Abstract
Mushrooms or fruiting bodies of many basidiomycetes are commonly produced in solid-state fermentation, generally after 20–60 days of growth. However, it is also possible to produce biomass from these fungi, in submerged fermentation in shorter time. This work was aimed at evaluating biomass production with the basidiomycete Pleurotus sajor-caju, in a submerged process and to determine the proportion of chemical components of this biomass. Initially, an optimization of the culture medium was done to produce a faster growth of microbial mass by changing the concentrations of ammonium sulfate, soy protein and yeast extract. Using the optimized culture medium, values of approximately 5.5 g L−1 of biomass in a medium with 10 g L−1 of glucose were attained. When the optimized culture medium was tested in a 5-L stirred tank bioreactor, using 10 g L−1 of glucose or sucrose as carbon source, values of 8.18 and 5.94 g L−1 of biomass concentration were obtained, respectively. In the medium with glucose, high yields (0.82 g g−1) and productivity of 0.085 g L−1 h−1 were obtained. The exopolysaccharide content (1.58 g dry matter L−1) in the culture was higher in the fermentation with sucrose. The nutritional composition of the biomass obtained in the submerged fermentation was similar to that of the fruiting body in terms of quantities of total carbohydrates, ash and calories, but total fat and protein were higher.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mushrooms have been appreciated and consumed for many years, by oriental cultures [9, 24]. They are considered delicate in terms of texture, with characteristic color and pleasant flavor [1–14]. The cultivation of mushrooms using biotechnological processes, employing agro-industrial substrates, is an important practice of modern societies, since the systematic and regular production of fruiting bodies with high organoleptic and nutritional quality is possible [23]. The Pleurotus genus is one of the most commercialized groups of mushrooms in the world and close, in commercial importance, to the Agaricus and Lentinula genera [20]. Pleurotus spp. are saprophytic fungi, commonly known as oyster mushrooms, which develop in nature on dead tree trunks, since they have the ability to degrade lignocellulosic residues [21]. Their fruiting body is mainly composed of carbohydrates and proteins, with low lipid contents, besides containing mineral salts [2].
The submerged culture of these mushrooms represents an alternative form of fast and efficient production of the biomass [25, 26], for food purposes [23] and also the production of exopolysaccharides (EPS) [8, 10]. The interest in the production of EPS by microorganisms, especially mushrooms, is due to their biological and pharmacological activities, such as immunostimulation, antitumoral and hypoglycemic activities [15]. In this study, the results for optimization of growth medium for submerged culture in relation to the concentrations of ammonium sulfate, soy protein and yeast extract are reported for the production of the Pleurotus sajor-caju biomass. The study also presents the data on biomass composition and quantification of soluble polysaccharides in the culture medium.
Materials and methods
Fungal strain and inoculum
P. sajor-caju strain PS-2001, used in the commercial production of edible mushrooms, was obtained from the fungus collection of the Institute of Biotechnology, University of Caxias do Sul. The culture was maintained on Petri dishes at 4 °C in a medium (per liter) of Pinus spp. sawdust (20 g), ground wheat bran (20 g), calcium carbonate (2 g), and agar-agar (20 g) per liter. The medium used for the inoculum contained (per liter) glucose (5 g), soy protein (1 g), and mineral solution (100 mL). The inoculum was developed in 500-mL Erlenmeyer flasks containing 100 mL of medium for 72 h under shaking at 180 rpm at 28 ± 2 °C. Plastic spheres (15 units) of 8 mm diameter were placed in the flasks in order to avoid the formation of mycelial pellets. The inoculum consisted of 5% (v/v) of working volume of the bioreactor. The mineral solution of nutrients and micronutrients used in the culture media was based on the formulation of Mandels and Reese [17], containing (per liter) KH2PO4 (20 g), (NH4)2SO4 (14 g), CO(NH2)2 (3 g), MgSO4·7H2O (3 g), CaCl2 (3 g), FeSO4·7H2O (50 mg), MnSO4·H2O (15.6 mg), ZnSO4·7H2O (14 mg), and CoCl2 (20 mg).
Culture medium and conditions
The culture medium was formulated with glucose (10 g L−1), soy oil (1 mL L−1), and mineral solution (100 mL L−1). For optimizing the concentrations of soy protein, yeast extract and ammonium sulfate, nutrients considered important in the culture medium, the experimental standard procedure was used based on the response surface method of Box and Wilson [3]. The three parameters, with a number of combinations of 23 = 8 treatments (each treatment being carried out in triplicate), were used in the experimental factorial design (Table 1).
This stage of the experiment was carried out in 500-mL Erlenmeyer flasks containing 100 mL of medium, for 96 h, under shaking at 180 rpm, at 28 ± 2 °C. Plastic spheres of 8 mm diameter were placed in the flasks to avoid the formation of mycelial pellets. In addition, cultures were also carried out in a 5-L stirred tank bioreactor (STR) with an operational volume of 4 L. The initial frequency of agitation was 100 rpm with an aeration rate of 0.5 vvm; however, after the first 4 h of growth, when in the mid-exponential, the frequency of agitation and aeration rate was increased to 250 rpm and to 0.75 vvm, respectively, in order to maintain dissolved oxygen levels higher than 30% saturation. The temperature was maintained at 28 ± 2 °C and without pH control. Glucose or sucrose (10 g L−1) was used as the main carbon source, samples (50 mL) of the culture broth being collected every 24 h for analyses in duplicate.
Determination of total reducing sugars and sucrose
The concentration of soluble reducing sugars was estimated with the use of the DNS (3,5-dinitrosalicylic acid) reagent, according to the method of Miller [19]. For sucrose dosing, its hydrolysis was carried out using a concentrated solution of Saccharomyces cerevisiae invertase.
Mycelium quantification
The biomass was estimated after filtration of 50 mL of the broth through microfibrous material with variable pores (≥50 μM). The solids were washed with 500 mL of distilled water. The filters with mycelium were dried at 80 °C for 24 h and weighed.
Analysis of centesimal composition of mycelium
The content of carbohydrates, fiber, total fats, saturated fats, trans fats, protein, moisture, ash, sodium, pH, acidity, and calorific value were evaluated according to official methodologies of the National Health Surveillance Agency (ANVISA - Brazil), resolution RDC 360 of December 23, 2003 [4].
Exopolysaccharide quantification
The dosing of exopolysaccharides (EPS) was carried out according to Kim et al. [12]. The samples collected were centrifuged at 10,000g for 20 min and a fourfold volume of absolute ethanol was added to the supernatant. The solutions were then homogenized and kept for 18 h at 4 °C. The precipitate was centrifuged at 10,000g for 20 min and the supernatant was discarded. The precipitate was dried at room temperature (28 ± 2 °C) until constant weight and then weighed.
Preparation of the crude ethanolic extract of mycelium
The preparation of the crude extract of mycelium was carried out as follows: for every 3 g of dry mycelium, ground in a knife mill, 30 mL of absolute ethanol were added, giving a 10% (m/v) suspension. The mixture was kept in a 50-mL beaker, which was later sealed and wrapped completely in aluminum foil, in order to shelter it from light. The mixture was maintained under slow shaking for 24 h at room temperature. After 24 h, the broth was filtered using Whatman no. 1 filter paper and the volume was made up to 30 mL with ethanol. For the DPPH• (1,1-diphenyl-2-picrylhydrazyl) tests, the original 10, 1 and 0.01% (v/v) dilutions were used.
Preparation of the crude extract of exopolysaccharides
Five milliliter of DMSO (dimethyl sulfoxide) were added to each 250 mg of EPS and this mixture was kept at 60 °C for 2 h. It was then filtered and the volume made up to 5 mL with DMSO. The dilutions of 0.5 and 0.05% (v/v) were prepared from this solution (5%). The crude extract preparation of exopolysaccharide was later analyzed for the antioxidant properties.
Evaluation of the antioxidant capacity of the mycelium
The evaluation of the in vitro antioxidant activity was carried out by measuring the reduction capacity of the free radical DPPH• (1,1-diphenyl-2-picrylhydrazyl). For this, 200 μL of samples of dry mycelium extract using ethanol were mixed with 800 μL of a 100 mM Tris-HCl (Merck®) buffer solution, pH 7. To this mixture, 1,000 μL of an ethanolic solution of 250 μM DPPH• (Sigma®) were added, and the tubes were kept sheltered from light for 20 min. The absorbance measurements were carried out using UV–vis spectrophotometry at 517 nm. For the blank, the samples were replaced with distilled water. At least three repetitions were carried out and the results were expressed as concentration of reduced DPPH• [5].
Conversion factors of substrate to biomass, yield, productivity, and statistical analysis
The substrate to biomass conversion factor was calculated and the results expressed in grams of biomass formed per gram of substrate consumed (g g−1). The substrate to biomass yields were expressed in grams of biomass formed per gram of substrate initially present in the culture medium (g g−1). The productivity in biomass was expressed in grams of mycelial mass formed per liter of culture medium per hour (g L−1 h−1).
The statistical tests were carried out through one-way analysis of variance (one-way ANOVA) and Tukey’s post hoc test, using a probability level of P < 0.05. The Student’s t test was also used for the analysis of two variables, using probability level of P < 0.05.
Results and discussion
Optimization of biomass production of P. sajor-caju PS-2001 in submerged fermentation, combining soy protein, yeast extract, and ammonium sulfate
The results for biomass production are shown in Table 2, whose different treatments (Table 1) consisted of basic medium containing the variables soy protein, yeast extract, and ammonium sulfate in two concentration extremes. Table 2 presents the values of biomass concentration in columns, according to concentrations +1 or −1, the values of the sum of the columns, the difference between the sum of the columns (P*), and the values of the coefficients (CV*) of the variables (P*/8). The positive CV* values for soy protein (+0.43) and yeast extract (+0.19) indicate that the concentrations of these components can be increased; however, for ammonium sulfate the negative value (−0.1) indicates the possibility of reducing this component.
The orders of magnitude by which these components should be increased or decreased in the culture medium were calculated. The product between the coefficients of the variables (CV*) (Table 2) and the respective units of variation (UV) (Table 1) was obtained, corresponding to 0.21 g [0.43 g(CV*sp) × 0.5 g(UVsp)] for soy protein, 0.09 g [0.19 g(CV*ye) × 0.5 g(UVye)] for yeast extract, and 0.06 g [0.1 g(CV*as) × 0.6 g(UVas)] for ammonium sulfate. Since soy protein was the variable that gave the highest value for the coefficient of the variable (CV*), we chose to vary soy protein concentration in multiples of one amount, corresponding to the X1 index. Then, the arbitrary choice value for X1 was 0.1 g L−1.
Using 0.1 g L−1 as value of X1 (variable soy protein), 0.045 g was then determined as value of the X2 (variable yeast extract) utilizing a proportion of 0.21/0.09 = X1/X2, while −0.028 g was determined as value of X3 (variable ammonium sulfate) utilizing a proportion of 0.21/−0.06 = X1/X3.
Table 3 shows the concentration of soy protein, yeast extract, and ammonium sulfate in the eight new different treatments. The concentrations were based on the values of X1 and X2 [where additions (in g L−1) of soy protein and yeast extract, respectively, were made], and X3 [where decreases (in g L−1) of ammonium sulfate were made].
Figure 1 shows that the highest average of biomass concentration (5.49 g L−1) was obtained for treatment T8, which statistically differed from T0, T1, T2, and T3, which showed the lowest values. It was observed that when increasing soy protein and yeast extract in the culture medium there were small increases in the average biomass production values. Thus, if there were a further nutrient increase in the culture medium, it would be possible to obtain higher mycelial concentrations. However, this would compromise the economic feasibility of the medium, since the cost of soy protein and yeast extract can be significant, when in higher concentrations in the broth.
Production of mycelial biomass of Pleurotus sajor-caju strain PS-2001 in shaken flasks. The values in the bar correspond to the average of three flasks. Bars with distinct letters indicate that the averages differed statistically according to Tukey’s post hoc test (P < 0.05). Treatments are shown in Table 3
Production of P. sajor-caju PS-2001 biomass and exopolysaccharides in the stirred tank bioreactor
A constant airflow rate of 0.5 vvm was applied during the whole cultivation period, but the initial turbine agitation speed of 100 rpm had to be increased to 250 rpm in order to maintain a dissolved oxygen concentration above 30% saturation. In this experiment, pH for glucose or sucrose treatment was reduced from 6.5 (value at the beginning of the cultivation) to 5 at the end of the incubation period.
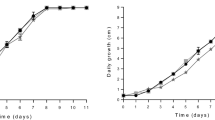
Figure 2a and b show the results obtained during the submerged culture of P. sajor-caju PS-2001 in the stirred tank bioreactor, using the formulation of treatment 8 (Table 3). Two experiments were carried out; the first used 10 g L−1 of glucose (Fig. 2a) and the second used sucrose under the same conditions (Fig. 2b). It was observed that for both treatments biomass production increased with the decrease in the carbon source, which was totally consumed after 112 h and 144 h of fermentation with glucose and sucrose, respectively. On analyzing the data referring to biomass production, it can be observed that the highest result (P < 0.05) was obtained with the medium containing glucose (8.18 g L−1) when compared with medium with sucrose (5.94 g L−1). Because of high biomass, the culture containing glucose showed the highest yield (0.82 g g−1) and productivity (0.085 g L−1 h−1) when compared to the data obtained with sucrose, 0.59 g g−1 and 0.041 g L−1 h−1, respectively.
Mycelial biomass and substrate consumption during submerged cultivation of Pleurotus sajor-caju PS-2001 in media optimized for concentrations of soy protein, yeast extract, and ammonium sulfate. Medium containing glucose (Fig. 2a) or sucrose (Fig. 2b) (10 g L−1), soy oil (1 mL L−1), salt solution (100 mL L−1), soy protein (2.3 g L−1), yeast extract (1.86 g L−1), and ammonium sulfate (1.576 g L−1)
The values observed herein for the medium containing sucrose (5.94 g L−1) were lower than those observed by Xu and Yun [27] who obtained 35.3 g L−1 of mycelial mass after 10 days when cultivating Auricularia polytrichae in a 5-L bioreactor under optimized conditions of 50 g L−1 of sucrose. With regard to other Pleurotus species, the values for P. sajor-caju biomass obtained in the present study were apparently lower than those obtained by Burns et al. [6], who observed values of 9.7 g L−1 for P. florida, with the difference that he used a medium with twice the glucose concentration [20% (w/v)] in a 2-L bioreactor. Rosado et al. [20], aiming at the production of P. ostreatus biomass in submerged culture, obtained 22.8 g L−1 of dry weight of mycelium after 9 days of incubation, however, in a medium with six times the glucose concentration used in the present work (60 g L−1).
It is important to point out that at the end of the culture in the bioreactor, when the fungal mass of P. sajor-caju was separated from the broth according to separation methodologies described by Kim et al. [12], the broth showed a concentration of exopolysaccharides of 1.18 g L−1 (glucose) or 1.58 g L−1 (sucrose). These values were similar to those found by Rosado et al. [20] for the P. ostreatus, which after 7 days of incubation produced 1.4 g L−1 of EPS, when cultivated in a liquid medium with 60 g L−1 of glucose; but they were lower than the EPS values (5.8 g of dry weight L−1) found for P. ostreatoroseus cultivated under the same conditions. The higher EPS result obtained with the sucrose treatment is in agreement with the results of Leifa et al. [16] for producing EPS by Agaricus brasiliensis, when comparing sucrose, glucose, fructose and maltose. In this study, the authors also observed that, in general, disaccharides when used as substrate are better than monosaccharides for producing EPS by A. brasiliensis.
The in vitro studies of antioxidant activity of the EPS thus obtained did not show a reducing capacity in the free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH•); however, the crude extract of mycelium showed only a small (5%) reduction. In contrast to the present data, the in vitro evaluation of the antioxidant activity of several concentrations of aqueous extract (hot water), methanol extract and ethyl acetate extract of the P. florida fruiting body revealed that for the ethyl acetate and methanol extracts there was a high capacity of hydroxyl radical scavenging and inhibition of lipid peroxidation induced by the Fe2+-ascorbate in rat liver [11].
Chemical composition of the Pleurotus sajor-caju PS-2001 mycelium produced in submerged culture
Table 4 shows the proportion of main components found in 100 g of dry mycelium of P. sajor-caju PS-2001 produced in submerged culture containing glucose and compares these data with those found for the fruiting body of the same strain obtained by Silva et al. [24]. With regard to soluble carbohydrates, the mycelium showed only 4.1%, while the fruiting body showed 19.55%. An inverse situation could be observed for the fiber proportion, where the mycelium showed 46.4% and the fruiting body showed 35.8%. Thus, the sum of both components (soluble carbohydrates and fiber), considered as total carbohydrates, was 55.5% (mycelium) and 55.35% (fruiting body). These data reveal similarities in carbohydrate content of both fungal structures analyzed and they may be compared with the sum of values of carbohydrates and fiber found in the literature for other fruiting bodies. Bisaria et al. [1] analyzed the total carbohydrate content of the fruiting body of P. sajor-caju cultivated on different agricultural wastes and found values varying between 41.2 and 471%.
The protein contents of mycelium produced in submerged culture (32.1%) and of the fruiting body cultivated on eucalyptus sawdust (36.36%) are similar. These values are higher than the data of Manu-Tawiah and Martin [18] who reported only 25.7% of protein in mycelium of P. ostreatus cultivated in a liquid medium with 45 g L−1 of glucose. The protein value obtained in a liquid culture with glucose was higher than that found in the literature for the fruiting body, since Bonatti et al. [2], on analyzing the nutritional composition of the P. sajor-caju fruiting body cultivated on banana tree straw and rice straw, observed lower protein values corresponding to 18 and 13% for each substrate, respectively.
The total fat content (10.2%) of P. sajor-caju mycelium was also similar to the value determined in P. ostreatus mycelium cultivated in a synthetic medium by Manu-Tawiah and Martin [18] in submerged culture. However, it can be observed that the mycelium was richer in total fats when compared with the values reported in the literature and with the fruiting body of the same strain, i.e., 2.3% [22].
On analyzing the fixed mineral residue of the mycelium and fruiting body (7.14 and 5.97% per 100 g of dry mass, respectively) (Table 4), it was observed that values were higher for mycelium and similar for fruiting bodies when compared with those described in the literature. Bonatti et al. [2] found contents of fixed mineral residue of 5.14 and 5.59% for the fruiting body of P. sajor-caju cultivated in banana tree straw and rice straw, respectively. The caloric value of mycelium found was high (362.6 kcal per 100 g of dry mycelium), while the values found in the literature for the fruiting body of the same fungus varied between 198 and 300 kcal per 100 g of dry mycelium [1, 7].
Thus, similarities were found between the chemical analyses of the mycelium of P. sajor-caju PS-2001 cultivated in a submerged process and of the fruiting body cultivated by Silva et al. [22] in eucalyptus sawdust, concerning quantities of total carbohydrate and fixed mineral residue. The contents of total fats and calories were higher in the mycelium than in the fruiting body. The differences found in relation to the chemical composition of the mycelium and the fruiting body of P. sajor-caju may be associated with the differences in the cultivation conditions established in each process.
From the data presented herein, it can be concluded that it was possible to develop an optimized medium with regard to concentration of soy protein, yeast extract, and ammonium sulfate to produce biomass of P. sajor-caju. High biomass concentration (8.18 g L−1) and high yield (0.82 g g−1) were obtained in culture bioreactor using the optimized medium with 10 g L−1 of glucose as carbon source. The biomass components presented values similar to those found in the literature for the fruiting body produced in solid culture, regarding the quantities of total carbohydrates, ash, and calories. However, the total fats and protein contents showed higher values for the mycelium than for the fruiting body. The use of glucose in a concentration of 10 g L−1 led to a higher mycelium biomass production of P. sajor-caju PS-2001 when compared to sucrose in the same concentration.
References
Bisaria R, Madan M, Bisaria VS (1987) Biological efficiency and nutritive value of Pleurotus sajor-caju cultivate on different agro-wastes. Biol Wastes 19:239–255. doi:10.1016/0269-7483(87)90058-9
Bonatti M, Karnopp P, Soares HM, Furlan SA (2004) Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem 88:425–428. doi:10.1016/j.foodchem.2004.01.050
Box GEP, Wilson KB (1951) On the experimental attainment of optimum conditions. J R Stat Soc 13:1–45
Brasil (2003) Resolução RDC 360. ANVISA (Agência Nacional de Vigilância Sanitária) 23 de dezembro de 2003
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. Lebenson Wiss Technol 28:25–30. doi:10.1016/S0023-6438(95)80008-5
Burns PJ, Yeo P, Keshavarz T, Roller S, Evans CS (1994) Physiological studies of exopolysaccharide production from the basidiomycete Pleurotus sp. Florida. Enzyme Microb Technol 16:566–571. doi:10.1016/0141-0229(94)90120-1
Chang ST, Lau OW, Cho KY (1981) The cultivation and nutritional value of Pleurotus sajor-caju. Eur J Appl Microb Biotechnol 12:58–62. doi:10.1007/BF00508120
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood-degrading mushrooms of genus Pleurotus. Appl Microbiol Biotechnol 58:582–594. doi:10.1007/s00253-002-0930-y
Gern RMM, Wisbeck E, Rampinelli JR, Ninow JL, Furlan SA (2008) Alternative medium for production of Pleurotus ostreatus biomass and potential antitumor polysaccharides. Bioresour Technol 99:76–82. doi:10.1016/j.biortech.2006.11.059
Hwang H-J, Kim S-W, Choi J-W, Yun J-W (2003) Production and characterization of exopolysaccharides from submerged culture of Phellinus linteus KCTC 6190. Enzyme Microb Technol 33:309–319. doi:10.1016/S0141-0229(03)00131-5
Janardhanan NJKK (2000) Antioxidant and antitumour actvity of Pleurotus florida. Curr Sci 70:941–943
Kim DH, Yang BK, Jeong SC, Park JB, Cho SP, Das S et al (2002) Production of hipoglycemic, extracellular polysaccharide from the submerged culture of the mushroom Phellinus linteus. Biotechnol Lett 23:513–517. doi:10.1023/A:1010312513878
Kim SW, Hwang HJ, Xu CP, Choi JW, Yun JW (2003) Effect of aeration and agitation on the production of mycelia mass and exopolysaccharides in an enthomopathogenic fungus Paecilomyces sinclairii. Lett Appl Microbiol 36:321–326. doi:10.1046/j.1472-765X.2003.01318.x
Kotwaliwale N, Bakane P, Verma A (2007) Changes in textural and optical propertiers of oyster mushroom during hot air drying. J Food Eng 78:1207–1211. doi:10.1016/j.jfoodeng.2005.12.033
Lee BC, Bae JT, Pyo HB, Choe TB, Kim SW, Hwang HJ et al (2004) Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible basidiomycete Grifola frondosa. Enzyme Microb Technol 35:369–376. doi:10.1016/j.enzmictec.2003.12.015
Leifa F, Soccol AT, Pandey A, Soccol CR (2006) Effect of nutritional and environmental conditions on production of exo-polysaccharide of Agaricus brasiliensis by submerged fermentation and its antitumor activity. Food Sci Technol Int 40:30–35
Mandels M, Reese ET (1957) Induction of cellulase in Trichoderma viride as influenced by carbon source and metals. J Bacteriol 73:269–278. doi:10.1002/path.1700730133
Manu-Tawiah W, Martin AM (1987) Chemical composition of Pleurotus ostreatus mycelial biomass. Food Microbiol 4:303–310. doi:10.1016/S0740-0020(87)80004-7
Miller GL (1959) Use of dinitrosalicilic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Rosado FR, Germano S, Carbonero ER, Costa SM, Iacomini M, Kemmelmeier C (2003) Biomass and exopolysaccharide production in submerged cultures of Pleurotus ostreatoroseus Sing. and Pleurotus ostreatus “florida” (Jack.: Fr.) Kummer. J Basic Microbiol 43:230–237. doi:10.1002/jobm.200390026
Shah ZA, Ashraf M, Ishtiaq CM (2004) Comparative study on cultivation and yield performance of oyster mushroom (Pleurotus ostreatus) on different substrates (wheat straw, leaves, sawdust). Pak J Nutr 3:158–160
Silva CO, Poletto NP, Cassini C, Munari FM, Dillon AJP, Salvador M (2004) Capacidade antioxidante e análise nutricional de Pleurotus sajor-caju produzido na Região Sul do Brasil. In: XII Congresso Estadual de Farmacêuticos e Bioquímicos, X Congresso Catarinense de Farmacêuticos e Bioquímicos, IV Encontro de Farmacêuticos do Mercosul e I Encontro Nacional de Farmacêuticos do SUS
Silva OS, Costa SMG, Clemente E (2002) Chemical composition of Pleurotus pulmonarius (Fr.) Quél., substrates and residue after cultivation. Braz Arch Biol Tech 45:531–535
Smith JE, Rowan NJ, Sullivan R (2002) Medicinal mushrooms: a rapidly developing area of biotechnology for cancer therapy and other bioactivities. Biotechnol Lett 24:1939–1945. doi:10.1023/A:1020994628109
Wu J-Z, Cheung PCK, Wong K-H, Huang N-L (2003) Studies on submerged fermentation of Pleurotus tuber-regium (Fr.) Singer. Part 1: physical and chemical factors affecting the rate of mycelial growth and bioconversion efficiency. Food Chem 81:389–393. doi:10.1016/S0308-8146(02)00457-0
Wu J-Z, Cheung PCK, Wong KH, Huang N-L (2004) Studies on submerged fermentation of Pleurotus tuber-regium (Fr.) Singer. Part 2: effect of carbon-to-nitrogen ratio of the culture medium on the content and composition of the mycelial dietary fibre. Food Chem 85:101–105. doi:10.1016/j.foodchem.2003.06.009
Xu CP, Yun JW (2003) Optimization of submerged-culture conditions for mycelial growth and exo-biopolymer production by Auricularia polytricha (wood ears fungus) using the methods of uniform design and regression analysis. Biotechnol Appl Biochem 38:193–199. doi:10.1042/BA20030020
Acknowledgments
This study was financially supported by the University of Caxias do Sul (UCS) and Conselho de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Confortin, F.G., Marchetto, R., Bettin, F. et al. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J Ind Microbiol Biotechnol 35, 1149–1155 (2008). https://doi.org/10.1007/s10295-008-0394-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0394-x