Abstract
Two inhibitors, triethanolamine (TEA) and monoethanolamine (MEA), were tested for their ability to prevent the de novo formation of polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) on sinter plant fly ash. The amounts of both PCDDs and PCDFs, formed by thermal treatment of the fly ash, decreased when inhibitors were added. Up to 90% reduction of the PCDD/Fs formation was reached when 2 wt % monoethanolamine was mixed with fly ash. The temperatures tested, 325 and 400 °C, did not affect the inhibition activity. However, a longer reaction time, 4 h instead of 2 h, gave higher percentages of PCDD/Fs reduction. The laboratory results show that ethanolamines reduce the dioxins formation on sinter plant fly ash under various conditions of temperature and reaction time. Moreover, factory tests performed in parallel at a sinter plant are in good agreement with the laboratory experiments, thus confirming that the use of ethanolamine inhibitors is an appropriate technique for the prevention of dioxins emissions from sintering processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) are highly toxic compounds produced by some natural processes, such as forest fires and volcanic eruptions, and different human activities. Dioxins structure is depicted in Fig. 1. These pollutants consist of a group of chlorinated tricyclic aromatic compounds with different degrees and positions of chlorination on the molecule skeleton, thus leading to the occurrence of 75 congeners of PCDDs and 135 congeners of PCDFs, usually termed 'dioxins'. Some congeners such as 2,3,7,8-tetrachlorodibenzodioxin (2,3,7,8-TCDD) are known to be among the most toxic compounds with potential carcinogenic and mutagenic effects.
Anthropogenic sources of dioxins include the incineration of waste and most combustion processes. Since the discovery of PCDD/Fs in the flue gas and fly ash of municipal waste incinerators by Kees Olie (1977), the formation of these toxic compounds has been studied intensively. Some reviews summarized the most important trends and results (Addink and Olie 1995; Tuppurainen et al. 1998). Two different pathways have been proposed to explain the occurrence of dioxins (PCDD/Fs) in the emissions of incinerators and other combustion processes: the synthesis from precursors and the de novo synthesis. The first pathway involves the formation of PCDD/Fs by reactions of chemical precursors such as chlorophenols, which are formed initially by incomplete combustion. The second de novo synthesis pathway involves PCDD/Fs formation from macromolecular carbon present in the fly ash. Indeed heterogeneous reactions between the gas phase and the fly ash are catalyzed by some constituents of the fly ash such as copper and iron chlorides. Many authors postulate that this synthesis could take place essentially in the post combustion zone of the incinerators at a temperature around 300 °C (Vogg and Stieglitz 1986). The de novo formation of PCDD/Fs is supposed to be strongly correlated with the metal-catalyzed oxidation of carbon in the fly ash (Huang and Buekens 1996). The oxidative degradation of the carbon structure gives mainly gaseous products such as CO2 and CO as well as, in a minor pathway, some small aromatic compounds including PCDD/Fs.
Although iron and steel industries are known to be important sources of PCDD/Fs in different countries (Lahl 1993; Bröker et al. 1993), most studies concerning the formation of these highly toxic compounds deal only with municipal waste incineration. Relatively few data are available for industrial and metallurgical processes, in particular for sintering plants (Buekens et al. 1998, 1999a, 1999b, 1999c, 2001; Stieglitz and Buekens 1999; Stieglitz et al. 1999; Weber et al. 1999).
The sintering process is an essential step in an integrated iron metallurgical plant. In this process, the iron ore is converted to larger fragments acceptable in the blast furnace. The sinter plant consists of a 50–100 m long, 3–5 m wide, horizontal strand, which supports the feed of hematite ores, cokes and lime, and is slowly moving (see Fig. 2). Burners initiate the process by igniting the feed layer on top and ambient air is sucked through the layer that moves the burning front downwards. The sinter is then cooled and broken before its use in the blast furnace. Separate chambers, called wind boxes and located below the strand, collect the off-gas prior to filtering in appropriate dust collectors. The detailed mechanism as well as the place of PCDD/Fs formation in the sintering process remain unknown although all the necessary ingredients are present: carbon from the coke, oxygen in the air sucked through the cake, chlorine and catalytic metals available in the ores. Considering the very large gas flow volumes (up to 106 m3/h) discharged from these industrial processes, the contribution of the dioxin pollution by these sources is important. In Belgium, it can be estimated from the data by Wevers and De Fre (1995) that, in 1995, the industry sector was responsible for 34% of the total dioxin emissions; among the different industries, 24% of the dioxin emissions estimated for the industrial sector came from sintering.
Schematic representation of a typical sinter plant (Anderson et al. 2001). This process is the core of an integrated steel plant where ore fines and ferrous reverts are converted into a feedstock acceptable in the blast furnace. High dioxins concentrations have been measured in the stack of different plants (Bröker et al. 1993) and, in most of the European countries, sintering process is now recognized as the major source of dioxins (European Dioxin Inventory-Stage I, 1997). Most dioxins emitted by this industrial process are supposed to be formed by de novo synthesis on fly ash in the wind boxes and the pipes, in particular on fly ash immobilized on walls (Xhrouet et al. 2001a, 2002b)
PCDD/Fs emissions can be controlled by means of flue gas cleaning systems, which remove the PCDD/Fs present in the gas, or by the way of the inhibition technique, which tries to avoid or reduce the formation of PCDD/Fs in the process. A recent review presents a comparative evaluation of techniques for controlling the formation and emission of PCDD/Fs in municipal waste incinerators (Buekens and Huang 1998).
Although PCDD/Fs formation in combustion processes is being studied widely, studies on inhibition are quite sparse, especially on sinter plants. This prevention technique is, however, adequate for this particular industrial process: the inhibitors can simply be mixed with the raw material and the additional cost will be insignificant. Various inhibitors have been tested both in laboratory and in pilot plants to reduce the PCDD/Fs formation relating to incineration processes. Different studies reveal the inhibition ability of some basic compounds such as NH3 (Ruokojärvi et al. 1998; Vogg et al.1987), CaO (Naikwadi and Karasek 1989), NaOH and KOH (Naikwadi et al. 1993). Based on the concept of "poisoning" the catalyst, another group of compounds is used as inhibitors for the PCDD/Fs formation: compounds able to form a complex with a catalyst like the functionalized amines. Ethanolamine and triethanolamine are very effective (Dickson et al. 1989; Naikwadi et al. 1993). Urea has also been examined in this way by several research teams as a potential PCDD/Fs inhibitor (Dickson et al. 1989; Ruokojärvi et al. 1999, 2001; Samaras et al. 2001; Tuppurainen et al. 1999; Xhrouet et al. 1999; Yli-Keturi et al. 1999).
In the present paper, we report on a series of inhibition experiments carried out with sinter plant fly ash. We have showed in a previous study that this fly ash is very active in de novo formation of PCDD/Fs and we investigate here the possibility to prevent this formation by using inhibitors (Xhrouet 2002; Xhrouet et al. 1999, 2001a). Two different inhibitors are examined: triethanolamine (TEA) and monoethanolamine (MEA). Different parameters such as the amount of inhibitors, the temperature and the reaction time are investigated. Preliminary results of our investigation have been published before (Xhrouet et al. 2001b). To get a better understanding of the inhibition mechanism, the homologue and full isomer distributions were also examined and are detailed elsewhere (Xhrouet 2002; Xhrouet et al. 2002).
Experimental
Materials
The following materials were used: solution of 2,3,7,8-Cl-substituted 13C12-labeled PCDD/Fs (EPA 1613 LCS, Campro Scientific, Veenendaal, The Netherlands); toluene (p.a., Baker); hexane (p.a., Baker); dichloromethane (p.a., Vel); dodecane (Merck); sulfuric acid (95–97%, Baker); sodium chloride (p.a., Merck); potassium hydroxide (p.a., Merck); sodium sulfate anhydrous (Baker); aluminum oxide (activated, neutral, type 507c, Aldrich); glass wool (silane treated, Alltech Europe); technical dry air (Air Liquide, Belgium), triethanolamine (97%, Acros), and monoethanolamine (99%, Acros).
Fly ash
Fly ash was collected in the electrostatic precipitator of a Belgian sintering plant. This electrofilter consists of three fields and operates at 120–130 °C. The fly ash used in this study comes from field 3 and was stored at ambient temperature prior to lab experiments. Around 72.5 wt % of the fly ash has a size under 40 μm. The fly ash composition was (wt %): Mg (1.04), Al (2.17), Si (3.62), P (0.24), S (4.07), Cl (9.55), K (9.07), Ca (7.83), Cr (0.04), Mn (0.31), Fe (49.90), Cu (0.17), Zn (0.34), Pb (5.98), C (3.34). All experiments were conducted with extracted fly ash in order to minimize potential interferences from adsorbed organic precursors and native PCDD/Fs. Prior to experiments, all fly ashes were Soxhlet extracted with toluene (2×24 h), rinsed with hexane, and air-dried at room temperature. This fly ash, cleared of native PCDD/Fs, is called "extracted fly ash" or "fly ash" in the rest of the paper as opposed to the "original fly ash", which refers to the fly ash that comes directly from the sintering plant without any pretreatment and contains the native PCDD/Fs. Only trace amounts of PCDD/Fs were found in the extracted fly ash.
Inhibitors
Two different inhibitors have been tested in this study: triethanolamine (TEA) and monoethanolamine (MEA). Extracted fly ash was mixed with 0.5, 1 or 2 wt % of inhibitors. The inhibitor was preliminarily dissolved in methanol, then mixed with the fly ash and the methanol evaporated.
Experimental apparatus
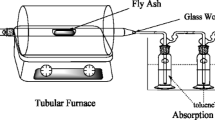
5 g of sample (fly ash or fly ash+inhibitor) was packed into a horizontal glass tube reactor (16 cm long, 3 cm diameter) with glass wool as plugs. The tube was placed in a chromatographic furnace, and the samples were heated under a flow of technical air (100 ml/min). Three kinds of experiments were performed: 325 °C during 2 and 4 h, 400 °C during 2 h. Products evaporated from the fly ash were collected using two cold-traps in series (100 ml of toluene cooled with ice). Each experiment was performed in duplicate or triplicate; mean values±range are reported.
Cleanup
The slightly modified EPA-8280 was followed for classical PCDD/Fs analysis. Detailed procedures are described elsewhere (Xhrouet et al. 2001a).
Analysis
All analyses were performed by gas chromatography-mass spectrometry (GC-MS) using Mat95-XL high-resolution mass spectrometer and Hewlett-Packard 6890 Series gas chromatograph. GC conditions were optimized to separate most PCDD/Fs as followed: SP2331 capillary column [Supelco, 60 m×0.25 mm i.d., 0.2 µm film thickness, poly (80% biscyanopropyl/20% cyanopropylphenyl)siloxane]; splitless injection of 2 µL extract at 275 °C; temperature: 150 °C (1 min), 150–200 °C (15°/min), 200–273 °C (1.2°/min), then 273 °C (18 min). Helium carrier gas. The mass spectrometer was operated in the electron impact ionization mode using selected ion monitoring. The mass spectrometer was tuned to a minimum resolution of 10000 (10% valley), and was operated in a mass drift correction mode using FC5311 to provide lock masses. The two most abundant ions in the chlorine clusters of the molecular ion were recorded for each congener of native and labeled PCDD/Fs. The source temperature was 270 °C.
Identification and quantification
Most dibenzodioxins substituted with 4 to 8 Cl and dibenzofurans substituted with 4 to 7 Cl were analyzed. No analyses of the species bearing less than 4 Cl were performed. Native concentration was determined by isotopic dilution using the 2,3,7,8-Cl-substituted 13C-labeled PCDD/Fs to quantify all the native isomers within homologues assuming equal response for all isomers within an isomer group and no isomer-selective losses during the cleanup. The isomers were identified according to Ryan et al. (1991).
Results and discussion
Two ethanolamine inhibitors, monoethanolamine (TEA) and triethanolamine (MEA), were tested to study their effect on the catalytic activity of the sinter plant fly ash towards the prevention of dioxins (PCDD/Fs) formation by de novo synthesis. The inhibition tests were compared to control experiments, which consisted of thermal experiments performed without inhibitors mixed with the fly ash. Percentages of inhibition were calculated from the difference with control tests. Results obtained are summarized in Table 1. Figure 3 shows a part of the results (325 °C and 2 h). The authors have paid particular attention to presenting their results with margins of error (tables and figures). These are too often absent from publications in the field, although it is known that such experiments are not easy to reproduce. In our case, the margins of errors are generally acceptable except for one test run (325 °C, 4 h, 0.5%TEA). This result has, in spite of that, been included in the table. However, the reader should be aware of the margin of error reported.
The results indicate that a clear reduction in both PCDDs and PCDFs concentrations occurred when inhibitors are used (Table 1, Fig. 3). Depending on the temperature and the reaction time investigated, the global inhibition yields are up to 90%. The results obtained are generally better with monoethanolamine (MEA) than with triethanolamine (TEA), and with the highest amount of inhibitors studied (2 wt %). Surprisingly, the inhibition test run at 325 °C, 2 h, with 0.5% MEA, shows an increase in PCDFs concentrations (inhibition yield: −56±31%). The margin of error relating to the PCDFs amount is however very small, only ±2 ng/g, corresponding to a relative error of 0.06%. This error is not realistic since errors of 10–20% are generally accepted for the only measure of PCDD/Fs in samples. It is indeed odd that MEA at low concentrations increases PCDD/Fs formation since it is supposed to block the de novo catalyst and can not easily be considered as a precursor to PCDD/Fs. Problems with the experimental conditions in this test run are thus strongly suggested.
Inhibition as a function of the temperature
Effect of the temperature on the inhibition of dioxins formation was also investigated. It can be observed from Table 1 that, for TEA, the temperature of reaction has no effect on the percentage of inhibition obtained. For example, the inhibition yields observed for the PCDDs with 1 wt % of inhibitor are 38 and 39% for experiments performed at 325 and 400 °C, respectively. For MEA, it seems that the inhibition is slightly better at 400 °C than at 325 °C (inhibition yield of 83% versus 63% for the PCDDs with 1 wt % of MEA). The same trend was observed for the PCDFs. These results are quite surprising since we thought that a higher temperature of reaction would induce a partial vaporization or degradation of the inhibitors and thus, a decrease in the inhibition. Nevertheless, these results are very encouraging relating to the sinter plant itself. Indeed, the sintering process presents a large range of temperature along the strand and in the different wind boxes located below the strand that collect the off-gas. The results suggest that the great variation of temperature along the process will not affect the performance of the inhibitors and that the inhibition remains effective at different temperatures.
Inhibition as a function of the reaction time
Table 2 summarizes the inhibition yields obtained for experiments with MEA as a function of the reaction time. Results show that longer reaction time (4 h instead of 2 h) gives better inhibition yields. The difference between the percentages of inhibition obtained at 2 and 4 h is particularly significant when the inhibition at 2 h is low (with a small amount of inhibitors). The percentage of PCDDs inhibition with MEA goes up from 12% at 2 h to 76% at 4 h for 0.5 wt % of inhibitor, although it goes only from 75% at 2 h to 85% at 4 h for 2 wt % of inhibitor. The same trend was observed with TEA for the PCDFs: inhibition yields go up from 12% at 2 h to 41% at 4 h for 0.5 wt % of TEA. For the PCDDs, there is no clear influence of the reaction time: with 1 wt % TEA, the inhibition yields obtained for the PCDDs are 38% at 2 h and 40% at 4 h. It can be concluded that longer reaction times do not involve loss of inhibitory activity through evaporation or destruction of the inhibitor, which is an advantage for the use of this technique in the industrial process, where various reaction conditions may exist, notably for the residence time.
Inhibition tests performed at a real sintering process
Parallel to these laboratory experiments, inhibition tests were performed in an industrial sinter plant. The same two inhibitors were tested: TEA and MEA. The way the inhibitors were introduced, however, was totally different: the inhibitors were dissolved in water and introduced into the operating process by way of spraying nozzles placed in wind boxes located below the strand. Reference tests were performed without inhibitor injection and compared to inhibition experiments in which the inhibitor was injected continuously and measurements carried out after different times.
The results obtained at the sinter plant are in good agreement with the laboratory experiments. Mono- and triethanolamines were both effective in preventing the dioxins (PCDD/Fs) formation in the industrial process. Monoethanolamine gives better results with percentages of inhibition up to 90% (the inhibition yields were calculated on the basis of dioxins concentrations taking only the 17 toxic congeners of dioxins affected by their toxic equivalent factor into account, Van den Berg et al. 1998). Better results were obtained for longer times between the beginning of the inhibitor injection and the sampling. This latency is not surprising since time is necessary to obtain a good spreading of the inhibitor in the process and especially on the walls where adsorbed fly ash can produce great amounts of PCDD/Fs by de novo synthesis.
Conclusions
Mono- and triethanolamines are both effective inhibitors to prevent the de novo formation of dioxins (PCDD/Fs) on sintering fly ash. Longer reaction times or higher temperatures do not involve loss of inhibitory activity through evaporation or destruction of the inhibitor, which is an advantage for the use of this technique in industrial processes, where various reaction conditions may exist. This study confirms that inhibition is a suitable and very cheap technique, necessitating only a few technical devices, for the reduction of PCDD/Fs emissions from sintering process, the major source of these pollutants in the environment.
References
Addink R, Olie K (1995) Mechanisms of formation and destruction of polychlorinated dibenzo-p-dioxins and dibenzofurans in heterogeneous systems. Environ Sci Technol 29:1425–1435
Anderson DR, Fisher R, Roworth MC, Wilson DT, Southern SM, Fray TAT (2001) Formation and suppression of PCDD/Fs in iron ore sintering. Organohalogen Compd 54:110–114
Bröker G, Bruckmann P, Gliwa H (1993) Systematic monitoring of PCDD and PCDF emissions of industrial installations. Organohalogen Compd :303–306
Buekens A, Huang H (1998) Comparative evaluation of techniques for controlling the formation and emission of chlorinated dioxins/furans in municipal waste incineration. J Hazard Mater 62:1–33
Buekens A, Stieglitz L, Huang H, Cornelis E (1998) Formation of dioxin in industrial combustors and pyrometallurgical plants. Environ Eng Sci 15:29–36
Buekens A, Prakhar P, Rivet F, Stieglitz L (1999a) Dioxins from the sintering process. (V) Characterisation, analysis and 'de novo' testing of samples. Statistical correlations. Organohalogen Compd 41:121–123
Buekens A, Prakhar P, Stieglitz L, Jacobs P (1999b) Dioxins from the sintering process. (VII) Adsorption/desorption data from 'de novo' tests. Activation energy of (presumed) equilibrium values. Organohalogen Compd 41:97–99
Buekens A, Huang H, Stieglitz L (1999c) Dioxins from the sintering process. (I) Particle characterisation and SEM/wet analysis of samples. Organohalogen Compd 41:109–112
Buekens A, Stieglitz L, Hell K, Huang H, Segers P (2001) Dioxins from thermal and metallurgical processes: recent studies for the iron and steel industry. Chemosphere 42:729–735
Dickson L C, Lenoir D, Hutzinger O, Naikwadi K P, Karasek F W (1989) Inhibition of chlorinated dibenzo-p-dioxin formation on municipal incinerator fly ash by using catalyst inhibitors. Chemosphere 19:1435–1445
European Dioxin Inventory-Stage I (1997) Identification of relevant industrial sources of dioxins and furans in Europe. Final report, n°43, 936 pp. Landesumweltamt Nordrhein-Wesphalen, Essen, Germany
Huang H, Buekens A (1996) De novo synthesis of polychlorinated dibenzo-p-dioxins and dibenzofurans. Proposal of a mechanistic scheme. Sci Total Environ 193:121–141
Lahl U (1993) Sintering plants of steel industry—the most important thermical PCDD/F source in industrialized regions? Organohalogen Compd :311–314
Naikwadi KP, Karasek FW (1989) Prevention of PCDD formation in MSW incinerators by inhibition of catalytic activity of fly ash produced. Chemosphere 19:299–304
Naikwadi KP, Albrecht ID, Karasek FW (1993) Mechanism of formation of PCDD/PCDF in industrial waste incinerator and a method of prevention of their formation. Chemosphere 27:335–342
Olie K, Vermeulen PL, Hutzinger O (1977) Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some municipal incinerators in the Netherlands. Chemosphere 8:455–459
Ruokojärvi P H, Halonen I A, Tuppurainen K A, Tarhanen J, Ruuskanen J (1998) Effect of gaseous inhibitors on PCDD/F formation. Environ Sci Technol 32:3099–3103
Ruokojärvi PH, Aatamila M, Tuppurainen K, Halonen IA, Ruuskanen J (1999) The effect of chemical inhibitor on PCDD/F concentrations in different particle size fractions. Organohalogen Compd 40:555–558
Ruokojärvi PH, Aatamila M, Tuppurainen KA, Ruuskanen J (2001) Effect of urea on fly ash PCDD/F concentrations in different particle sizes. Chemosphere 43:757–762
Ryan JJ, Conacher HBS, Panopio LG, Lau BPY, Hardy JA, Masuda Y (1991) Gas chromatographic separations of all 136 tetra- to octa-polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans on nine different stationary phases. J Chromatogr 541:131–183
Samaras P, Blumenstock M, Lenoir D, Schramm K-W, Kettrup A (2001) PCDD/F inhibition by prior addition of urea to the solid fuel in laboratory experiments and results statistical evaluation. Chemosphere 42:737–743
Stieglitz L, Buekens A (1999) Dioxins from the sintering process. (III) Operating factors influencing upon 'de novo' formation of field 2 dust. Organohalogen Compd 41:129–132
Stieglitz L, Polzer J, Hell K, Weber R, Buekens A, Prakhar P, Rivet F (1999) Dioxins from sintering process. (II) Samples and their propensity to form dioxins, as derived by a 'de novo' laboratory test. Organohalogen Compd 41:113–115
Tuppurainen K, Halonen I, Ruokojarvi P, Tarhanen J, Ruuskanen J (1998) Formation of PCDDs and PCDFs in municipal waste incineration and its inhibition mechanisms: a review. Chemosphere 36:1493–1511
Tuppurainen K, Aatamila M, Ruokojarvi P, Halonen I, Ruuskanen J (1999) Effect of liquid inhibitors on PCDD/F formation. Prediction of particle-phase PCDD/F concentrations using PLS modeling with gas-phase chlorophenol concentrations as independent variables. Chemosphere 38:2205–2217
Van den Berg M, Birnbaum L, Bosveld A, Brunström B, Cook P, Feeley M, Giesy J, Hanberg A, Hasegawa R, Kennedy S, Kubiak T, Larsen J, Van Leeuwen R, Liem D, Nolt C, Peterson R, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T (1998) Toxic Equivalency Factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106:775–792
Vogg H, Stieglitz L (1986) Thermal behavior of PCDD/PCDF in fly ash from municipal incinerators. Chemosphere 15:1373–1378
Vogg H, Metzger M, Stieglitz L (1987) Recent findings on the formation and decomposition of PCDD/PCDF in municipal solid waste incineration. Waste Manag Res 5:285–294
Weber R, Buekens A, Segers F, Rivet F, Stieglitz L (1999) Dioxins from the sintering process. (IV) Characterisation, analysis and 'de novo' testing of sintering belt siftings. Influence of temperature, hydrogen chloride and activated carbon addition. Organohalogen Compd 41:101–104
Wevers M, De Fre R (1995) Estimated evolution of dioxin emissions in Belgium from 1985 to 1995. Organohalogen Compd 24:105–108
Xhrouet C (2002) Thesis, Contribution à l'étude d'une technique de réduction de la formation des dioxines dans le procédé d'agglomération des minerais de fer
Xhrouet C, Pirard C, De Pauw E (1999) De novo synthesis of polychlorinated dibenzofurans on fly ash from a Belgian sintering belt and its inhibition. Organohalogen Compd 41:307–310
Xhrouet C, Nadin C, De Pauw E (2001a) De novo synthesis of polychlorinated dibenzo-p-dioxins and dibenzofurans on fly ash from a sintering process. Environ Sci Technol 35:1616–1623
Xhrouet C, Nadin C, Pirard C, De Pauw E (2001b) Prevention of PCDD/Fs de novo formation on sintering process fly ash. Organohalogen Compd 54:123–127
Xhrouet C, Nadin C, De Pauw E (2002) Amines compounds as inhibitors of PCDD/Fs de novo formation on sintering process fly ash. Environ Sci Technol 36:2760–2765
Yli-Keturi, Ruokojärvi, Asikainen, Ruuskanen, Halonen, Hänninen (1999) Urea as PCDD/F inhibitor in incineration of RDF. Organohalogen Compd 41:311–314
Acknowlegments
The authors would like to thank Mr. J.-M. Brouhon from the C.R.M (Centre de Recherches Metallurgiques de Liege) for interesting discussions, as well as C. Nadin for performing some of the experiments. C. Xhrouet was funded as fellow by the F.N.R.S (Fonds National de la Recherche Scientifique Belge).
Author information
Authors and Affiliations
Corresponding author
Additional information
C Xhrouet: Recipient of the 2001 ACE Environmental Chemistry Award
Rights and permissions
About this article
Cite this article
Xhrouet, C., De Pauw, E. Prevention of dioxins de novo formation by ethanolamines. Environ Chem Lett 1, 51–56 (2003). https://doi.org/10.1007/s10311-002-0011-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-002-0011-6