Abstract
The physiology of Lactobacillus delbrueckii ssp. bulgaricus and Lactobacillus casei, extensively used in the dairy industry, was studied in order to evaluate key parameters in the synthesis of exopolysaccharides and to improve their production through novel fermentation processes. Selected strains were studied in shake flasks and in fermentor experiments using glucose and lactose as main carbon sources and bacto casitone as the only complex component, in a temperature range between 35 and 42°C. The production of exopolysaccharides was monitored and correlated to the growth conditions using both a colorimetric assay and chromatographic methods. Fermentor experiments in batch mode yielded 100 mg l−1 of EPS from L. bulgaricus and 350 mg l−1 from L. casei. Moreover, the use of a microfiltration (MF) bioreactor resulted in exopolysaccharides (EPS) concentrations threefold and sixfold those of batch experiments, respectively. The monosaccharidic composition of the two analyzed polymers differed from those previously reported. The optimization of the production of EPSs using the MF fermentation strategy could permit the use of these molecules produced by generally recognised as safe (GRAS) microorganisms in the place of other polysaccharides in the food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many microorganisms synthesize exopolysaccharides (EPSs), which either remain attached to the cell surface or are found in the extracellular medium in the form of amorphous slime. Several such microbial polysaccharides are now widely accepted products of biotechnology, while others are in various stages of development [1]. A number of lactic acid bacteria (LAB) are known to produce EPSs that can be beneficial for the texture of dairy products. It has been demonstrated that consumers’ preferences have moved towards sweeter and thicker yogurts, and EPSs improve the rheology of stirred yogurt, especially those containing a lower fat content [2]. Generally, they may replace polysaccharides used in the food industry as thickeners, stabilizers, emulsifiers, bodying agents, foam enhancers and gelling agents. In addition, EPSs can be produced in situ resulting in a natural product with no need for additives to improve the texture. Moreover, some studies have indicated that EPSs from LAB may be beneficial for human health; there are a few claims regarding the ability of these molecules to function as prebiotics [3]. Furthermore, it is interesting to determine for each probiotic strain whether they actually have the ability to produce EPSs, as they are believed to mediate the adhesion of the microorganisms to the gut wall. Hence, the probiotic effect might be related to the secretion of polysaccharides that have been shown to stimulate the immune response and exhibit antitumor activity [4]. Therefore, in this study, L. delbrueckii ssp. bulgaricus and L. casei were compared with regard to their ability to synthesize EPSs under different growth conditions. Fermentor batch cultures were used to analyze the influence of aeration and temperature conditions, and microfiltration (MF) experiments were exploited to improve production. The polysaccharides secreted into the medium were then recovered, purified and characterized as to their composition and chemical structure.
Materials and methods
Yeast nitrogen base without amino acids and bacto casitone peptone were purchased from Difco (Becton Dickinson, Le Pont De Claix, France). De Man, Rogosa and Sharpe Medium (MRS), M17 medium, bacteriological agar as well as the atmosphere generation system AnaeroGen Compact for solid-state incubation on petri dishes were from Oxoid (Basingstoke, England). All other chemicals needed to prepare the semi-defined medium and buffers were purchased from Sigma-Aldrich (Milan, Italy). A kit of standards, containing acetic acid, lactic acid, citric acid, butyric acid iso-butyric acid, succinic acid, oxalic acid and maleic acid was obtained from Supelco (Milan, Italy) for quantification of organic acids.
Microorganisms and media
L. delbrueckii ssp. bulgaricus (ATCC 11842; DSM, 20081) and L. casei (ATCC 393; DSM, 20011) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). The freeze-dried cultures were inoculated into Rogosa’s Medium (MRS) [5]. Petri dishes containing MRS agar were inoculated and incubated in strictly anaerobic or micro-aerophilic conditions at 37 and 35°C, respectively. The microorganisms were maintained as suspended culture (stabs) at −80°C using glycerol (20% w/v) as a cryoprotectant. These stabs were used to inoculate either flasks (1 l) containing 250 ml of medium or Pyrex bottles (250 ml) filled to the top and incubated on a rotary shaker (HT Aquatron, Infors, Switzerland) at 160 rpm. Cell growth, lactic acid and EPS production under different aeration conditions over a period of 24–30 h and at temperatures ranging from 35 to 42°C were studied. The semi-defined medium used for cultivations was developed by Kimmel and Roberts [6] and in some experiments we added a bacto casitone supplement (30 g l−1, SDMP medium). For the EPS production with L. casei, a modified SDM medium was developed (SDM-BM), containing a mineral salts solution: NaCl 0.1 g l−1, C2H2O2Na 0.5 g l−1, l-cysteine 0.1 g l−1, (NH4)2C6H5O7 0.1 g l−1, MnCl2·4H2O 0.033 g l−1, FeSO4·7H2O 0.013 g l−1, prepared as a tenfold concentrated solution and sterilized by microfiltration (Millex GP 0.2 μm filter units, Millipore).
Fermentor experiments
The fermentor used was a Biostat CT, Braun Biotech International (Melsungen, Germany), 2 l working volume, equipped with two polypropylene MF modules as previously described [7], provided with a digital control unit and connected to a PC for remote control via MFCS-win software. L. delbrueckii ssp. bulgaricus and L. casei were grown at T=35–42°C, pH=6.5; the stirring velocity was initially set between 100 and 200 rpm and increased up to 300 rpm during the experiments. The medium was sparged with nitrogen for at least 30 min after sterilization and prior to inoculation. Experiments in batch mode were carried out using the SDMP medium, or the SDMP-BM/lactose composition for L. casei, controlling the pH by addition of NH4OH (2.5 M). MF experiments were performed using SDM, starting in batch mode, and switching after 8–10 h to fed-batch and approximately 4 h later in MF mode. Each phase duration was determined after the evaluation of lactate formation, carbon source consumption rate and their influence on growth rates, in order to start the fed-batch phase when glucose concentration was lower than 5 g l−1, and the MF phase when lactate was above 15 g l−1. Filtrate exhaust medium was replaced via a pump, coupled with a level controller to maintain a constant fermentation volume, with a fresh salt solution. In this way microorganisms were held in the vessel and fed through appropriate profiles (e.g. step or linear, generally ranging from 1 to 5 g l−1 h−1) [7]. However, different from our previous paper, the C/N ratio in the nutrient solution was lowered from one-fourth to one-sixteenth during the MF phase.
Analytical methods
Cell growth was followed during experiments by measuring absorbance at 600 nm on a Beckman DU 640 Spectrophotometer (Milan, Italy). Samples collected every hour were spun down in an ALC PK 131R centrifuge at 2,000g; wet weight was measured after centrifugation and washing in saline solution (0.9% NaCl w/v). A calibration curve was constructed to relate the absorbance value to the cell dry weight. The latter was measured by drying the washed pellet overnight (16–18 h) at 85°C. One gram per liter of dry cell weight corresponded to 1.9 OD600. This correlation was extrapolated for many different fermentation experiments. The supernatant (1 ml) was ultrafiltered in a Centricon tube (10 kDa Mw cut–off, Millipore) at 5,000g to prepare the samples for analytical quantification.
Glucose and lactose concentrations were analyzed by HPAEC-PAD (Dionex model DX 500); the organic acids from the culture broth and the permeate solutions were analyzed by HPLC as previously described [7]. A quick off-line determination was obtained using Haemo-Glukotest 20–800 strips (Boehringer-Manheim, In vitro diagnosticum).
Yield coefficients were evaluated for each fermentation phase considering the overall production of LA and EPSs and the consumption of glucose or lactose.
Yield coefficients estimation:
-
Yxs=yield coefficient biomass/substrate
$$ Y_{{{\text{xs}}}} = \frac{{({\text{OD}}_{2} - {\text{OD}}_{1} ) \times 0.52}} {{(C_{{{\text{G1}}}} - C_{{{\text{G}}2}} )}} $$ -
Yps=yield coefficient product/substrate
$$ Y_{{{\text{ps}}}} = \frac{{({\text{LA}}_{2} - {\text{LA}}_{1} )}} {{(C_{{{\text{G}}1}} - C_{{{\text{G2}}}} )}} $$ -
YEPS/x=specific yield coefficient EPS produced/wet biomass weight
$$ Y_{{{\text{EPS/x}}}} = \frac{{{\text{EPS}}}} {{{\text{OD}} \times 0.52}} $$
where OD is the absorbance reading at 600 nm, C G the glucose (or lactose) concentration, LA the lactic acid concentration and EPS the exopolysaccharide concentration.
EPS isolation and quantification
After removal of cells from the fermented broth, the supernatants were recovered to purify the EPSs. To treat small volumes of supernatant (e.g., 4–10 ml) Centricon Amicon tubes (cut-off 10 kDa) were used. Samples were diafiltered with twice the volume of ultrapure water (MilliQ, Millipore), using Centricon tubes (5,000g for 60–180 min). The retentate was resuspended in deionized water to the initial sample volume and analyzed for EPS.
The procedure for EPS purification from larger broth volumes (50–1,000 ml) was modified as follows: the fermentation supernatant was ultrafiltered using an Amicon hollow fiber system, with polyethersulfone capillaries (cut-off 30 kDa) to achieve a tenfold concentration. Successively, the retentate was diafiltered in Amicon cells with polyethersulfone membranes (cut-off 10 kDa). To keep the flux constant, the N2 pressure was increased during the process from 0.5 to 2 atm; in this first step for each volume of retentate two volumes of deionized water were used to diafilter and remove low molecular weight solutes. In the second step of diafiltration the retentate was resuspended in water to reach the initial sample volume and finally precipitated overnight with ethanol (five volumes) at −20°C, and the EPS was collected after centrifugation at 10,000g before analysis.
For EPSs quantification, the anthrone/H2SO4 method was used [8]. A standard solution of carbohydrates was first prepared with a mixture of three monosaccharides: galactose, glucose and rhamnose in a 5:1:1 ratio, according to the composition reported in the literature for L. bulgaricus RR’s EPS [6].
The monosaccharide composition in EPSs was analyzed by GLC of alditol acetate derivatives. Briefly, polysaccharide samples were hydrolyzed with 2 M CF3CO2H at 120°C for 1 h, reduced with sodium borohydride for 16 h and acetylated by acetic anhydride in pyridine. The alditol acetates were identified by GLC, using inositol as an internal standard, on a SP2330 capillary column (Supelco, 30 m×0.25 mm i.d.; flow rate 1 ml min−1, N2 as carrier gas), at 235°C.
Results
Shake flasks experiments
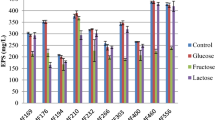
In order to evaluate the influence of the main culture parameters on the production of EPSs, shake flasks experiments were performed both on L. casei and L. bulgaricus. In all cases 30 g l−1 of BC and anaerobic conditions were used [6]. Table 1 shows the average values for optical density, growth rate, EPS and lactic acid (LA) production and the specific EPS yield for shake flasks cultivations using different substrates and temperatures. L. bulgaricus experiments showed that by increasing the initial content of glucose the final cell density increased at all temperatures. Generally, the higher the temperature, the lower the growth rate and the biomass yield. On the other hand, an increase in temperature improved the production of EPS. For the evaluation of EPS production from the strain L. casei, a similar set of experiments was carried out with increasing concentrations of substrates (glucose and lactose) at 35 and 42°C (Table 1b). In this case the lower temperature resulted in a higher biomass yield and EPS production. As shown in Table 1, during cultivation of both strains LA concentration was higher at higher initial substrate, while temperature did not significantly affect its production. In particular in shake flasks experiments on lactose a greater amount of EPS was formed, achieving a concentration of 1 g l−1 at 35°C, with a final OD600 of 3.3. These experiments also showed that EPS specific yield was greater at higher temperatures, especially when using lactose as the carbon source: 969 mg g−1 was formed for L. bulgaricus and 940 mg g−1 for L. casei.
The kinetics of production of EPSs at the optimal conditions was then determined for both strains. As shown in Fig. 1 EPSs are secreted in the medium and accumulate in the early stationary phase, and their concentrations seemed to decrease when the carbon concentration in the medium dropped below 1 g l−1 (from 35–42 h, data not shown).
Batch experiments
To investigate whether pH-stat and homogeneous conditions could affect EPS production, L. bulgaricus was grown in batch fermentor experiments using SDMP. Figure 2a, b shows the growth curves with the corresponding production of EPSs at 37 and 42°C. The final OD600 readings were 8.0 and 5.6, respectively, while LA achieved 25 g l−1 at the higher temperature and 15 g l−1 at 37°C. It can be easily recognized that the EPSs production at 42°C was outstandingly superior (0.92 g l−1, Fig. 2b); in addition, it was evident that the accumulation of EPSs in the medium is growth related. For L. casei, batch experiments were performed with SDMP-BM medium at 35 and 42°C; LA and biomass production were evaluated together with EPSs. As shown in Fig. 3a, a final OD600 of about 11 was achieved, which is fivefold the corresponding shake flasks experiments (average values from sets of four), and the final LA content was 42 g l−1 when 50 g l−1 lactose was used as substrate. At the higher temperature the maximal OD600 was 5.8 OD600 and LA concentration was 40 g l−1. In addition, a growth-related production of EPSs was confirmed, and the final concentration of exopolysaccharides obtained under controlled conditions was higher, achieving over 1.7 g l−1.
MF experiments
In order to evaluate the performance of the MF bioreactor, in comparison to traditional fermentations, experiments were undertaken using a leaner medium than the one described for previous batches. In fact, the feeding strategy during MF experiments aimed to reduce the amount of complex components, in order to contain fermentation costs. The MF experiments typically lasted 50–65 h, and they ended when the high cellular density achieved, together with the EPSs gel layer on the membrane capillaries, slowed the medium exchange to 20% of the maximum rate, causing the accumulation of lactic acid above the critical inhibition level of 35 g l−1 (Fig. 4a) [7]. L. bulgaricus batch experiments with a basic SDM yielded 2.5 g l−1 of dry biomass and 100 mg l−1 of polysaccharide (Table 2a) while in the MF experiments the biomass yield was increased sixfold, obtaining a final EPS concentration of 0.350 g l−1 (throughput of 0.800 g). In L. casei SDMP-BM batch experiments, 3 g l−1 of dry biomass was obtained and 350 mg l−1 of EPS, while in MF experiments, the biomass yield was only threefold but an EPS concentration of 2.2 g l−1 was achieved, improving the product throughput almost sevenfold (Fig. 4b).
Discussion
Microbial EPSs probably have a protective function in the natural environment and also play a major role in cell recognition and adhesion to surfaces [9]. Polysaccharide secretion is related to various environmental responses, such as stress and growth conditions, and varies widely among different species, even for strains of the same species. We analyzed the influence of parameters such as dissolved oxygen, temperature and medium composition (BC concentration) on exopolysaccharide production. In the preliminary experiments it was found that higher bacto casitone concentration and anaerobic conditions favor the production of EPSs. A few other research groups have investigated the effect of temperature on EPS production by various strains of L. delbrueckii ssp. bulgaricus [10, 11, 13] and some of these studies reported higher EPS production at growth temperatures lower than optimum (37 vs. 43°C). Kimmel and Roberts [6] found that the maximal production was similar when experiments were carried out at 30 or 40°C, but the productivity increased with temperature. Our results showed that the absolute amount produced at 42°C is definitely greater than that obtained at lower temperatures for L. bulgaricus; in addition, an increase in temperature reduced the growth rate and final biomass yield, possibly making the essential factors of the medium more available for EPS biosynthesis. This is also supported by the data shown in Table 1, where an increase in substrate concentration resulted in a higher yield coefficient EPS/biomass (YEPS/x). On the other hand, for L. casei the optimal conditions for growth, in particular a lower temperature, were also suitable to EPS production, as has been reported for other bacterial strains [12]. However, the specific production of EPS (YEPS/x) for both the strains we studied was positively influenced by a temperature increase.
Experiments on L. casei were particularly interesting because this strain has often been reported to produce a lower amount of EPS (120–200 mg l−1) with respect to L. delbrueckii ssp. bulgaricus, while here we demonstrated it could reach up to 476 mg l−1 in shake flasks using glucose as substrate (Table 1). Mozzi et al. [14] reported that the EPS production for L. casei CRL 87 was higher on galactose than on glucose. In our experiment, when lactose was used as main carbon source, the yields were higher than those obtained on glucose; however, we never detected either galactose or glucose in the media.
Production kinetics in shake flasks (Fig. 1) as well as in batch experiments (Figs. 2, 3) showed a growth-related pattern. Also, in the stationary phase, no accumulation of EPS was observed. This may be due to EPS utilization as an alternative nutrient source during cell starvation.
In order to improve EPSs yield and productivity, we applied the MF strategy, which had been proved successful in high cell density cultivations of lactic acid bacteria [7]. As shown in Table 2a, L. bulgaricus experiments carried on with continuous removal of lactic acid, resulted in a threefold EPS yield in comparison with batch fermentation, reaching a sixfold greater biomass yield. EPS was mainly recovered in the fermentation broth; only a smaller amount was detected in the MF filtrate. This finding was surprising since the MF capillaries have a 0.22 μm cut-off, and total recovery of the EPS in the filtrate could be expected. It can be argued that a gel layer formation, together with the cellular cake deposition on the capillary surfaces, introduces a further resistance to EPS transport through the membrane that resulted in EPS retention inside the vessel. This is challenging for EPS production, because the volume to treat in the downstream phase can be dimensioned without considering the permeate solution (e.g., in this case 2 l in place of 6–8 l).
Table 2b shows the MF strategy benefits on L. casei: almost a sevenfold EPS yield was achieved compared to the batch experiments in standard SDM. On the other hand, the lower improvement in biomass yield could be attributed to the lower concentration of lactose in the nutrient solution, due to its lower solubility.
The monosaccharide analysis of both EPSs was carried out by GLC of the alditol acetate derivatives. L. bulgaricus EPS was composed of mannose and, in very low amounts, galactose, 2-amino-2-deoxy-glucose, glucose and rhamnose. Likewise, L. casei monosaccharide composition showed, besides a high amount of mannose, the presence of glucose, 2-amino-2-deoxy-glucose, galactose, 2-amino-2-deoxy-galactose, rhamnose and ribose. The characterization of the primary structure of both EPSs is in progress in our laboratory.
Conclusion
Two lactobacilli closely related with probiotic strains of the L. bulgaricus and L. casei families were compared for their ability to produce exopolysaccharides from different carbon sources, and the productivity of these natural thickeners was increased substantially by finding optimal conditions for production and exploiting a microfiltration technique in fermentation experiments. The optimization of the production of EPSs could permit the use of these molecules produced by GRAS microorganisms in the place of other polysaccharides in the food industry.
References
Sutherland IW (1998) Novel and established applications of microbial polysaccharides. Trends Biotechnol 16(1):41–46
Mollet B (1999) Genetically improved starter strains: opportunities for the dairy industry. Int Dairy J 9:11–15
Roberfroid MB (1998) Prebiotics and symbiotic: concepts and nutritional properties. Br J Nutr 80:S197–S202
Kitazawa H, Toba T, Itoh T, Yamaguchi T (1991) Antitumoral activity of slime-forming encapsulated Lactococcus lactis ssp. Cremoris. J Dairy Sci 74:2082–2088
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23:130–135
Kimmel SA, Roberts RF (1998) Development of a growth medium suitable for exopolysaccharide production by Lactobacillus delbrueckii ssp. bulgaricus RR. Int J Food Microbiol 40:87–92
Schiraldi C, Adduci V, Valli V, Maresca C, Giuliano M, Lamberti M, Carteni M, De Rosa M (2003) High cell density cultivation of probiotics and lactic acid production. Biotechnol Bioeng 82(2):213–222
Scott TA Jr, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 25(11):1656–1661
Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12:163–171
Garcia-Garibay M, Marshall VME (1991) Polymer production by Lactobacillus delbrueckii ssp. bulgaricus. J Appl Bacteriol 70:325–328
Grobben GJ, Sikkema J, Smith MR, de Bont JAM (1995) Production of extracellular polysaccharides by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in a semidefined medium. J Appl Bacteriol 79:103–107
Degeest B, Janssens B, De Vuyst L (2001) Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0–1: production kinetics, enzyme activities and EPS yields. J Appl Microbiol 91(3):470–477
Bouzar F, Cerning J, Desmazeaud M (1996) Exopolysaccharides production in milk by Lactobacillus delbrueckii ssp. bulgaricus CNRZ 1187. J Dairy Sci 79:205–211
Mozzi F, Rollan G, Savoy de Giori G, Font de Valdez G (2001) Effect of galactose and glucose on the exopolysaccharide production and the activities of biosynthetic enzymes in Lactobacillus casei CRL 87. J Appl Microbiol 91:160–167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schiraldi, C., Valli, V., Molinaro, A. et al. Exopolysaccharides production in Lactobacillus bulgaricus and Lactobacillus casei exploiting microfiltration. J IND MICROBIOL BIOTECHNOL 33, 384–390 (2006). https://doi.org/10.1007/s10295-005-0068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0068-x