, Mitani JC (2002) Hunting behavior of chimpanzees at Ngogo, Kibale National Park, Uganda. Int J Primatol 23:1–28. doi:10.1023/A:1013270606320
Acknowledgements
T.F. and E.B.O. thank FUMDHAM and Niede Guidon for their support during the research in SCNP, and the field assistants Francisco “Chico” Reinaldo and George Reinaldo. M.V., N.S., O.M.F., P.I. and E.V. thank the family Gomes de Oliveira for permission to work at FBV, and their field assistants Arisomar, Josemar, Renato, Marcos and Marino Junior. We thank Luciano Candisani for permission to use one picture, and Harry Greene for the assistance with the identification of the snakes. We are grateful to the review feedback by L.A. Isbell and W.C. McGrew, which enriched the manuscript. The following grants supported this study: 2006/07187-5 (T.F.), 2006/07190-6 (E.B.O.), 2006/51578-9 (M. P. V.), 2008/52293-3 (O.M.F.) and 2008/55684-3 (P.I.), São Paulo Research Foundation (FAPESP); Università La Sapienza di Roma (N.S.); EU FP6 NEST Programme ANALOGY (No. 029088) (N.S.); Istituto di Scienze e Tecnologie della Cognizione del CNR di Roma (N.S.); CNPq (E.B.O.); Capes (E.B.O., M.P.V., O.M.F.). The research was only observational and complied with protocols approved by the Animal Research Ethical Committee of the Institute of Psychology, University of Sao Paulo, and fully adhered to Brazilian law under authorizations IBAMA/ICMBio—037/2007 and 14825-1 (T. F.), 21406 (P.I., O.M.F.), 28689 (P.I., M.P.V., N.S., E.V.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 45340 kb)
Supplementary material 4 (MP4 37750 kb)
About this article
Cite this article
Falótico, T., Verderane, M.P., Mendonça-Furtado, O. et al. Food or threat? Wild capuchin monkeys (Sapajus libidinosus) as both predators and prey of snakes. Primates 59, 99–106 (2018). https://doi.org/10.1007/s10329-017-0631-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-017-0631-x