Abstract
Bacterial contamination of commercial fermentation cultures is a common and costly problem to the fuel ethanol industry. Antimicrobials such as virginiamycin (VIR) and penicillin (PEN) are frequently used to control contamination but there are little data available on the susceptibility of bacterial contaminants to these agents. A survey of bacterial contaminants from a wet-mill ethanol plant with no history of using antibiotics and a dry-grind facility that periodically doses with VIR found that the majority of contaminants were species of Lactobacillus. Thirty-seven isolates of Lactobacillus species from the wet-mill and 42 isolates from the dry-grind facility were tested for antimicrobial susceptibility using broth dilution and agar dilution methods. In general, the Lactobacillus isolates from the dry-grind plant had higher minimum inhibitory concentrations (MICs) for the tested agents than the isolates from the wet-mill facility. The MIC90 for VIR was 4 μg/ml for the dry-grind isolates versus 0.25 μg/ml for the wet-mill isolates; and for PEN, the MIC90’s were >8 and 2 μg/ml for the dry-grind and wet-mill isolates, respectively. Sixteen Lactobacillus isolates from the dry-grind plant but none from the wet-mill possessed vatE, a gene that encodes a streptogramin acetyltransferase associated with resistance to virginiamycin. Despite decreased susceptibility to virginiamycin, most dry-grind isolates had MICs lower than the maximal recommended application rate of 6 ppm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial contamination is a major cause of reduced yields in the fuel ethanol industry [7]. The offending microbes are usually species of lactic acid bacteria (LAB), whose fast growth rate and tolerance to alcohol and low pH allow them to quickly outnumber the culture yeast. In addition to diverting carbohydrates for bacterial growth and competing with yeast for growth factors in the media, the LAB produce deleterious end products such as lactic and acetic acids that inhibit the growth of Saccharomyces cerevisiae [19, 21]. Despite efforts to prevent contamination with extensive cleaning and disinfecting procedures, saccharification tanks and continuous yeast propagation systems can act as reservoirs of bacteria that can continually reintroduce contaminants. For this reason, antimicrobials may be used to treat or prevent contamination [9].
A number of antimicrobial agents have been described to control bacterial contamination in ethanol fermentations under laboratory conditions. Urea hydrogen peroxide reduced the numbers of five species of Lactobacillus while providing assimilable nitrogen and oxygen to aid performance by the yeast [22]. In alcoholic fermentation of wheat mash and sugar beet molasses mash, hop acids inhibited the growth of two strains of lactobacilli without reducing ethanol yields [25]. Strains of Bacillus and Lactobacillus isolated from Brazilian industrial fermentation units were shown to be susceptible to penicillin and the ionophore antibiotic monensin [28]. Other antibiotics including penicillin, virginiamycin, and tetracycline have also been shown to control contamination by select strains of lactic acid bacteria in experimentally infected alcoholic fermentations [3, 5, 9, 15]. In industrial fermentations, however, the most common commercially available products used to control contamination are based on the antibiotics virginiamycin or penicillin [7, 18].
A recent survey of bacterial contaminants of corn-based fuel ethanol plants in the USA found that bacterial loads in a wet mill facility were approximately 106 CFU/ml, while those at dry-grind facilities could reach 108 CFU/ml [26]. The identified isolates included species of Bifidobacterium, Lactococcus, Leuconostoc, Pediococcus, and Weisella. But the predominant contaminating genus was Lactobacillus, which constituted between 36 and 77% of all isolates depending on sample time and location.
There are very little data available on the antimicrobial susceptibility of bacterial contaminants from fuel ethanol plants. Indeed, most susceptibility studies on lactic acid bacteria in the scientific literature are related to food-associated strains [8, 11]. The present study was initiated to assess the level of antimicrobial resistance among bacterial contaminants in fuel ethanol facilities by examining the antimicrobial susceptibility patterns of Lactobacillus species isolated from either a wet-mill ethanol plant with no history of using antibiotics or from a dry-grind facility that uses virginiamycin.
Materials and methods
Isolation of bacterial strains
Samples (50–100 ml) were obtained from fermentors at either a continuous wet mill fuel ethanol production facility or a dry-grind ethanol facility. Both facilities were located within the Midwestern United States. The wet-mill facility had no history of using antibiotics, whereas the dry-grind facility periodically dosed the fermentation tank with virginiamycin throughout the fermentation. Samples were diluted in MRS media and plated onto MRS agar supplemented with cycloheximide (10 μg/ml) to suppress yeast growth. Random colonies were picked, and streaked for isolation three times prior to testing for identification. Lactobacillus isolates were preliminarily identified using a combination of API 50 CHL test kits and the Biolog system as previously described [26]. The following control strains from the ARS Culture Collection at the National Center for Agricultural Utilization Research, Peoria, IL, USA were used to validate the API and Biolog tests: Lactobacillus brevis strain NRRL B-4527, L. casei subsp. casei strain NRRL B-1922, L. delbrueckii strain NRRL B-763, L. fermentum strain NRRL B-4524, L. paracasei subsp. paracasei strain NRRL B-4564, L. plantarum strain NRRL B-4496, and L. rhamnosus strain NRRL B-442.
Partial 16 s rRNA genes were amplified from each strain by PCR using one of the following sets of universal primers. For wet-mill isolates, Ana1F (GCCTAACACATGCAAGTCGA) and K2R (GTATTACCGCGGCTGCTGG) primer sets were used to amplify a 480 bp product [16]. For dry-grind isolates, the U1 (CCAGCAGCCGCGGTAATACG) and U2 (ATCGGYTACCTTGTTACGACTTC) primer sets were used to amplify a 1,000 bp product [17]. The resulting PCR products were purified using a Qiagen PCR purification kit, and one strand was sequenced by standard methods with either the Ana1F or the U1 primers. The sequences obtained were compared with those in the GenBank database by using the BLASTN program [1] available at the National Center for Biotechnology Information (available at http://www.ncbi.nlm.nih.gov). More than 98% identity to a known species was considered a positive match.
Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MICs) were determined by antimicrobial susceptibility methods analogous to those described by the Clinical Laboratory Standards Institute [23]. For virginiamycin, the agar dilution method was performed on MRS agar plates containing twofold serial dilutions of virginiamycin starting with 16 μg/ml. Virginiamycin was purchased from Research Products International Corporation, Mt. Prospect, IL, USA. Inocula were diluted to a density of 0.5 McFarland units, then spotted on agar plates and incubated at 37°C in an anaerobic chamber. For all other antimicrobials, MICs were determined by the broth microdilution method using a GPN susceptibility panel manufactured by Trek Diagnostic Systems, Westlake, OH, USA. Inocula were diluted to a density of 0.5 McFarland units, then diluted 100-fold in anaerobic MRS media. Each well of the GPN panel was inoculated with 50 μl of culture, sealed, and incubated at 37°C in an anaerobic chamber. Enterococcus faecalis ATCC 29212 was used as a quality control strain for broth microdilution susceptibility testing. Results for the reference antibiotics (ampicillin, chloramphenicol, penicillin G, and tetracycline) and strain ATCC 29212 were within acceptable quality control limits [23].
Breakpoints for lactic acid bacteria have not been established by the CLSI. We therefore used the following breakpoints as proposed by the European Commission’s Scientific Committee on Animal Nutrition [2] and by Danielson and Wind [8] to interpret resistance: ampicillin, 2 μg/ml; chloramphenicol, 16 μg/ml; penicillin G, 4 μg/ml; synercid (quinupristin/dalfopristin), 4 μg/ml; tetracycline, 16 μg/ml. Virginiamycin is a streptogramin antibiotic and similar in composition to synercid; for that reason, we used the same breakpoint for virginiamycin as that for synercid (4 μg/ml).
Polymerase chain reaction
Genomic DNA was prepared from each strain using a DNeasy Tissue kit (Qiagen, Inc., Valencia, CA, USA), according to the manufacturer’s instructions. Each isolate was screened for the vatD and vatE genes by polymerase chain reaction with gene-specific primer sets [27]. For vatD, the following primer set was used: satA1-GCTCAATAGGACCAGGTGTA and satA2-TCCAGCTAACATGTATGGCG. For vatE, the following primer set was used: satG1-ACTATACCTGACGCAAATGC and satG2-GGTTCAAATCTTGGTCCG. Each isolate was also screened for the class 1 integrase gene intI1 using the following primer set: intI1F-CCTCCCGCACGATGATC and intI1R-TCCACGCATCGTCAGGC [4].
Results and discussion
Isolation and identification of isolates
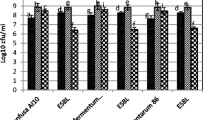
Samples were taken at strategic points along the production line from either a continuous wet-mill ethanol facility or a dry-grind operation with a single batch fermentation tank. Approximately 80 bacterial isolates from each facility were typed using phenotypic methods (API strips and the Biolog system). Wet-mill isolates included species of Bacteroides, Bifidobacterium, Clostridium, Lactobacillus, Leuconostoc, Pediococcus, and Propionibacterium. Dry-grind isolates included species of Fusobacterium, Lactobacillus, Leuconostoc, Pediococcus, Propionibacterium, and Weisella. The relative distribution of isolates in the identified genera was in agreement with that reported by Skinner and Leathers [26]. More than 50% of the isolates were identified as belonging to the genus Lactobacillus, but species identifications by the two methods did not always agree and a number of isolates were not identified by either method. A high level of phenotypic variability has previously been observed among Lactobacillus species, resulting in misidentification of lactic acid bacteria even up to the genus level [6, 14]. We therefore confirmed the identity of 37 wet-mill and 42 dry-grind isolates of Lactobacillus by sequencing partial 16 S rRNA genes. Tables 1 and 2 list the identifications of the isolates by genotypic and phenotypic methods.
From the rRNA typing data, it would appear that the diversity of Lactobacillus species is greater in the dry-grind isolates than in those from the wet-mill. The apparent homogeneity in the wet-mill isolates may merely reflect the differences in primer sets used for amplification of the partial rRNA genes. Horz et al. [13] reported that “universal” primer sets for PCR amplification of rRNA genes differ in their coverage of the domain Bacteria. We found that the U1/U2 primer set successfully produced rRNA gene amplicons from all genomic DNA templates of the dry-grind Lactobacillus isolates but not from all of the wet-mill isolates. The Ana1F/K2R primer set worked with the wet-mill isolates, but produced only a 460 bp amplicon rather than the 1,000 bp amplicon produced with the U1/U2 primer set. The use of the shorter sequence may reduce the ability of BLAST searches to discriminate between Lactobacillus species with highly identical rRNA sequences.
Antimicrobial susceptibility patterns
The Lactobacillus isolates were tested for susceptibility to select antimicrobials, and the ranges of minimum inhibitory concentrations are listed in Tables 1 and 2. MIC ranges were wider in isolates from the dry-grind plant than in those from the wet-mill, and only isolates from the dry-grind plant had MICs that exceeded the highest tested concentrations. Lower susceptibility to these agents in isolates from the dry-grind plant is also reflected in the MIC50 and MIC90 values, the concentrations of antimicrobial required to inhibit growth of 50 and 90% of the isolates, respectively (Table 3). Overall, most dry-grind isolates were less susceptible than wet-mill isolates to all drugs tested. Of particular interest are the elevated MIC90’s for penicillin and virginiamycin, the two antibiotics most frequently used commercially to control bacterial contamination in the fuel ethanol industry.
Table 3 also lists the percentage of isolates that are “resistant” to each antibiotic. It should be noted that there are no Clinical Laboratory Standards Institute guidelines for interpreting resistance in Lactobacillus species. But in a report on the safety of bacterial products intended for use as feed additives, the European Commission’s Scientific Committee on Animal Nutrition (SCAN) recommends the following resistance breakpoints for Lactobacillus: AMP, 2 μg/ml; CHL, 16 μg/ml; TET, 16 μg/ml; SYN, 4 μg/ml [2]. Although no breakpoints for penicillin or virginiamycin are recommended in the SCAN report, we chose breakpoints of 4 μg/ml for both agents based on studies of lactic acid bacteria from the dairy industry [8], and on the breakpoint for synercid, an antimicrobial similar in composition to virginiamycin. This is also a practical resistance breakpoint because the dosing range for both these agents in industrial ethanol fermentations is generally 0.25 to 2.0 ppm. Growth of isolates with an MIC for penicillin and virginiamycin of ≥4 μg/ml would not be inhibited under the general dosing regimen, and thus would be resistant to these agents.
Although resistance to PEN was prevalent among the dry-grind isolates (27 of 42 isolates with MIC ≥4 μg/ml), only five were also resistant to VIR. Four of the five VIR-resistant strains had an MIC for VIR below the maximal recommended application rate of 6.0 ppm. This suggests that simultaneous treatment with both agents would be sufficient to suppress most contaminating strains, and this approach is commercially used. However, penicillin is less stable than virginiamycin under ethanol fermentation conditions. Furthermore, it seems likely that this strategy would eventually select for multi-drug resistant strains. Indeed, one of the dry-grind isolates from the present study had MICs for VIR and PEN of >16 and 8 μg/ml, respectively.
Screening for antimicrobial resistance genes
The streptogramin antibiotics are mixtures of two components (A and B) that act synergistically to inhibit the growth of Gram-positive bacteria. Acquired resistance to either component may occur by a variety of mechanisms including enzymatic modification of the drug, active drug efflux, and modification of the drug’s target. Marked reduction in susceptibility to streptogramins requires only resistance to the A component. Virginiamycin is a streptogramin antibiotic consisting of a mixture of two cyclic lactone peptolides, factors M and S, produced by Streptomyces virginiae. It functions by binding to the 50 s subunit of the ribosome and inhibiting protein synthesis. Resistance to virginiamycin is associated with enzymes encoded by the genes vat(D) and vat(E) that inactivate the A component of virginiamycin (factor M) by acetylation of the hydroxyl group [10, 24, 29]. We screened all Lactobacillus isolates for the presence of vat(D) and vat(E). Sixteen of the dry-grind isolates (38%) but none of the wet-mill isolates possessed vat(E). The vat(D) gene was not detected in any isolates.
Interestingly, chi square analysis did not indicate a significant association (P > 0.05) between the presence of vat(E) in the dry-grind isolates and the resistance to either virginiamycin or synercid. It has previously been reported that a strain of Lactobacillus fermentum isolated from raw milk possessed vat(E) on plasmid pLME300, and that strains harboring pLME300 were resistant to dalfopristin, the A component of the streptogramin antibiotic synercid [12]. Although the vat(E) streptogramin resistance determinant was shown to be active in species of Lactobacillus, it may not be the dominant resistance determinant present in the Lactobacillus isolates from the dry-grind ethanol plant.
Localization of vat(E) to plasmid pLME300 demonstrates a possible mechanism for dissemination of resistance to other strains [12]. In the present study, however, we were unable to isolate plasmids from the vat(E) positive strains using a lyzozyme/alkaline lysis mini-prep procedure. Our continuing investigation of virginiamycin resistance in these isolates includes mapping the genetic location of the gene and screening flanking regions for sequences of mobile genetic elements such as transposons. Although Gram-positive bacteria may possess Class 1 antibiotic resistance integrons and have been shown to be major reservoirs of Class 1 integrons in poultry litter [20], no isolates from the fuel ethanol plants possessed the Class 1 site-specific integrase gene intI1. This suggests that Class 1 integrons do not play a major role in mediating antibiotic resistance in lactic acid bacteria from fuel ethanol plants.
Conclusions
In general, MICs to AMP, CHL, PEN, SYN, and VIR were higher in isolates from the dry-grind facility than in those from the wet-mill. There are many fundamental differences between the continuous fermentation at wet-mill plant and the batch fermentation at the dry-grind, but antimicrobial usage is the most significant difference in regard to the present study. Antibiotics were not used at the wet-mill facility, while virginiamycin was the only antibiotic known to be used at the dry-grind plant. Five VIR resistant strains were also resistant to PEN, and a thorough characterization of resistance genes and their localization in these isolates are warranted to investigate the possibility that VIR use may co-select for resistance to other drugs. It should be noted that neither the wet-mill nor the dry-grind facility was experiencing active contamination problems at the time of sampling. Thus the data presented here reflect the chronic state of contamination in each respective plant rather than an acute production problem. And although MICs were generally higher for the dry-grind isolates, most isolates are still susceptible to virginiamycin in the recommended application range of 0.25–2.0 ppm.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Anonymous (2003) Opinion of the Scientific Committee on Animal Nutrition on the criteria for assessing the safety on micro-organisms resistant to antibiotics of human clinical and veterinary importance. European Commission Health & Consumer Protection Directorate-General, Brussels. Available at http://www.ec.europa.eu/food/fs/sc/scan/out108_en.pdf
Aquarone E (1960) Penicillin and tetracycline as contamination control agents in alcoholic fermentation of sugar cane molasses. Appl Microbiol 8:263–268
Bass L, Liebert CA, Lee MD, White DG, Summers AO, Thayer SG, Maurer JJ (1999) The incidence and characterization of integrons, genetic elements associated with multiple drug resistance, in avian Escherichia coli. Antimicrob Agents Chemother 43:2925–2929
Bayrock DP, Thomas KC, Ingledew WM (2003) Control of Lactobacillus contaminants in continuous fuel ethanol fermentations by constant or pulsed addition of penicillin G. Appl Microbiol Biotechnol 62:498–502
Boyd MA, Antonio MAD, Hillier SL (2005) Comparison of API 50 CH strips to whole-chromosomal DNA probes for identification of Lactobacillus species. J Clin Microbiol 43:5309–5311
Connolly C (1997) Bacterial contaminants and their effects on alcohol production. In: Jacques KA, Lyons TP, Kelsall DR (eds) The alcohol textbook, 3rd edn. Nottingham University Press, Nottingham, UK, pp 317–334
Danielson M, Wind A (2003) Susceptiblity of Lactobacillus spp. to antimicrobial agents. Int J Food Microbiol 82:1–11
Day WH, Serjak WC, Stratton JR, Stone L (1954) Antibiotics as contamination-control agents in grain alcohol fermentations. J Agric Food Chem 2:252–258
De Meester C, Rodelet J (1976) Microbial acetylation of M factor of virginiamycin. J Antibiot 29:1297–1305
Florez AB, Delgada S, Mayo B (2005) Antimicrobial susceptibility of lactic acid bacterial isolated from a cheese environment. Can J Microbiol 51:51–58
Gfeller KY, Roth M, Meile L, Teuber M (2003) Sequence and genetic organization of the 19.3-kb erythromycin- and dalfopristin-resistance plasmid pLME300 from Lactobacillus fermentum ROT1. Plasmid 50:190–201
Horz HP, Vianna ME, Gomes BPFA, Conrads G (2005) Evaluation of universal probes and primer sets for assessing total bacterial load in clinical samples: general implications and practical use in endodontic antimicrobial therapy. J Clin Microbiol 43:5332–5337
Huys G, Vancanneyt M, D’Haene K, Vankerckhoven V, Goossens H, Swings J (2006) Accuracy of species identity of commercial bacterial cultures intended for probiotic or nutritional use. Res Microbiol 157:803–810
Hynes SH, Kjarsgaard DM, Thomas KC, Ingledew WM (1997) Use of virginiamycin to control the growth of lactic acid bacteria during alcohol fermentation. J Ind Microbiol Biotechnol 18:284–291
Khan AA, Nawaz MS, Robertson L, Khan SA, Cerniglia CE (2001) Identification of predominant human and animal anaerobic intestinal bacterial species by terminal restriction fragment patterns (TRFPs): a rapid, PCR-based method. Mol Cell Probes 15:349–355
Lu J, Perng C, Lee S, Wan C (2000) Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J Clin Microbiol 38:2076–2080
Lushia W, Heist P (2005) Antibiotic resistant bacteria in fuel ethanol fermentations. In: Ethanol Producer Magazine, pp 80–82
Makanjuola DB, Tymon A, Springham DG (1992) Some effects of lactic acid bacteria on laboratory-scale yeast fermentations. Enzyme Microbiol Technol 14:350–357
Nandi S, Maurer JJ, Hofacre C, Summers AO (2004) Gram-positive bacteria are a major reservoir of Class 1 antibiotic resistance integrons in poultry litter. Proc Natl Acad Sci USA 101:7118–7122
Narendranath NV, Hynes SH, Thomas KC, Ingledew WM (1997) Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl Environ Microbiol 63:4158–4163
Narendranath NV, Thomas KC, Ingledew WM (2000) Urea hydrogen peroxide reduces the numbers of Lactobacilli, nourishes yeast, and leaves no residues in the ethanol fermentation. Appl Environ Microbiol 66:4187–4192
National Committee for Clinical Laboratory Standards (1999) Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard (M31-A). National Committee for Clinical Laboratory Standards, Wayne
Rende-Fournier R, Leclercq R, Galimand M, Duval J, Courvalin P (1993) Identification of the satA gene encoding a streptogramin A acetyltransferase in Enterococcus faecium BM4145. Antimicrob Agents Chemother 37:2119–2125
Rückle L, Senn T (2006) Hop acids can efficiently replace antibiotics in ethanol production. Int Sugar J 108:139–147
Skinner KA, Leathers TD (2004) Bacterial contaminants of fuel ethanol production. J Ind Microbiol Biotechnol 31:401–408
Soltani M, Beighton D, Philpott-Howard J, Woddford N (2000) Mechanisms of resistance to quinupristin-dalfopristin among isolates of Enterococcus faecium from animals, raw meat, and hospital patients in western Europe. Antimicrob Agents Chemother 44:433–436
Stroppa CT, Andrietta MGS, Andrietta SR, Steckelberg C, Serra GE (2000) Use of penicillin and monensin to control bacterial contamination of Brazilian alcohol fermentations. Int Sugar J 102:78–82
Werner G, Witte W (1999) Characterization of a new enterococcal gene, satG, encoding a putative acetyltransferase conferring resistance to streptogramin A compounds. Antimicrob Agents Chemother 43:1813–1814
Acknowledgments
The authors thank the fuel ethanol companies that participated in this study, who requested that their contributions remain anonymous. Expert technical assistance was provided by Melinda S. Nunnally, Eric Hoecker, and Imran Khan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may be suitable.
Rights and permissions
About this article
Cite this article
Bischoff, K.M., Skinner-Nemec, K.A. & Leathers, T.D. Antimicrobial susceptibility of Lactobacillus species isolated from commercial ethanol plants. J Ind Microbiol Biotechnol 34, 739–744 (2007). https://doi.org/10.1007/s10295-007-0250-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-007-0250-4