Abstract
Adsorption of vanadate(V) from aqueous solution onto industrial solid ‘waste’ Fe(III)/Cr(III) hydroxide was investigated. HCl treated Fe(III)/Cr(III) hydroxide was found to be more efficient for the removal of vanadate(V) compared to untreated adsorbent. The adsorption follows second-order kinetics. Langmuir and Freundlich isotherms have been studied. The Langmuir adsorption capacity (Q 0) of the treated and untreated adsorbents was found to be 11.43 and 4.67 mg g−1, respectively. Thermodynamic parameters showed that the adsorption process was spontaneous and endothermic in the temperature range 32–60°C. Maximum adsorption was found at system pH 4.0. The adsorption mechanism was predominantly ion exchange. Effect of other anions such as phosphate, selenite, molybdate, nitrate, chloride, and sulfate on adsorption of vanadium has been examined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vanadium is widely distributed in Earth’s crust and has been recognized as a potentially dangerous pollutant and it is listed on the United States Environment Protection Agency (USEPA) candidate contaminant list (Naeem et al. 2007). Vanadium is mostly found along with phosphorus ores (Jansson-Charrier et al. 1996). The production of phosphoric acid using these minerals results in the extraction of large amount of vanadate. Vanadium compounds are also present in significant concentration in fuel oil (Jansson-Charrier et al. 1996) and combusting this in thermal power plants produces toxic fly ash. These wastes cannot be disposed directly into the environment because rain water easily leaches vanadate (Naeem et al. 2007). It has been reported that contamination of groundwater occurs with vanadate from industrial mining and natural sources (Ortiz-Bernard et al. 2004). Vanadium/phosphoric acid system is also used as corrosion inhibitor in the place of Cr(VI) in steel industries (Blackmore et al. 1996). Vanadium compounds have shown harmful effects on the circulatory system and can disturb the metabolism of human (Zagulski et al. 1980) and lower the food glucose, cholesterol, and triglycerides in diabetic rats (Nukatsuka et al. 2002).
Various biological and chemical treatments have been studied to remove vanadate (Kunz et al. 1976). Blackmore et al. (1996) have shown that vanadium could be removed chemically through adsorption and co-precipitation with FeO(OH). Guzman et al. (2002) also reported that vanadium was removed by chitosan through adsorption process. Although many studies have been conducted in treating vanadium, few studies are available to clearly depict the removal mechanism.
Baes and Mesmer (1976) have extensively studied vanadium species at different pH values. Speciation diagram suggests that vanadium exists in cationic form below pH 3.0 and anionic form between pH 3.5 and 11. Adsorption of oxyanions onto metal(oxy)hydroxide can be described by inner sphere or outer sphere anion surface complexation (Hayes et al. 1988). Surface complex mechanism of anionic species onto metal(oxy)hydroxide can be represented as follows (Su and Suarez 2000; Kreller et al. 2002):
Outer sphere complex mechanism
Inner sphere complex mechanism
where M is the hydroxylated mineral surface and OH is a reactive surface hydroxyl group. Ln− is the oxyanion. Protonation of the reactive hydroxyl group of metal(oxy)hydroxide is represented by Eqs. 1 and 2. Complex formation of background electrolyte ions and the surface is shown in Eqs. 3–5. Outer sphere complex mechanism is represented by Eqs. 6–9, which shows that anion reacts similar to the background electrolyte. Inner sphere coordination mechanism of anions is shown in Eqs. 10–13.
Chemical industries use hexavelant chromium compounds as corrosion inhibitors in the cooling water systems. Periodically fresh Cr(VI) solutions are injected and the spent cooling water is let out. Chromium(VI) in the water is toxic and is reduced to less toxic Cr(III) using ferrous ion produced through an electrolytic process or ferrous sulfate under acidic conditions. The trivalent chromium and iron are precipitated as chromium hydroxide and ferric hydroxide under alkaline conditions. The resultant Fe(III)/Cr(III) hydroxide settles as sludge in lagoons and is removed periodically. The Fe(III)/Cr(III) hydroxide sludge is discarded as waste in the industries. The re-use of solid waste from wastewater treatment plants may reduce the cost of treating vanadate. The use of this solid waste has been applied to removing heavy metals (Namasivayam and Senthilkumar 1998), dyes (Namasivayam et al. 1994), phenol (Namasivayam and Sumithra 2004), phosphate (Namasivayam and Prathap 2005), selenite (Namasivayam and Prathap 2006), and silica (Namasivayam and Prathap 2007). The purpose of this study was to use the solid waste Fe(III)/Cr(III) hydroxide to remove vanadate(V). Effects of contact time, pH, and temperature were also studied. In this study, effects of the competitive ions such as phosphate, selenite, and molybdate on adsorption of vanadate(V) were also investigated.
Materials and methods
‘Waste’ Fe(III)/Cr(III)hydroxide was obtained from the Southern Petrochemical Industries Corporation Limited (SPIC), Tuticorin, Tamil Nadu, India. It was grinded and washed with deionized water to remove very fine powder. Then it was dried at 65°C for 12 h. The dried material was sieved to 75–150 μm size and used as adsorbent. The characteristics of waste Fe(III)/Cr(III) hydroxide are shown in Table 1 (Namasivayam and Ranganathan 1994). This material has a fractal dimension usually known for mineral surfaces. The specific surface area (SSA) is made of very small non-micro pores particles. The pore size distribution shows that the particles alone are not porous but the total pore volume for pores is mostly due to interparticle pores. A broad distribution with pores larger than the estimated particle diameter (a maximum around 3 nm) was observed, which most likely corresponds to the small pores created by spheres with a diameter of 10 nm adjacent to each other and such building makes a porous aggregate. Table 1 shows that neither Fe3+ nor Cr3+ goes into solution in the pH range 3.5–10.0. Ammonium vanadate was obtained from s.d. Fine Chemicals, Mumbai, India.
Batch mode adsorption experiments
An aliquot of 500 mg of untreated adsorbent and 50 ml of ammonium vanadate solution of desired concentration were transferred into 250 ml high-density polyethylene bottles and agitated at 160 rpm, 32°C on a mechanical shaker. At the end of the predetermined time intervals, the bottles were withdrawn and the supernatant was centrifuged at 20,000 rpm for 20 min. Then the residual vanadate(V) was analyzed by colorimetric method (Jansson-Charrier et al. 1996) on UV–Vis spectrophotometer (Hitachi, Model U-3210, Japan and/Analytik Jena AG, Specord 200, Germany). Effect of pH was studied in the range 4.0–10.0 using 1 M HCl and 1 M NaOH solutions by means of a pH meter (Elico, Mode LI-107, Hyderabad, India).
Treated adsorbent was also used to study the adsorption process. Treated adsorbent was prepared by shaking the adsorbent, Fe(III)/Cr(III) hydroxide, with distilled water at pH 4.0 adjusted using 1 M HCl. Shaking and adjustment of the supernatant pH to 4.0 were continued until the supernatant pH remained at 4.0. Then the wet adsorbent was separated from the solution and used for adsorption experiments.
In the case of untreated adsorbent, the equilibrium pH (final pH after agitation) was 7.9–8.7 for the initial pH range 4.0–10.0. Since the pHzpc of the adsorbent is 8.1, the surface sites are mostly negatively charged at pH > 8.1, which is less favorable for the adsorption of vanadate anion due to the electrostatic repulsion between the negatively charged surface and vanadate anion. In the case of treated adsorbent, equilibrium pH was found to be 6.5–7.8 for the initial pH range 4.0 to 10.0. At this condition, surface sites of the adsorbent exist mostly as positively charged, which favors the adsorption of vanadate anion by electrostatic attraction between the positively charged adsorbent surface and negatively charged adsorbate. This surface property of the treated adsorbent enhances the adsorption capacity. Effect of foreign anions on adsorption was studied using equimolar concentrations (0.78 mmol l−1) of vanadate(V) and foreign anions.
Desorption studies
The adsorbent that was used for the adsorption of 10 and 20 mg l−1 of vanadate(V) solution was separated from the solution by suction–filtration using Whatmann filter paper and washed gently with water to remove unadsorbed vanadate(V). Then the spent adsorbent was agitated for equilibrium time with 50 ml of desorption media. The desorption media were prepared with water at different pH values (4.0–12.0) adjusted using HCl/NaOH solutions. Then the desorbed vanadate(V) was estimated as before.
Temperature study
An aliquot of 50 ml of vanadate solution was added into 500 mg of the untreated adsorbent in a 100-ml conical flask. The concentrations of vanadate solution used are 10, 20, 30, 40, and 50 mg l−1 at initial pH 4.0 and the experiments were carried out at 32, 40, 50, and 60°C in a thermostated rotary shaker. The final pH ranged from 7.9 to 8.2.
Effect of foreign ions
Solutions of NO3 −, SO4 2−, Cl−, PO4 3−, SeO3 2−, and MoO4 2− were prepared from their sodium salts. To study the effect of these ions on the removal of vanadate, the sorption of vanadate alone and in the presence of foreign anion were determined. Equimolar quantities (0.78 mM) of vanadate(V) and foreign anions were used to study the foreign ion effect.
Preliminary experiments showed that there was no adsorption of vanadate(V) due to container walls. Experiments were carried in duplicates and mean values were used for calculations. Maximum deviation was found to be 3.5%.
Results and discussion
Adsorption kinetics
In order to investigate the mechanism of adsorption and rate-controlling steps, the kinetic data were modeled using Lagergren first-order (Lagergren 1898) and second-order models (Mckay and Ho 1998)
The Lagergren first-order model can be represented as:
where q e and q t are the amounts of vanadate(V) adsorbed (mg g−1) at equilibrium and at time t, respectively, and k 1 is the rate constant of first-order adsorption (min−1). Values of q e and k 1 were calculated from the slope and intercept of the plots of log(q e − q t ) versus t (Fig. 1).
The second-order kinetic model is represented as:
Values of the second-order rate constant, k 2 and q e, were calculated from the slope and intercept of the plots of t/q t versus t (Fig. 2).
Table 2 shows that q e values calculated using the first-order kinetic model do not agree with the experimental q e values even though the plots have high correlation coefficients. This suggests that the adsorption of vanadate(V) does not follow first-order kinetic model. It was found that the q e values calculated using the second-order model agree with the experimental q e values with high correlation coefficients (Table 2). So, the adsorption system studied belongs to the second-order kinetic model. Similar results have been observed in the adsorption of phosphorus on alunite (Ozacar 2003) and Fe(III)/Cr(III)hydroxide (Namasivayam and Ranganathan 1994).
Adsorption isotherms
The equilibrium of a solute separated between liquid and solid phase is described by various models of adsorption isotherms such as the Langmuir (Langmuir 1918) and Freundlich (Freundlich 1906) models. The Langmuir isotherm has widely been used to describe single-solute systems. It is based on the assumption that intermolecular forces decrease rapidly with distance and consequently it predicts monolayer coverage of the adsorbate on the outer surface of the adsorbent. This model also suggests that there is no lateral interaction between the sorbed molecules. Once a molecule occupies a site, no further adsorption can take place at that site.
The Langmuir isotherm can be expressed as:
where q e is the solid-phase adsorbate concentration at equilibrium (mg g−1), C e is the concentration of vanadate(V) solution (mg l−1) at equilibrium. The constant Q 0 gives the theoretical monolayer adsorption capacity (mg g−1) and b is related to the energy of adsorption (l mg−1). A linear expression for the Langmuir equation is expressed as:
Langmuir isotherms are shown in Figs. 3 and 4 for untreated and treated adsorbents, respectively. Langmuir constants are given in Table 3. In the case of untreated adsorbent, the monolayer adsorption capacity, Q 0, was found to be 4.93 mg g−1 and b was 0.10 l mg−1 at room temperature (Table 3), whereas for the treated adsorbent the Q 0 and b were found to be 11.43 mg g−1 and 0.82 l mg−1, respectively. Low adsorption capacity of the untreated adsorbent is due to the higher equilibrium pH (final pH > 8.1) compared to the treated adsorbent. Above pH 8.1, the adsorbent surface modified with hydroxide ions does not favor adsorption of oxyanion, since the pHzpc of the adsorbent is 8.1 (Namasivayam and Sumithra 2004). In the case of treated adsorbent, the equilibrium pH (final pH) is 6.5, and positively charged adsorbent surface favors the adsorption of vanadate anion. The value of Q 0 obtained in this study was lower than that reported by Guzman et al. (2002) for chitosan (400–450 mg g−1). The high adsorption capacity of chitosan may be due to lower particle size (0–125 μm), surface functional groups, and maintenance of the equilibrium pH (final pH) at 3–3.5. In this study, final pH was not maintained during adsorption. For all the different initial pH values studied, the final pH was >8.0 and 7.0 for the untreated and treated adsorbents, respectively. At pH >8.0, chitosan gave Q 0 values of less than 20 mg g−1. Guibal et al. (1998) reported adsorption capacity for the adsorption of vanadate onto chitosan beads of varying particles size: 402.5, 250.4, 165.2, and 146.8 mg g−1 for particle size, 0.95 mm, 0–125, 125–250, and 250–500 μm, respectively. So, it is expected that combined influence of functional groups, particle size, and system pH is responsible for good adsorption.
Freundlich model assumes heterogeneous adsorption due to the diversity of sorption sites or the diverse nature of the metal ions sorbed, free, or hydrolyzed species.
The Freundlich model equation is expressed as:
where k f denotes adsorption capacity and n is related to intensity of adsorption. Freundlich isotherms are shown in Figs. 3 and 4. Values of the Freundlich constants, k f and n, were found to be higher for treated adsorbent compared to untreated adsorbent (Table 4). Experimental equilibrium data appear to fit better with Langmuir isotherm than with Freundlich isotherm for both the untreated and treated adsorbents.
Effect of temperature
Effect of temperature on the adsorption isotherm was investigated in the temperature range 32–60°C. Figure 5 shows the temperature dependence of vanadate adsorption on to untreated Fe(III)/Cr(III) hydroxide. Values of the Langmuir constants obtained from the best fit of Langmuir adsorption isotherm are listed in Table 3. Results show that the value of b increases with increase in temperature. Value of Q 0 remains more or less same with increase in temperature (Sontheimer et al. 1988). Similar results have been reported for the adsorption of Zn(II) from aqueous solution by fly ash (Weng and Huang 2004).
Using the Lagergran rate equation, the first-order rate constants (k 1) and correlation coefficients were calculated. The calculated q e values obtained from the first-order kinetics do not agree with the experimental q e values at different temperatures (Table 2). These results indicate that the system does not follow first-order kinetic model. Second-order kinetic model shows that the calculated q e values agree with the experimental q e values at different temperatures (Table 2). This indicates that the adsorption follows second-order kinetic model at different temperatures used in this study. On increasing the temperature from 32 to 60°C, the amount adsorbed at equilibrium, q e, increased from 2.23 to 2.46 mg g−1. The increase in the equilibrium sorption of vanadate(V) with temperature indicates that high temperature favors vanadate(V) removal. Thermodynamic parameters such as change of Gibbs free energy (ΔG o), entropy (ΔS o), and enthalpy (ΔH o) were calculated using the following thermodynamic equations (Sontheimer et al. 1988):
where R is the gas constant in J K−1 mol−1, T is the temperature in K, and b is equilibrium constant (Langmuir constant in l mol−1). Figure 6 shows van’t Hoff plot of ln b versus 1/T and the values of ΔS o and ΔH o were determined from the intercept and slope, respectively. The thermodynamical parameters are listed in Table 5. The negative values of ΔG o indicate the spontaneous nature of adsorption. ΔG increased with increase in temperature from 32 to 60°C, which shows that the process is favorable at higher temperature. The positive value of ΔH confirms the endothermic nature of adsorption. The positive value of ΔS shows the increased randomness at the solid/solution interface during the adsorption of vanadate(V) (Dursun et al. 2005).
The activation energy for the adsorption process was calculated using Arrhenius equation.
where k is the rate constant at given temperature, T, E a is the activation energy for the overall adsorption process (kJ mol−1), which must be overcome before adsorption takes place, A is the exponential factor, which is the measure of the accessibility of the reactive sites of the adsorbent, R is the universal gas constant (8.314 J K−1 mol−1), and T is the absolute temperature. A plot of log k versus 1/T yields a straight line, from which the E a and A were calculated based on the slope and intercept, respectively (data not shown). Value of E a was found to be 23.9 kJ mol−1, which is lower than 40 kJ mol−1 for chemical adsorption process (Ozcan and Ozcan 2004).
Effect of pH
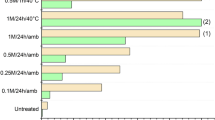
The species distribution of vanadium was studied by Baes and Mesmer (1976). According to their study, various forms of vanadium existed in solution. They were cationic VO2 +, neutral VO(OH)3, and anionic species, V10O26(OH)3−, V10O27(OH)5−, V10O28 6−, and other mono or poly vanadate species, VO2(OH) −2 , VO3(OH)2−, VO4 3−, V2O6(OH)3−, V2O7 4−, V3O9 3−, and V4O12 4−. The distribution of these forms was found to be pH dependent. In the pH range 4.0–10.0, vanadium(V) exists mainly as VO3 − (Blackmore et al. 1996). Figure 7 shows the adsorption of vanadate onto treated Fe(III)/Cr(III) hydroxide in the pH range 4.0–10.0. It was found that with increase in pH the removal of vanadate(V) decreases. Similar behavior has been reported for the adsorption of vanadate by iron hydroxide (Blackmore et al. 1996) and chitosan (Guzman et al. 2002). pHzpc plays an important role in adsorption process. The pHzpc of the Fe(III)/Cr(III) hydroxide used in the study is 8.1, below which the surface is positively charged. The removal efficiency is higher in acidic pH than that in basic pH conditions (Fig. 7). In the lower pH the adsorbent surface is modified by H+ ions. Due to the electrostatic attraction between the anionic vanadate species and the adsorbent surface, removal is maximum at pH 4.0. On increasing the pH, the electrostatic repulsion occurs between adsorbed OH− ions and anionic vanadate species, which hinder the adsorption of vanadate.
Effect of pH on removal of vanadate(V) by treated Fe(III)/Cr(III) hydroxide. Adsorbent dose, 200 mg, 50 ml−1; vanadate(V) concentration, 10 mg l−1; agitation time, 20 min; vanadate(V) concentration, 20 mg l−1; agitation time, 40 min; vanadate(V) concentration, 30 mg l−1; agitation time, 60 min; vanadate(V) concentration, 40 mg l−1; agitation time, 80 min; temperature 32°C
Desorption studies
Desorption studies were carried out with two different initial concentrations of vanadate(V) (10 and 20 mg l−1) in the initial pH range 4.0–12.0 for both untreated and treated adsorbents. It was found that desorption of vanadate increased with increase in pH for both untreated and treated adsorbents (Table 6). Very high desorption shows that the adsorption of vanadate on Fe(III)/Cr(III) hydroxide is reversible and ion exchange mechanism seems to predominate (Eqs. 11–13). Similar observation was reported for the adsorption of vanadate(V) onto chitosan (Guibal et al. 1998). Inner sphere complex mechanism was reported for the adsorption of vanadate(V) onto iron oxyhydroxide (Blackmore et al. 1996).
Vanadate anion was adsorbed onto Fe(III)/Cr(III) hydroxide through exchange of OH− or SO4 2− ions of the adsorbent. Release of the sulfate ions was experimentally observed and it is represented by:
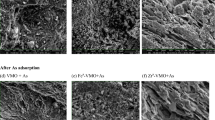
However, there was no detectable pH increase after adsorption. This may be attributed to a small increase in OH− concentration in the system, due to low concentration of vanadate(V) used (Jackson and Miller 2000) (Eq. 10) or due to the release of water molecules (Eqs. 11–13). The adsorbent Fe(III)/Cr(III) hydroxide is similar to iron oxyhydroxide (Kreller et al. 2002). Since the final pH for the untreated adsorbent ranged from 7.99 to 8.8 for the initial pH range 4.0–10.0, the adsorbent surface is dominated with –MOH species. Hence adsorption of vanadate follows Eqs. 10–12. Since the final pH for the treated adsorbent ranged from 6.2 to 7.8 for the initial pH range 4.0–10.0, the adsorbent surface is dominated with \( {{-}\text{MOH}}_{2}^{+} \) species. Hence, adsorption of vanadate follows Eq. 13. Chemisorption plays a minor role in the adsorption of vanadate(V). This is substantiated by the fact that ΔH for adsorption was found to be less than 40 kJ mol−1 (Alkan et al. 2004) and desorption was high at pH 12.0. Also FTIR spectrum of vanadate(V)-loaded adsorbent did not show any band specific for chemisorbed vanadate(V) species.
Effect of foreign anions
The extent of vanadate adsorption by untreated and treated Fe(III)/Cr(III) hydroxide in the presence of other ions is shown in Table 7. Anions, PO4 3−, MoO4 2−, and SeO3 2− interfered with the adsorption of vanadate for both the adsorbents. This agrees with the result proposed by Blackmore et al. (1996) in the adsorption of vanadate by iron oxyhydroxide. The order of adsorption among the oxyanions studied was V(V) > PO4 3− > SeO3 2− > MoO 24 based on the percent removal of anions at equimolar concentrations (0.78 mM). This may be due to the preference of the adsorbent sites for those anions in that order (Rayden et al. 1987). Competition between the anions occurs for the common sites and results will be published elsewhere. Anions—SO4 2−, Cl−, and NO3 −—showed no effect on the adsorption of vanadate, because these ions were not adsorbed onto both the untreated and treated adsorbents. Hingston et al. (1971) studied the adsorption of phosphate, selenite, and arsenate on goethite, and proposed that there are a number of sorption sites available to a particular anion on goethite. The adsorption of vanadate onto the adsorbent surface can be described by inner sphere surface coordination mechanism, since the adsorption was not affected by change in ionic strength using KNO3 in the concentration range 0.5–50 mM (Blackmore et al. 1996).
Conclusions
This study demonstrates that the solid waste Fe(III)/Cr(III) hydroxide is effective in removing vanadate(V) from aqueous solutions. The vanadate adsorption was found to be spontaneous and endothermic in nature. The adsorption mechanism was primarily associated with ion exchange, which was best predicted by second-order kinetic model. The experimental data appeared to fit better with Langmuir isotherm than Freundlich isotherm. High temperature favored adsorption and maximum adsorption occurred at pH 4.0. Although SO4 2−, Cl−, and NO3 − did not interfere with vanadate adsorption, other ions like selenite, molybdate, and phosphate showed negative effect on the adsorption for both treated and untreated Fe(III)/Cr(III) hydroxides.
References
Alkan M, Demirbas O, Celikcapa S, Dogan M (2004) Sorption of acid red 57 from aqeuous solution onto sepiolite. J Hazard Mater B 116:135–145
Baes CF Jr, Mesmer RE (1976) Hydrolysis of cation. Wiley, New York
Blackmore DPT, Ellis J, Riley PJ (1996) Treatment of vanadium containing effluent by adsorption/coprecipitation with iron oxyhydroxide. Water Res 30:2512–2516
Dursun G, Cicek H, Dursun AY (2005) Adsorption of phenol from aqueous solution using carbonized beet pulp. J Hazard Mater B 125:175–182
Freundlich H (1906) Uber die adsorption in losungen. Z Phys Chem 57:387–470
Guibal E, Milot C, Tobin JM (1998) Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Ind Eng Chem Res 37:1454–1463
Guzman J, Saucedo J, Navarro R, Revilla J, Guibal E (2002) Vanadium interaction with chitosan: influence of polymer protection and metal speciation. Langmuir 18:1567–1573
Hayes KF, Papelis C, Leckie JO (1988) Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interface. J Colloid Interface Sci 125:717–726
Hingston FJ, Posner AM, Quirk JP (1971) Competitive adsorption of negatively charged ligands on oxide surfaces. Discuss Faraday Soc 53:334
Jackson BP, Miller WP (2000) Effectiveness of phosphate and hydroxide for desorption of arsenic and selenium species from iron oxides. Soil Sci Soc Am J 64:1616–1622
Jansson-Charrier M, Guibal E, Delaghe B, Le Clorec P (1996) Vanadium (IV) sorption by chitosan: kinetics and equilibrium. Water Res 30:465–475
Kreller DI, Gibson G, van Loon GW, Horton JH (2002) Chemical force microscopy investigation of phosphate adsorption on the surface of Fe(III) oxyhydroxide articles. J Colloid Interface Sci 254:205–213
Kunz RG, Giannelli JF, Stensel HD (1976) Vanadium removal from industrial wastewaters. J WPCF 48:762–770
Lagergren S (1898) Zur throrie der sogenannten adsorption geloester stoffe. K Sven Vetenskapsakad Hadl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 40:1361
Mckay G, Ho YS (1998) The adsorption of dyes from aqueous solution by peat. Chem Eng J 70:115–124
Naeem A, Westerhoff P, Mustafa S (2007) Vanadium removal by metal (hydr)oxide adsorbents. Water Res 41:1596–1602
Namasivayam C, Prathap K (2005) Recycling Fe(III)/Cr(III) hydroxide- and industrial solid waste for the removal of phophate from water. J Hazard Mater 123:127–134
Namasivayam C, Prathap K (2006) Removal of selenite using waste Fe(III)/Cr(III) hydroxide: Adsorption kinetics and isotherms. Toxicol Environ Chem 88:85–98
Namasivayam C, Prathap K (2007) Adsorptive removal of silica onto waste Fe(III)/Cr(III) hydroxide: kinetics and isotherms. Colloids Surf A Physicochem Eng Asp 295:55–60
Namasivayam C, Ranganathan K (1994) Recycling of waste Fe(III)/Cr(III) hydroxide for the removal of nickel from wastewater: adsorption and equilibrium studies. Waste Manage 14:709–716
Namasivayam C, Senthilkumar S (1998) Removal of arsenic(V) from aqueous solution using industrial solid waste: adsorption rates and equilibrium studies. Ind Eng Chem Res 37:4816–4822
Namasivayam C, Sumithra S (2004) Adsorptive removal of catechol on waste Fe(III)/Cr(III) hydroxide: equilibrium and kinetic study. Ind Eng Chem Res 43:7581–7587
Namasivayam C, Jayakumar R, Yamuna RT (1994) Dye removal from wastewater by adsorption on Fe(III)/Cr(III) hydroxide. Waste Manage 14:643–648
Nukatsuka I, Shimiza Y, Ohzeki K (2002) Determination of V(IV) and V(V) by electrothermal atomic absorption spectrometry following selective solid-phase extraction and the study on the change in the oxidation state of vanadium species in seawater during the sample storage. Anal Sci 18:1009–1014
Ortiz-Bernard I et al (2004) Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl Environ Microbiol 70:3091–3095
Ozacar M (2003) Equilibrium and kinetic modeling of adsorption of phosphorus on calcined alunite. Adsorption 9:125–132
Ozcan AS, Ozcan A (2004) Adsorption of acid dyes from aqueous solution onto acid activated-bentonite. J Colloid Interface Sci 276:39–46
Rayden JC, Syres KJ, Tilman RW (1987) Inorganic anion sorption and interaction with phosphate sorption by hydrous ferric oxide gel. J Soil Sci 38:211–217
Sontheimer H, Crittendren JC, Summers S (1988) Activated carbon for water treatment, chap. 3. Description of adsorption equilibria. DVGW Forschungstelle, Karlsruhe, Germany, p 112
Su C, Suarez DL (2000) Selenate and selenite sorption on iron oxides: an infrared and electrophoretic study. Soil Sci Soc Am J 64:101–111
Weng CH, Huang CP (2004) Adsorption characteristics of Zn(II) from dilute solution by fly ash. Colloids Surf A Physicochem Eng Asp 247:137–143
Zagulski I, Pawlowski L, Cichocki A (1980) Physicochemical methods for water and wastewater treatment. In: Pawlowski L (ed) Proceeding of the second international conference, Lublin 1979. Pergamon Press, Oxford, UK
Acknowledgments
The authors are grateful to Dr. P. Weidler, Institute for Technical Chemistry, Karlsruhe Research Center (Germany), for the analysis of surface area and pore size distribution of the adsorbent. Grateful acknowledgement is due to DAAD, Germany, for the Equipment Grant, which facilitated the experimental work. The authors are also grateful to the anonymous reviewers for the useful comments/suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prathap, K., Namasivayam, C. Adsorption of vanadate(V) on Fe(III)/Cr(III) hydroxide waste. Environ Chem Lett 8, 363–371 (2010). https://doi.org/10.1007/s10311-009-0234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-009-0234-x