Abstract
Colletotrichum nymphaeae, causal agent of celery stunt anthracnose, has caused severe damage to celery production in Nagano Prefecture, Japan. For elucidating the infection cycle, celery seeds in the prefecture were screened on a selective medium and found to harbor C. nymphaeae as did seeds harvested from plants with diseased leaves. When seed samples with 1 and 10 % of the seeds infested were sown and grown, stunt anthracnose developed on seedlings, then finally spread extensively to other plants, suggesting that primary transmission occurred from seeds to seedlings, followed by secondary infection to other plants. These results strongly suggest that the disease cycle of the celery stunt anthracnose is completed through seed transmission in Nagano Prefecture. To disrupt the infection cycle, seed sterilization with hot water was tested. Conidia of C. nymphaeae in suspensions were killed by treatment at 50 °C for 40 min or at >55 °C for 10 min. Seedlings grown from seeds treated at 50 °C for 30 min did not develop any symptoms, and germination was not affected. Thus, hot water treatment at 50 °C for 30 min was the best method to eradicate the stunt anthracnose of celery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nagano Prefecture is a major area for celery (Apium graveolens var. dulce) production in Japan, especially in summer and autumn. In August 2007, a new disease, designated stunt anthracnose, emerged on celery in this prefecture (Fujinaga et al. 2011). Its causal agent was first identified as Colletotrichum simmondsii R. G. Shivas and Y. P. Tan (Fujinaga et al. 2011), then re-identified as Colletotrichum nymphaeae (Passerini) Aa (Sato and Moriwaki 2013). The pathogen causes severe chlorotic leaf spots and dwarfness with leaf curl, resulting in significant yield losses. Thus, the celery stunt anthracnose has become a serious problem in celery production in Nagano Prefecture. In this prefecture, celery is usually seeded in May–June, transplanted in July–August, and harvested in October–November. The disease is observed mainly during harvest in farm fields.
Celery seedlings are grown in greenhouse nurseries. To grow healthy seedlings, celery producers have tried to keep pathogen-free conditions in nurseries by removing debris of diseased plants thoroughly, sterilizing soils and cultivating materials, etc. Therefore, there seems to be a very low possibility that the pathogen remains in the nurseries. Nevertheless, celery stunt anthracnose was found in nursery greenhouses kept warmer than usual. This led us to assume that the source of the primary infestation may be celery seeds contaminated with the pathogen.
Additional lines of circumstantial evidence suggested that this disease may have spread through seed transmission of the pathogen. Almost all celery producers in the major production area in Nagano Prefecture had used celery seeds that were harvested by several farmers participating in seed production in the area and stored for more than 1 year. Around 2006, however, farmers began to use seeds collected the previous year due to a shortage of stored seeds. Concomitantly, the outbreak of the celery stunt anthracnose occurred. In addition, this disease is the most prevalent in an area where so-called “home-raised” seeds are used.
Colletotrichum simmondsii sensu lato including C. nymphaeae is separated from the species complex of Colletotrichum acutatum J. H. Simmonds (Damm et al. 2012; Shivas and Tan 2009). Celery leaf curl caused by C. acutatum was documented in Australia (Heaton and Dullahide 1993). Bob and Persley (2010) reported that the source of primary infection was the fungus surviving on celery residues left in the soil. However, seed transmission has not been reported so far. The first objective of this study was to test the hypothesis that C. nymphaeae has spread through seed transmission.
The second objective was to establish a strategy for controlling the disease. If its causal agent is actually transmitted with seeds, the most efficient way to break its infection cycle is seed sterilization. Chemical and physical methods have been developed for seed sterilization, but when preventive measures are urgent, chemical methods may not be suitable because newly selected pesticides cannot be used until they are registered. Moreover, the use of chemicals may lead to the development of fungicide-resistant strains. Therefore, methods other than chemical treatments to sterilize seeds are needed to control celery stunt anthracnose.
A typical physical method for seed sterilization is heat treatment. Several methods of heat treatment, either wet-heat or dry-heat types, have been reported (Kuniyasu 1999). Nagai and Takeuchi (1975) controlled watermelon anthracnose by seed sterilization with dry heat; we thus considered that the method may also be effective against celery stunt anthracnose. However, the dry heat treatment requires expensive equipment, and celery seeds have low heat resistance and are vulnerable to dry heat (Nakamura et al. 1981). Consequently, the dry heat sterilization appeared to be unsuitable for celery seeds. In this study, therefore, we tested a wet-heat type, treatment with hot water, for control of celery stunt anthracnose.
Materials and methods
Detection of C. nymphaeae in local seeds raised for commercial cultivation
Celery seeds produced by seed production farmers were collected in the central area of Nagano Prefecture in 2006 (Lot A), 2007 (Lot B), 2008 (Lot C) and 2011 (Lot D–K). Harvested seeds were dried, sieved, and stored in a refrigerator at 8 °C until use. In 2011, these seeds were incubated at 25 °C for 10 days on the medium developed by Uematsu et al. (2009) for specific detection of C. gloeosporioides and C. acutatum from soil with some modification: potato dextrose agar (PDA, Becton–Dickinson, New Jersey, USA) amended with 50 mg (a.i.)/L mepronil, 200 mg (a.i.)/L validamycin, 10 mg (a.i.)/L iprodione, 100 mg (a.i.)/L sodium choleate, 100 mg (a.i.)/L streptomycin sulphate, and 100 mg (a.i.)/L chloramphenicol.
C. nymphaeae colonies that developed on this medium were gray to white, cottony on the surface, and pale gray to pale orange on the reverse side. Conidia were observed with a light microscope (Olympus, Tokyo, Japan). If C. acutatum-like conidia were observed, these colonies were transferred to fresh PDA plates (9 cm diameter) and incubated for 7 days. A conidial suspension (103/mL) prepared from the plates was sprayed onto three 60-day-old, healthy celery seedlings (cv. Cornell 619) grown in separate pots (9 cm diameter) for each pathogenicity test. The inoculated seedlings were covered with a plastic bag and kept in a greenhouse for 1 day, then uncovered and grown for 10 days under the same conditions. Pathogenic isolates were then identified based on morphological characters according to a dichotomous key (Sato and Moriwaki 2013) for member species of the Colletotrichum acutatum species complex.
Confirmation of seed infection on plants with diseased leaves
In July 2009, purchased seeds of celery (cv. Cornell 619) were sown in soil in pots and grown for 81 days in a greenhouse. In October 2009, a pathogenic strain Gom-1 of C. nymphaeae (MAFF 242590) was cultured on PDA plates at 25 °C for 10 days. Conidia were harvested and suspended in sterile water at 107/mL. The conidial suspension was sprayed onto the 81-day-old healthy plants. The inoculated plants were covered with a plastic bag, kept in the greenhouse for 1 day, uncovered, and grown further in the same conditions. Fourteen days after inoculation, leaf symptoms were observed. Inoculated plants were grown further in the pots in the greenhouse for 8 months. In June 2010, seeds produced on these plants were harvested, dried, sieved, and stored in a refrigerator at 8 °C for 18 months. These seeds were designated as Lot X. A portion (n = 1020) of these seeds were retrieved and incubated on the detection medium mentioned above at 25 °C for 10 days.
We then checked the possibility that C. nymphaeae was present on the surface of the seeds and did not colonize them in a second trial. In August 2011, purchased seeds of celery (cv. Cornell 619) were again sown in pots in soil and grown for 80 days in a greenhouse. In October 2011, as described already, a conidial suspension of strain Gom-1 (107/mL) was sprayed onto the 80-day-old healthy plants, which were grown in the pots in the greenhouse for another 8 months. In June 2012, seeds were harvested, dried, sieved, and stored in a refrigerator at 8 °C for 11 months. These seeds were designated as Lot Z. A portion (n = 594) of these seeds were retrieved and surface-sterilized with a 30-min soak in water, 30-min soak in a suspension of 50 % (w/v) agent of iprodione (500 × dilution), 15-min water wash, and 5-min soak in 3 % (v/v) antiholmin solution (Chikuo and Sugimoto 1984). After sterilization, the seeds were incubated on the detection medium at 25 °C for 10 days.
Conidia on developed colonies were observed with a light microscope. C. acutatum-like conidia were transferred to fresh PDA plates (9 cm diameter) and incubated further. A conidial suspension prepared from the subcultures was sprayed onto healthy celery seedlings (cv. Cornell 619) as described to confirm their pathogenicity.
Confirmation of primary and secondary infection
About 100 seeds from the inoculated plants and the seeds from a farmer (Lot E) were sown in cell trays in May 2012 and grown in a chamber at 22 °C (12 h light/dark). Fifty-nine days after sowing, disease incidence was recorded.
The development of any primary and secondary infection was also examined using artificially infested seeds. Purchased celery seeds (cv. Cornell 619) were dipped in a conidial suspension (107/mL) of strain Gom-1 for 10 min at 17.5 °C. These artificially infested seeds were mixed with uninfested seeds so that the ratio of infested seeds was 0 % (infested seeds: n = 0; uninfested seeds: n = 300), 1 % (infested seeds: n = 3; uninfested seeds: n = 297), or 10 % (infested seeds: n = 30; uninfested seeds: n = 270). These seeds were sown in cell trays in May 2012. Twenty-nine days after sowing, seedlings were potted and grown in a greenhouse (average temperature around 25 °C) separately to prevent cross-infection. Twenty-eight and 67 days after sowing, disease incidence was recorded. In July (72 days after sowing), 100 seedlings from each were transplanted to a field with 50 and 45 cm of interrow and interrow spacing, respectively (1 plot/treatment). Each plot (1.5 m × 25 m) consisting of 100 seedlings was 2 m apart in the field. Thirty-four, 50, and 80 days after transplanting, disease incidence was recorded again. Celery plants were watered every day from 50 days after transplanting.
Determination of temperature and duration of hot water treatment required to kill C. nymphaeae conidia in suspension
A conidial suspension (1.6 × 104/mL) of strain Gom-1 was prepared using conidia produced on PDA culture plates. Test tubes containing 9 mL of sterile water were submerged in a water bath (SANSO THERMO PRO.SCD-301; Sansho, Tokyo, Japan) set at 45, 50, 55, 60, or 65 °C. When the temperature of the sterile water in the tubes reached that of the water bath, 1 mL of the conidial suspension was poured into each of the tubes, which were then incubated for 10, 20, 30, 40, 50, and 60 min. After the incubation, a sample (100 μL per plate) of each suspension was spread on a PDA plate and incubated at 25 °C. Seven days after incubation, colonies of C. nymphaeae were counted. Two PDA plates were used for each treatment.
Determination of temperature and duration of hot water treatment required to eradicate C. nymphaeae on seeds
Purchased celery seeds (cv. Cornell 619) were dipped in the conidial suspension (107/mL) of strain Gom-1 for 10 min at 20.5 °C. These artificially infested seeds were submerged in hot water at 50, 55, or 60 °C and kept for 10, 20, and 30 min. After the treatment, these seeds were immediately cooled with running water (23.4 °C) for 10 min, and then incubated on the detection medium at 25 °C. Also, artificially infested seeds were incubated without these treatments as control. After 10 days of incubation, the number of seeds with C. nymphaeae colonies was recorded.
Effect of hot water treatment on seed germination
For checking whether the treatment with hot water has any ill effects on celery seeds, purchased celery seeds (cv. Cornell 619) were submerged in water at 9.5 °C for 30 min or hot water at 50, 55, or 60 °C for 10, 20, and 30 min. After the treatment, these seeds (n = 50) were immediately cooled with running water (19.8 °C) for 10 min, placed on two-layered filter papers (ADVANTEC No. 2, Toyo Roshi Kaisya, Tokyo, Japan; TANEPITA, Fujihirakogyo, Tokyo, Japan) moistened with 4 mL of distilled water in a Petri dish, and incubated at 20 °C in the dark. Also, untreated seeds (n = 50) were incubated as a control. The number of germinated seeds was counted after 10 days. Each treatment consisted of three Petri dishes.
Effect of hot water treatment on incidence of stunt anthracnose
Purchased celery seeds (cv. Cornell 619) were dipped in conidial suspensions (107 mL or 105/mL) of isolate Gom-1 for 10 min 20.5 °C. The seed samples inoculated with 107 mL and 105/mL conidia were designated as Lot L and M, respectively. A portion of these artificially infested seeds were submerged in hot water at 50 °C for 30 min, and then cooled with running water (23.4 °C) for 10 min. Another portion of these artificially infested were also used without these treatments as control. They were immediately sown in cell trays in May 2012 and grown in a greenhouse (average temperature around 25 °C). Twenty days after sowing, seedlings were potted and grown further in the greenhouse. Disease incidences were recorded in June 2012 (Lot L) or July 2012 (Lot M).
The seeds from a farmer (Lot E) and those from the inoculated plants mentioned above (Lot X) were also tested. They were submerged in hot water at 50 °C for 30 min, and then cooled with running water (23.4 °C) for 10 min. Untreated seeds were used as controls. They were sown in cell trays in May 2012 and grown in a greenhouse (average temperature around 25 °C). Twenty days after sowing, seedlings were potted and grown further in the greenhouse (average temperature around 25 °C). Sixty-six days after sowing (in July), seedlings were transplanted to a field with 50 and 45 cm of interrow and interrow spacing, respectively, (1 plot/treatment) and grown further. Each plot (1.5 m × 25 m) consisting of 100 seedlings was located 2 m apart in the field. Disease incidence was recorded in August.
Results
Detection of C. nymphaeae in locally raised seeds
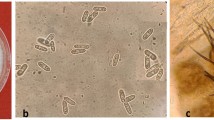
Colonies typical of C. nymphaeae were detected on seeds from Lot E, H, and J, which were harvested in 2011 (Fig. 1a). Microscopic observations revealed that length and L/B ratio of conidia on the colonies were similar to those of C. nymphaeae. On the basis of colony appearance and conidial morphology, we identified these isolates as C. nymphaeae. The highest rate of infested seeds was 0.49 % (5 of 1023 seeds tested) in Lot E (Table 1). All eight isolates from the eight infested seeds in Lot E, H, and J produced chlorotic spots on all celery seedlings, which were similar to the natural symptoms of the disease. C. nymphaeae colonies were not detected in seeds harvested before 2008 (Table 1).
Colonies of Colletotrichum nymphaeae that developed from celery seeds provided by seed farmers, after 10 days incubation on the detection medium (a) and symptoms of stunt anthracnose on celery plant (cv. Cornell 619) 10 days after inoculation with a C. nymphaeae isolate obtained from celery seeds (b)
Seed infection on plants with diseased leaves
Leaves of 81-day-old healthy celery plants were inoculated with strain Gom-1 and grown for 8.5 months. Fourteen days after inoculation, chlorotic spots were confirmed on the inoculated leaves. Of 1020 seeds (Lot X) harvested from the inoculated plants, 10 seeds (0.98 %) produced C. nymphaeae-like colonies on media. Even in the second trial with surface-sterilization, C. nymphaeae-like colonies were detected in 0.67 % of the seeds (4 of 594 Lot Z seeds tested) harvested from 80-day-old plants with inoculated, diseased leaves.
To confirm that these colonies were actually the causal agent of stunt anthracnose, fungal cultures were isolated from the 14 seeds (10 from the first trial + 4 from the second trial). When a spore suspension was sprayed on healthy seedlings, all 14 cultures produced chlorotic spots on all seedlings, which were similar to the natural symptoms of this disease (Fig. 1b).
Primary and secondary infection
The seeds obtained from inoculated plants in the first trial (Lot X containing 0.98 % of infested seeds) were grown in the chamber. Among 90 seedlings grown from these seeds, one showed typical symptoms of this disease. Typical symptoms were also observed on one of 111 seedlings grown from the seeds obtained from a farmer (Lot E, containing 0.49 % of infested seeds).
Seed samples with 0, 1, and 10 % contamination were prepared using artificially infested seeds and sown in the greenhouse. Twenty-eight days after sowing, disease incidence was 27 % on seedlings from the sample with 10 % contamination and 5 % on those from the sample with 1 % contamination (Fig. 2a). Disease incidence increased gradually after transplanting and sharply in September (Fig. 2b). In October (harvest), disease incidence in plants from the 10 % plot reached 87 %. Even in plants from the 1 % plot, disease incidence reached 75 %. These results suggest that even seeds with 1 % contamination can cause destructive epidemics or outbreaks. On the other hand, the disease was not observed on plants from the 0 % plot (Fig. 2b).
Temperature and time of hot water treatment required for killing C. nymphaeae conidia in suspension
After treatment of conidial suspensions at 45 °C, colonies grew on PDA irrespective of treatment duration, suggesting that C. nymphaeae can survive at this temperature (Table 2). On the other hand, the 50 °C treatment decreased the number of colonies markedly. When the suspension was treated for 40 min, no colonies formed. Above 55 °C, no colonies were produced even after just 10 min. From these results, we concluded that treatment with hot water above 50 °C is required to kill C. nymphaeae conidia.
Temperature and time of hot water treatment required to eradicate C. nymphaeae on seeds
C. nymphaeae on seeds was completely killed by treatments at 60 °C for 10–30 min and by treatments at 50 and 55 °C for 30 min (Table 3). Thus, the treatment at 50 °C for 30 min is sufficient to eradicate C. nymphaeae on seeds.
Influence of hot water treatment on seed germination
The germination rate of celery seeds was 64.7 % in the control (with no treatment) and was not affected by the treatment at 50 °C for 10–30 min (Table 4). However, it decreased severely when treated at 55 and 60 °C (Table 4).
Effect of hot water treatment on incidence of stunt anthracnose
When artificially infested seeds from Lot L were sown without any treatment and grown in the greenhouse, 46.7 % of the plants developed typical symptoms (Table 5). The symptoms were also observed on 17 % of plants grown from Lot M. When these seeds were treated at 50 °C for 30 min, however, no plants developed the symptoms (Table 5).
Similar effects were obtained in experiments using seeds collected from inoculated plants (Lot X) and those obtained from the farmer (Lot E) (Table 5). They yielded 11 and 2 % of diseased plants, respectively, when grown without treatments with hot water. On the other hand, no plants were diseased in either group when seeds were treated with hot water at 50 °C for 30 min.
Discussion
As mentioned earlier, circumstantial evidence suggested that stunt anthracnose may have spread through seed transmission of C.nymphaeae. In the present study, we successfully detected seeds harboring C. nymphaeae among locally grown seeds used for celery production in Nagano Prefecture. To examine whether this pathogen can complete an infection cycle by first colonizing seeds, we divided the possible infection cycle into the following three steps, and tested the possibilities one by one: (1) primary infection from conidia produced on infected seeds to plant leaves, (2) secondary infection from infected leaves to (leaves of) other individuals, and (3) transmission of the pathogen from leaves to seeds (infection of seeds from conidia produced on leaves).
First, we demonstrated that primary infection of seedlings occurred through infected seeds. Disease symptoms were observed on seedlings from the seeds collected from inoculated plants (containing 0.98 % of infested seeds) and obtained from a farmer (containing 0.49 % of infested seeds). It is unlikely that these symptoms were caused by C. nymphaeae that entered from an external source because the seedlings were maintained in isolated, controlled-environment chambers. Primary infection from infested seeds to seedlings was also confirmed by the experiment using artificially infested seeds (Fig. 2a). Second, disease development after transplanting in this experiment (Fig. 2b) showed that secondary infection occurred very efficiently. Even 1 % contamination of infested seeds led to destructive infection (disease incidence = 75 %) in October (harvest period). Third, by harvesting seeds on plants with diseased leaves, we demonstrated that C. nymphaeae can be transmitted from lesions on leaves to heads and infect the seeds. All these results strongly suggest that infested seeds are the source of the primary infection, which is followed by secondary infection and seed infection, resulting in the completion of the infection cycle of celery stunt anthracnose in Nagano Prefecture. The source of infection for celery leaf curl disease caused by C. acutatum sensu lato in Australia (Heaton and Dullahide 1993) was found to be the fungus surviving on celery residues in the soil (Bob and Persley 2010). The present study is the first report of seed transmission of C. nymphaeae in celery.
Fujinaga et al. (2011) showed that C. nymphaeae grew most rapidly at high temperatures (25–30 °C). Celery seedlings are usually cultivated in May to July when the climate is hot and humid in Nagano Prefecture. Conditions in the nursery field during this season seem to be suitable for this disease. In addition, the present study showed that disease development accelerated sharply in September. During the month before harvest, celery plants are watered every day. We consider that this watering contributed to the rapid disease spread in September. High temperature and watering may explain why the disease could spread quickly even when the frequency of contaminated seeds was low (Fig. 2b).
Effective methods for seed sterilization should be established to control this disease as soon as possible. Doornik (1992) reported that more than 99 % of Colletotrichum acutatum conidia were killed by treatment with hot water at 50–60 °C for 1 min. Bob and Persley (2010) reported that treatment with hot water at 50 °C for 30 min was effective against late blight and early blight disease of celery. The present study showed that conidia of C. nymphaeae were killed by treatment with hot water at 50 °C for more than 40 min or at 55 °C for 10 min (Table 2), suggesting that the minimum temperature required to kill the pathogen is around 50 °C. The best temperature and time must be selected based on the combination required to kill the pathogen without affecting seed viability. Webster (1921) reported that celery seed loses viability somewhere between 50 and 55 °C. We found that the germination of celery seeds was not affected by treatment at 50 °C for 30 min, but was severely affected at 55 °C or more (Table 4). Consequently, we concluded that the best condition to prevent seed transmission of the pathogen is at 50 °C for 30 min. Actually, the treatment of the infested seeds at 50 °C for 30 min suppressed stunt anthracnose completely (Table 5). This is the first report of seed sterilization for the control of celery stunt anthracnose. In this study we did not consider short-time treatments for less than 10 min because short treatments at a high temperature may be affected by temperature instability or a time lag.
Most farmers in Nagano Prefecture, as mentioned earlier, use celery seeds for commercial cultivation that were produced locally. Therefore, there is a threat that C. nymphaeae may spread rapidly through seeds in Nagano Prefecture when it occurs in fields of seed producers. We have recommended that farmers sterilize celery seeds with hot water using the conditions established in the present study. Consequently, the incidence of the celery stunt anthracnose has been reduced drastically where the farmers followed our suggestions (data not shown).
We have not elucidated which seed tissues is colonized by C. nymphaeae. However, the fungus is unlikely to colonize the inside of the seeds because the treatment with hot water is highly effective. Further studies are needed to determine the sites of colonization.
It should be noted that, in Nagano Prefecture, most farmers grow celery in the same fields every year. Once stunt anthracnose occurs in a field, therefore, celery residues may be left in the field and act as the primary inoculum in the next season. Plants that surround the field may also provide another source of inoculum because of the wide host range of C. nymphaeae (Sato and Moriwaki 2013). We are now working to develop cultural controls for this disease.
References
Bob D, Persley D (2010) Celery. In: Persley D et al (eds) Diseases of vegetable crops in Australia. CSIRO Publishing, Australia, pp 107–112
Chikuo Y, Sugimoto T (1984) Infection of sugar beet seed by Colletotrichum dematium f. spinaciae (in Japanese with English summary). Ann Phytopath Soc Japan 50:249–254
Damm U, Cannon PF, Woudenberg JHC, Crous PW (2012) The Colletotrichum acutatum species complex. Stud Mycol 73:3–113
Doornik AW (1992) Heat treatment to control Colletotrichun acutatum on corms of Anemone coronaria. Neth J Pl Path 98:377–386
Fujinaga M, Yamagishi N, Ogiso H, Takeuchi J, Moriwaki J, Sato T (2011) First report of celery stunt anthracnose caused by Colletotrichum simmondsii in Japan. J Gen Plant Pathol 77:243–247
Heaton JB, Dullahide SR (1993) Control of celery leaf curl disease caused by Colletotrichum acutatum. Australas Plant Path 22:152–155
Kuniyasu K (1999) Characteristics of the seed epidemic. In: Ohata K et al (eds) Ecology and control of seed borne disease (in Japanese). Japan Plant Protection Association, Tokyo, p 25
Nagai Y, Takeuchi T (1975) The dry heat seed disinfection method for the seed-borne disease damage of several sort vegetables. Ann Phytopath Soc Japan 41:269
Nakamura H, Kobayashi K, Yamada H (1981) Heat resistance of seeds in various vegetable crops (in Japanese with English summary). Bull Veg Ornam Crops Res Stn Japan Ser A 8:33–51
Sato T, Moriwaki J (2013) Molecular re-identification of strains in NIAS Genebank belonging to phylogenetic group A2 and A4 of the Colletotrichum acutatum species complex. Microbiol Cult Coll 29:13–23
Shivas RG, Tan YP (2009) A taxonomic re-assessment of Colletotrichum acutatum, introducing C. fioriniae comb. et stat. nov. and C. simmondsii sp. nov. Fungal Divers 39:111–122
Uematsu S, Mochizuki D, Tanaka C, Sato T (2009) A new selective medium for Colletotrichum gloeosporioides and C. acutatum (abstract in Japanese). Jpn J Phytopathol 75:187–188
Webster SK (1921) Treatment of celery seed for the control of Septoria blight. J Agric Res 21:369–372
Acknowledgments
Special thanks to Mr. Munetoshi Aoki, Nagano Vegetable and Ornamental Crops Experiment Station, for careful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamagishi, N., Fujinaga, M., Ishiyama, Y. et al. Life cycle and control of Colletotrichum nymphaeae, the causal agent of celery stunt anthracnose. J Gen Plant Pathol 81, 279–286 (2015). https://doi.org/10.1007/s10327-015-0598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-015-0598-7