Abstract
Industrial wastewater is often polluted by Cr(VI) compounds, presenting a serious environmental problem. This study addresses the removal of toxic, mutagenic Cr(VI) by means of microbial reduction to Cr(III), which can then be precipitated as oxides or hydroxides and extracted from the aquatic system. A strain of Staphylococcus epidermidis L-02 was isolated from a bacterial consortium used for the remediation of a chromate-contaminated constructed wetland system. This strain reduced Cr(VI) by using pyruvate as an electron donor under anaerobic conditions. The aims of the present study were to investigate the specific rate of Cr(VI) reduction by the strain L-02, the effects of chromate and nitrate (available as electron acceptors) on the strain, and the interference of chromate and nitrate reduction processes. The presence of Cr(VI) decreased the growth rate of the bacterium. Chromate and nitrate reduction did not occur under sterile conditions but was observed during tests with the strain L-02. The presence of nitrate increased both the specific Cr(VI) reduction rate and the cell number. Under denitrifying conditions, Cr(VI) reduction was not inhibited by nitrite, which was produced during nitrate reduction. The average specific rate of chromate reduction reached 4.4 μmol Cr 1010 cells−1 h−1, but was only 2.0 μmol Cr 1010 cells−1 h−1 at 20 °C. The maximum specific rate was as high as 8.8–9.8 μmol Cr 1010 cells−1 h−1. The role of nitrate in chromate reduction is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is a transition metal which is able to exist in several oxidation states. The most stable and common forms are the trivalent Cr(III) and hexavalent Cr(VI) species [6, 31]. Cr(VI) is considered to be the most toxic and carcinogenic form of Cr, and is usually associated with oxygen as chromate (CrO 2−4 ) or dichromate (Cr2O 2−7 ) ions. By contrast, Cr(III) forms insoluble oxides and hydroxides above pH 5 [25], is much less mobile, and mostly exists bound to organic matter in soil and aquatic environments [18]. The use of chromium in leather-tanning, electroplating, paint pigment and dye production, automobile manufacturing, the steel industry and other industries has led to Cr(VI) being discharged into natural ecosystems. Since high levels of Cr(VI) may overcome the reducing capacity of the environment, it persists as a pollutant. Problems of incremental chromate pollution and the relatively low cost of biological methods of heavy metal recovery have encouraged interest in both Cr(VI)-reducing microorganisms and chromate-resistant bacteria. A wide variety of bacteria belonging to various systematic and physiological groups are involved in Cr(VI) reduction under anaerobic conditions [1, 3, 8, 10, 16, 20, 21, 23, 24, 32]. However, the reduction of CrO 2−4 —a terminal electron acceptor during anaerobic respiration—may not produce enough energy to enable bacterial growth [14, 15]. Consequently, the distribution of chromate reduction in the bacterial world is not specific and can be affiliated to various physiological processes, such as simultaneous reductive processes with alternative electron acceptors (nitrate, nitrite, sulphate). These compounds are present in contaminated systems and often vary spatiotemporally. Cr(VI) reduction by bacterial consortiums has been shown to be related to sulphate and nitrate reduction [30]. It has also been suggested that the microbial reduction of nitrate, Cr(VI) and sulphate takes place consecutively [4, 13].
As far as bioremediation applications are concerned, it is important to know how various electron acceptors affect Cr(VI) reduction, and in turn how Cr(VI) affects other terminal electron-accepting processes. Hence, the goal of this work was to investigate the interaction of chromate and nitrate reduction by Staphylococcus epidermidis in model experiments.
Materials and methods
Microorganism
The pure bacterial culture, strain L-02, was isolated by the authors from the active chromium-reducing bacterial consortium obtained from the Grosskayna experimental wetland station in Merseburg, Germany [30]. The culture was identified at the German collection of microorganisms and cells (DSMZ). Its 100% similarity with the type strain of Staphylococcus epidermidis was determined by 16SrRNA sequencing [17, 26].
Reduction experiments
The basic medium for culturing S. epidermidis L-02 contained (g/L): KH2PO4, 0.9; Na2HPO4·2 H2O, 1.2; NH4Cl, 0.5; yeast extract, 0.9; Na pyruvate, 2.0; NaHCO3, 0.2; MgSO4·7 H2O, 0.5 (pH 7). The high concentration of phosphates resulted in no change of pH during experiments. The experiments were performed in 55 ml Wheaton glass serum bottles (Sigma) with 25 ml of the medium. The bottles were purged with N2, sealed with Wheaton butyl stoppers (Sigma), and sterilized in an autoclave at 105 °C for 20 min. Stock solutions of K2Cr2O4 (10 g Cr(VI)/L) and KNO3 (100 g/L) were sterilized separately and then added to the base medium either individually or combined to reach the final concentrations. All the supplements (inoculum, Cr (VI) and nitrate) were added by injection with sterile syringes. The ability of the isolated strain to reduce Cr(VI) was examined in cultures with 0.3 mmol (standard concentration) or 0.6 mmol (double concentration) Cr(VI) in the medium. The final working nitrate concentration was 3.2 mmol. Despite the strain’s optimal temperature of 30–37 °C, our experiments were carried out at 20 °C to expand the process dynamics over time.
Analytical methods
Chromium (VI) concentration was estimated with diphenylcarbazide reagent (1% in acetone) and the resulting colour was measured at 540 nm using the standard method [30]. Nitrate and nitrite concentrations were analysed by ion chromatography using a Dionex 100 (AS4A-SC column/AG4A-SC column) with UV detection at 215 nm (NO −3 /NO −2 ) [22]. Cell numbers were counted by direct microscopy in a counting chamber [34]. All the bottles were set up in duplicate.
Data analyses
Specific reduction rates were described as the relationship between the substrate concentration decrease per time unit and the number of bacterial cells. These specific rates can be expressed mathematically as: V sp = dC/N*t, where V sp is the specific reduction rate in μmol Cr 1010 cells−1 h−1, dC the difference in the reduced substrate concentrations in micromole, N the cell concentration in 1010/L, and t the time in hours. Analyses of parallel samples showed that variations never exceeded 5–8%.
Results and discussion
A representative of the Staphylococcus genus, namely S. cohnii, has already been reported to reduce chromate [27]. We found that our isolated S. epidermidis strain L-02 can also reduce chromate. Resistance to Cr(VI) has been shown for several species of the Staphylococcus genus including S. epidermidis [29]. Nevertheless, whether all the members of this genus possess chromate-reducing ability is yet unknown. Information on the reductive activity of Staphylococci suggests that one of the typical genus features is nitrate reduction. We tested the combined and separate effects of chromate and nitrate on the strain L-02 under anaerobic conditions in the presence of pyruvate, which provided an alternative possibility of growth with a fermentative process. In addition, pyruvate could be used as an electron donor in processes of nitrate or chromate reduction.
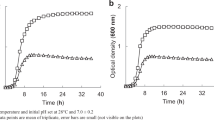
Changes in the cell numbers during the experiments showed that nitrate supplementation stimulated the growth of the culture. The increase in the cell numbers in the medium containing nitrate was faster than in the basic medium. Furthermore, cell numbers in the medium with nitrate and chromate increased faster than with just chromate (Fig. 1). By contrast, the presence of chromate inhibited the rate of multiplication and the cell yield. Thus, chromate depressed the growth of both S. epidermidis L-02 and various other bacterial species [2, 11].
Dynamics of S. epidermidis L-02 cell numbers during growth with various substrates. (Filled square medium without nitrate or chromate (fermentative growth), open triangle medium spiked with nitrate, filled triangle medium spiked with chromate, open diamond medium spiked with chromate and nitrate, open square medium spiked with nitrate and double concentration of chromate)
During experiments with 0.3 mmol Cr (VI), the strain S. epidermidis L-02 totally reduced chromate in 3 days at 30 °C. The maximum specific reduction rate at 30 °C was 1.025 mmol Cr 1010 cells−1 h−1, meaning the strain can clearly be classified as an active chromate-reducer. Reported or calculated values of maximum specific Cr(VI) reduction available in the literature for other microorganisms under anaerobic conditions are shown in Table 1. No Cr (VI) reduction occurred in cell-free control experiments. The data presented were obtained at 20 °C to show differences between the various experiments in more detail. Although the change of temperature did not alter the basic regularities of the processes, it slowed down reduction: 0.3 mmol chromate was totally reduced in just 13 days (Fig. 2). When the initial Cr (VI) concentration was twice as high (0.6 mmol), the beginning of the process was observed later, even though the same amount of Cr (VI) was reduced within 13 days (data not shown).
Kinetics of chromate reduction by S. epidermidis L-02 depending on available electron acceptors. (Filled rectangle Cr(VI) concentration in the medium spiked with chromate, open rectangle Cr(VI) concentration in the medium spiked with chromate and nitrate, filled triangle specific rate of Cr(VI) reduction in the medium spiked with chromate, open circle specific rate of Cr(VI) reduction in the medium spiked with chromate and nitrate)
The physiological features of the culture during the process of chromate reduction are characterized by the specific rate of reduction and its change in the experiments. The study on the specific rate of Cr(VI) reduction revealed a few stages of the process: (1) adaptation during the first 3 days (initial growth, enzyme induction), (2) a peak of activity on the fourth day (initial phase of active growth and the cell multiplication related to fast reductase formation as a protective mechanism), (3) a stable phase (5–13 days for the variant with no nitrate and 5–10 days for the variant supplemented with nitrate) of the reduction processes, (4) the appearance of a new peak of the specific nitrate reduction activity when chromate was nearly exhausted but nitrate was still present in the medium (only in the variant supplemented with nitrate). In the medium supplemented with nitrate, the biomass increase (Fig. 1) did not cause a corresponding increase in the specific Cr reduction rate. The same situation was observed in the medium supplemented with chromate only. (Fig. 2). Nevertheless, the specific rate of Cr reduction was fivefold higher in the absence of nitrate. This fact testifies to the fact of the nitrate competition as an alternative final electron acceptor to chromate. The chromate and nitrate reductions appear to be provided with the same reductase complex.
Nitrate was completely reduced within 4 days at 20 °C by the bacteria when Cr (VI) was not added to the medium (Fig. 3). Supplemented Cr (VI) exerted an influence on bacterial nitrate reduction by the strain: in experiments with 0.3 mmol Cr (VI), the nitrate concentration decreased drastically and vanished only after Cr (VI) had been completely reduced (Fig. 3).
Kinetics of nitrate reduction by S. epidermidis L-02 depending on available electron acceptors. (Filled rectangle NO −3 concentration in the medium spiked with nitrate, open rectangle NO −3 concentration in the medium spiked with chromate and nitrate, filled triangle specific rate of NO −3 reduction in the medium spiked with nitrate, open circle specific rate of NO −3 reduction in the medium spiked with chromate and nitrate)
Judged by the specific rate, the nitrate reduction process also showed different stages (Fig. 3). There was an increase at the beginning of growth and at the end of the stable phase when chromate was more or less exhausted (Fig. 2). The specific rate of nitrate reduction at log-phase was about 3.5 times higher in the absence of chromate (Fig. 3).
Nitrate reduction was accompanied by the appearance and accumulation of nitrite. In the final analyses, nitrite accounted for approximately 30% of the initial nitrate concentration at 20 °C and reached more than 60% at 30 °C. We discovered no relation between nitrite accumulation and chromate reduction (Fig. 4). It had been suggested that the interaction of nitrite with chromate can result in chemical chromate reduction associated with nitrite oxidation [19]. Then again, other authors [33] using a fixed initial Cr(VI) concentration of 0.04 mmol and varying the nitrite concentration in the range of 0–2 mmol have reported the inhibition of specific rates of Cr(VI) reduction with nitrite for Shewanella oneidensis MR-1. However, according to our experiments, this effect is only affiliated with their specific strain. None of these hypotheses were supported by our investigations into nitrite and chromate interaction in sterile medium. Neither our tests on Paracoccus denitrificans DSM 415 reducing nitrate via nitrite in the presence of chromate (data not shown) nor our sterile chemical experiments with nitrite and chromate in phosphate buffer significantly lowered the Cr(VI) concentration within a week. Moreover, the accumulation of nitrite in our experiments with S. epidermidis L-02 did not affect chromium reduction itself. We must thus conclude that our strain reduced chromate via a direct mechanism and not nitrite production. We tested the possibility of Cr(VI) reduction with biogenic nitrite: the bacterial cells were collected by centrifugation and removed from the liquid. This halted Cr(VI) reduction and the cultural cell-free medium did not enable the reduction process. These results also testified that the Cr(VI)-reducing enzymes were bound to the cells. Dmitrienko et al. [4, 5] studying chromate and nitrate reduction by Pseudomonas sps. reported that nitrate was reduced only after chromate reduction. This sequence may be explained by the redox potentials of the anion groups mentioned. They suggested that bacteria used both chromate and nitrate as electron acceptors to gain energy, and that chromate tends to be used before nitrate. Our experiments with S. epidermidis L-02 showed that the strain reduced chromate and nitrate simultaneously. Similar data were published for Bacilli sps.: chromate reduction was not affected by a 20-fold excess of nitrate serving as an alternative electron acceptor [9]. However, differences between specific rates of reduction in the presence or absence of the second electron acceptor may be evidence of the partial alternative use of these compounds with the same or related enzymes. In addition, the slight increase in the NO −3 reduction rate before Cr(VI) had been exhausted can be attributed to both the decrease in the Cr(VI)-inhibiting effect and the delivery of an additional nitrate reductase system already used for Cr(VI) reduction.
Nitrite production by S .epidermidis L-02 depending on available electron acceptors at 30 °C. (Filled rectangle NO −3 concentration in the medium spiked with nitrate, filled triangle NO −3 concentration in the medium spiked with nitrate and chromate, open rectangle NO −2 concentration in the medium spiked with nitrate, open triangle NO −2 concentration in the medium spiked with nitrate and chromate, open circle Cr(VI) concentration in the medium supplemented with nitrate and chromate)
Conclusions
This paper describes the pattern of chromate reduction by S. epidermidis. The average specific reduction rate was 4.4 μmol Cr 1010 cells−1 h−1 at 30 °C. The specific chromate reduction rate at 20 °C was generally only 2.0 μmol Cr 1010 cells−1 h−1, although at the maximum stages it reached 8.8 μmol Cr 1010 cells−1 h−1 without nitrate supplements and 9.8 μmol Cr 1010 cells−1 h−1 in the presence of nitrate. Nitrate also stimulated Cr(VI) reduction by S. epidermidis L-02 by increasing the cell numbers, too. The nitrite produced did not affect the process of Cr(VI) reduction under the experimental conditions. The mutual negative effect of nitrate and chromate on the specific reduction rate can be explained as the alternative use of the oxidizers by joint enzymes.
References
Bae WC, Kang TG, Kang IK, Won YJ, Jeong BC (2000) Reduction of hexavalent chromium by Escherichia coli ATCC 33456 in batch and continuous cultures. J Microbiol 38:36–39
Bopp LH, Ehrlich HL (1988) Chromate resistance and reduction in Pseudomonas fluorescens strain LB300. Arch Microbiol 150:426–431
Dhakephalkar PK, Bhide JV, Paknikar KM (1996) Plasmid mediated chromate resistance and reduction in Pseudomonas mendocina MCM B-180. Biotechnol Lett 18:1119–1122
Dmitrenko GN, Konovalova VV, Shum OA (2002) The consecution of bacterial Сr(VI) and NO3− at their simultaneous presence in cultivation medium. Khimiya i Vodnaya Tehnologiya 24:578–583 (in Russian)
Dmitrenko GN, Konovalova VV, Shum OA (2003) The reduction of Cr(VI) by bacteria of the genus Pseudomonas. Mikrobiologiya 72:370–373 (in Russian)
Fendorf S, Wielinga BW, Hansel CM (2000) Chromium transformations in natural environments: the role of biological and abiological processes in chromium(VI) reduction. Inter Geol Rev 42:691–701
Francis CA, Obraztsova AY, B.M.Tebo BM (2000) Dissimilatory metal reduction by the facultative anaerobe Pantoea agglomerans SP1. Appl Environ Microbiol 66:543–548
Ganguli A, Tripathi AK (2002) Bioremediation of toxic chromium from electroplating effluent by chromate-reducing Pseudomonas aeruginosa A2Chr in two bioreactors. Appl Microbiol Biotechnol 58:416–420
Garbisu C, Alkorta I, Llama MJ, Serra JL (1998) Aerobic chromate reduction by Bacillus subtilis. Biodegradation 9:133–141
Guha H, Jayachandran K, Maurrasse F (2001) Kinetics of chromium (VI) reduction by a type strain Shewanella alga under different growth conditions. Environ Pollut 115:209–218
Gvozdyak PI, Mogilevich NF, Rylsky AF, Grishchenko NI (1986) Reduction of chromium (VI) by collection bacterial strains. Mikrobiologiya 55:962–965 (in Russian)
Hissner F, Matusch J, Heinig K (1999) Determination of sulphur-containing inorganic anions by dual ion chromatography and capillary electrophoresis—application to the characterization of bacterial sulphur degradation. Fresenius J Anal Chem 365:647–653
Hong J, Sewell GW (1999) Organic electron donors for the microbial Cr(VI) reduction. In: Bioremediation of metals and inorganic compounds. Proceedings of the 5th international in-situ and on-site bioremediation symposium, San Diego, California 135–140
Lovley DR (1993) Dissimilatory metal reduction. Annu Rev Microbiol 47:263–290
Lovley DR, Coates JD (1997) Bioremediation of metal contamination. Curr Opin Biotechnol 8:285–289
Mabbett AN, Macaskie LE (2001) A novel isolate of Desulfovibrio sp. with enhanced ability to reduce Cr(VI). Biotechnol Lett 23:683–687
Maidak BL, Cole JR, Parker CT Jr, Garrity GM, Larsen N, Li B, Lilburn TG, McCaughey MJ, Olsen GJ, Overbeek R, Pramanik S, Schmidt TM, Tiedje JM, Woese CR (1999) A new version of the RDP (Ribosomal database Project). Nucl Acids Res 27:171–173
Marsh TL, Leon NM, McInerney MJ (2000) Physicochemical factors affecting chromate reduction by aquifer materials. Geomicrobiol J 17:2394–2399
Muller RF, Goeres D, Sturman P, Sears J (1998). Using microbial dynamics in-situ consortia in hydrocarbon reservoirs for the inhibition of souring. In: Proceedings of the 5th international petroleum environmental conference, Albuquerque, New Mexico, pp 1396–1414
Nepple BB, Kessi J, Bachofen R (2000) Chromate reduction by Rhodobacter sphaeroides. J. Industr Microbiol Biotechnol 25:198–203
Nozawa M, Hu HY, Fujie K, Tsuchida T, Urano K (1998) Population dynamics of chromate reducing bacteria in a bioreactor system developed for the treatment of chromate wastewater. Water Sci Technol 37:109–112
Ohtake H, Fujii E, Toda K (1990) Bacterial reduction of hexavalent chromium: kinetic aspects of chromate reduction by Enterobacter cloaceae HO1. Biocatalysis 4:227–235
Park CH, Keyhan M, Wielinga B, Fendorf S, Matin A (2000) Purification to homogeneity and characterization of a novel Pseudomonas putida chromate reductase. Appl Environ Microbiol 66:1788–1795
Pattanapipitpaisal P, Brown NL, Macaskie LE (2001) Chromate reduction by Microbacterium liquefaciens immobilised in polyvinyl alcohol. Biotechnol Lett 23:61–65
Rai D, Sass BM, Moore DA (1987) Chromium (III) hydrolysis constants and solubility of chromium (III) hydroxide. Inorg Chem 26:345–349
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. new. Int J Syst Bacteriol 46:1088–1092
Saxena D, Levin R, Firer MA (2000) Removal of chromate from industrial effluent by a new isolate of Staphylococcus cohnii. Water Sci Technol 42:93–98
Shen H, Wang YT (1994) Modeling hexavalent chromium reduction in Escherichia coli 33456. Biotechnol Bioeng 43:293–300
Ug A, Ceylan O (2003) Occurrence of resistance to antibiotics, metals, and plasmids in clinical strains of Staphylococcus spp. Arch Med Res 34:130–136
Vainshtein M, Kuschk P, Mattusch J, Vatsourina A, Wiessner A. (2003) Model experiments on microbial removal of chromium from contaminated groundwater. Water Res 37:1401–1405
Venitt S, Levy LS (1974) Mutagenicity of chromate in bacteria and its relevance to chromate carcinogenesis. Nature 250:493–495
Viamajala S, Peyton BM, Apel WA, Petersen JN (2002) Chromate reduction in Shewanella oneidensis MR-1 is an inducible process associated with anaerobic growth. Biotechnol Progress 18:290–295
Viamajala S, Peyton BM, Petersen JN (2003) Modeling chromate reduction in Shewanella oneidensis MR-1: development of a novel dual-enzyme kinetic model. Biotechnol Bioeng 83:790–797
Yagodka SN (1975) A new variant of method of direct count of bacteria in water. Mikrobiologiya 44:169–170 (in Russian)
Acknowledgement
The research was kindly supported by the Linkage NATO grant EST-CLG-978918.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vatsouria, A., Vainshtein, M., Kuschk, P. et al. Anaerobic co-reduction of chromate and nitrate by bacterial cultures of Staphylococcus epidermidis L-02. J IND MICROBIOL BIOTECHNOL 32, 409–414 (2005). https://doi.org/10.1007/s10295-005-0020-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0020-0